ABSTRACT
Immune checkpoint inhibitors (ICIs) are new therapeutic strategies for non-small cell lung cancer (NSCLC). We aimed to quantitatively evaluate the efficacy and safety of ICIs in NSCLC. Pubmed, Embase, Cochrane Library, and Web of Science were searched for randomized clinical trials comparing ICIs with control therapies in NSCLC. Data were pooled according to Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines. A total of 12 trails comprising 6,919 NSCLC patients were included in this meta-analysis. ICIs therapies significantly improved progression-free survival (PFS) (HR, 0.838; P < 0.001), overall survival (OS) (HR, 0.747; P < 0.001) and objective response rates (ORR) (RR, 1.311; P < 0.001) in NSCLC. Prognostic benefit was observed irrespective of age, sex, treatment line, performance status and histology. Survival improvement of ICIs was limited for NSCLC patients with non-smoker (PFS, P = 0.468; OS, P = 0.317) or central nervous system (CNS) metastasis (PFS, P = 0.209; OS, P = 0.090), or positive EGFR mutation (PFS, P = 0.083; OS, P = 0.522) or PD-L1 expression level less than 5% (PFS, P = 0.370; OS, P = 0.047). The relative risks of all-grade and high-grade (≥3) anemia, neutropenia, leukopenia, thrombocytopenia, stomatitis, nausea, pyrexia, asthenia and neuropathy were all decreased in patients received ICIs compared with control therapies. This meta-analysis provides clinical evidence that ICIs improve PFS, OS, and ORR in NSCLC with fewer adverse effects. Our data establish ICIs as a prefer treatment option for NSCLC patients with smoker, no CNS metastasis, wild type EGFR, and high PD-L1 expression.
Introduction
Traditional chemotherapy for non-small cell lung cancer (NSCLC) has limited outcomes benefit and the “one size fits all” treatment modality should be changed.Citation1 Advances of tumor biology promote the development of target therapies. Those targeted drugs block vital cellular signaling pathways, such as vascular endothelial growth factor and epidermal growth factor pathways. But limitations of target therapies should not been ignored. According to our previous studies, about 50% of NSCLC patients have no specific genetic mutations or identifiable targets.Citation2,Citation3 Limited patients (25%) seem to benefit from targeted therapies.Citation4 Novel modalities that are effective in a majority of NSCLC patients with less toxicity are urgently needed.
Over the past decades, the findings of immune checkpoint molecules had revolutionized anti-cancer therapies. Immune checkpoint inhibitors (ICIs) could restore anti-tumor immunity, which block immunosuppressive molecules such as programmed cell death protein 1 (PD-1), programmed cell death 1 ligand 1 (PD-L1) and cytotoxic T lymphocyte associated protein 4 (CTLA-4). Currently, ICIs therapies have been novel management options and changed the therapeutic paradigm in NSCLC. Several ICIs have been developed for NSCLC, such as nivolumab, pembrolizumab, atezolizumab, durvalumab, and ipilimumab.Citation5-Citation9 The clinical efficacy of ICIs in NSCLC, as a part of combination therapies or single agent had been evaluated.Citation5-Citation11 But the results were inconsistent. A previous meta-analysis reported that ICI immunotherapy is effective for patients with cancers.Citation12 However, the meta-analysis was across different tumor subtypes, included melanoma, and small cell lung cancer, not focused on NSCLC. Another meta-analysis suggested that ICIs are overall better tolerated than chemotherapy.Citation13 While, this study did not analyze the survival benefit and tumor response. In a recent meta-analysis, Sheng Z et al.Citation14 demonstrated that anti-PD-1/PD-L1 therapies could improve the progression free survival (PFS) (HR, 3.20; P < 0.001), but not overall survival (OS) (HR, 1.30; P = 0.180) compared with control therapies. However, the data resulted from indirect comparisons and excluded anti-CTLA4 therapies. These studies drew different even contradictory conclusions. The efficacy and safety of ICIs in NSCLC remain unclear. Above all, several novel ICIs clinical trials in NSCLC were emerging since then. Pooled analyses of currently available studies may provide clinically useful information and optimize the management of NSCLC. Therefore, we performed this meta-analysis of randomized controlled trials (RCTs) to summarize the up to-date evidence.
Methods
Search methods and study selection
Two investigators independently searched the PubMed, EMBASE, Cochrane Library and Web of Science databases with the following key words: “nivolumab”, “pembrolizumab”, “atezolizumab”, “durvalumab”, “ipilimumab”, “MDX-010”, “BMS-963558”, “MK-3475”, “MPDL3280A”, “OPDIVO”, “KEYTRUDA”, “cancer”, or “lung cancer”. Reference lists of original articles and reviews were also examined. The articles search was conducted up to 10 September 2017 and language was limited to English. Search results were double-checked by two investigators (Shuai Wang and Jiatao Hao) and the discrepancies were resolved by discussion to validate the accuracy of extraction. Articles from the initial search that match the criteria below were eligible. (1) The studies must be prospective phase II or phase III RCTs to investigate the usage of ICIs in NSCLC. (2) NSCLC must be histopathologically confirmed. (3) None of patients received ICIs treatment before the trials. (4) The reports must analyzed one of endpoints, such as PFS, OS, objective response rate (ORR), and treatment-related adverse effects (AEs). Studies were excluded if (1) patients had benign lung tumor or small cell lung cancer or metastatic cancer from the other organs; (2) data were not provided regarding baseline characteristics of NSCLC patients; (3) trials were not RCTs; (4) case studies, review articles, and animal or in vitro studies; (5) articles were presented only as meeting abstracts without full-text original articles. Reporting of AEs is needed to ensure completeness and transparency of RCTs. This would enable a more precise evaluation of therapeutic risks and benefits. Factors pertinent to the assessment of AEs such as frequency and severity were specifically incorporated into the meta-analysis. Treatment related AEs were assessed by Common Terminology Criteria for Adverse Events (CTCAE 3.0). For evaluation of AEs risk, we calculated RRs and their 95% CIs based on the number of patients with AEs from each RCT. Therefore, trails that did not provide the number of patients with AEs were excluded.
Data extraction and definition
The Preferred Reporting Items for Systematic Reviews and Meta analyses (PRISMA) statements were used to provide complete information of RCTs.Citation15 All data were independently extracted by two authors according to PRISMA. The primary end point was defined as PFS to standardize data collection. The secondary end points included OS, ORR, and common treatment related AEs. The DFS was defined as the time from random assignment to disease progression and OS time was calculated from random assignment to the date of death from any cause. Tumor response was defined as progressive disease, stable disease, partial response or complete response based on the Response Evaluation Criteria in Solid Tumors criteria.Citation16 The ORR was defined as the proportion of patients with complete or partial response. Central nervous system (CNS) metastasis of NSCLC at baseline was determined according to criteria reported by previous studies.Citation8,Citation10 Patients performance status was evaluated by Eastern Cooperative Oncology Group (ECOG) performance status score (on a 5-point scale, with higher numbers indicating greater disability). The quality of RCTs was evaluated based on Jadad scale.Citation17
PD-L1 biomarker analysis
The PD-L1 expression was evaluated through immunohistochemical assay using monoclonal anti-human PD-L1 antibody.Citation5-Citation7 PD-L1 expression should be detected in pretreatment specimens, sush as biopsy and resected samples. Positive PD-L1 expression was defined as staining of the tumor-cell membrane. PD-L1 expression was validated quantitatively at specified level of < 5%, ≥ 5%, or ≥ 50% of tumor cells in a section that included at least 100 tumor cells.Citation5-Citation7 Patients with PD-L1 expression level of at least 5% included those with PD-L1 expression level of at least 50%.
Statistical analysis
The choice of fixed or random-effects model was determined through Mantel-Haenszel method. Sensitivity analyses were performed by excluding each study at a time individually. The publication bias was assessed by Begg's funnel plots and Egger's linear regression test. P < 0.05 was defined as significant publication bias. Meta regression with random-effects model was performed to evaluate potential effects of clinical variables on outcomes. The restricted maximum likelihood method was carried out to evaluated the residual between-trial variance and heterogeneity degree. Monte Carlo permutation test was performed with 10,000 random permutations.Citation18 Stata 11.0 (Stata Corporation, College Station, TX, USA) was used in this meta-analysis. Differences were considered statistically significant at two sided P < 0.05.
Results
Eligible studies and characteristics
Our initial search retrieved 724 references. After carefully screening abstract and full text of references, 12 trails were finally included.Citation5-Citation11,Citation19-Citation23 The selection steps were summarized in the flow diagram (). These 12 RCTs enrolled a total of 6,919 NSCLC patients (ICIs arm: 3,598, control arm: 3,321). Among 12 RCTs, 7 were anti-PD-1 (4 on nivolumab;Citation5,Citation19-Citation21 3 on pembrolizumab,Citation6,Citation10,Citation22) 3 were anti-PD-L1 (2 on atezolizumab;Citation7,Citation23 1 on durvalumab,Citation8) and 2 were anti-CTLA-4 (2 on ipilimumab.Citation9,Citation11) These studies comprised 3 phase II, 1 phase II/III and 8 phase III clinical trials. ICIs were compared with placebo in one study. Eight studies were ICIs versus chemotherapy and 2 studies were chemotherapy plus ICIs versus chemotherapy alone. The trial quality was quite good with Jadad score of 5 in all RCTs. The characteristics of 12 RCTs were listed in Supplementary Table S1.
Progression free survival
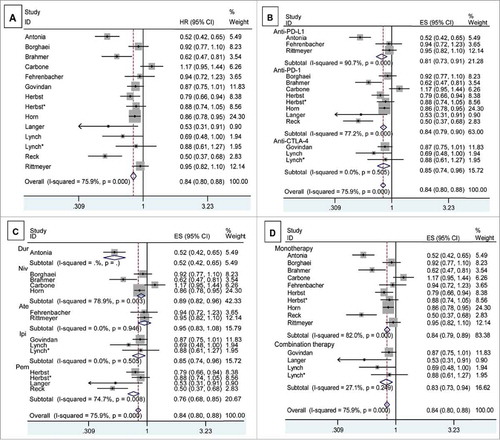
Twelve studies reported the PFS of NSCLC patients. Heterogeneity analysis revealed that there was significant between-study heterogeneity (chi-squared = 54.05, P < 0.001, I-squared = 75.9%). A statistically significant PFS improvement was observed in ICIs arm (HR, 0.838; 95% CI, 0.796 – 0.882; P < 0.001) (). Subgroups analyses were performed based on the target, drug and regimen (Supplementary Table S2). Significant PFS benefits were found in all targets, including anti-PD-1 (HR, 0.844; 95% CI, 0.792-0.901), anti-PD-L1 (HR, 0.812; 95% CI, 0.726-0.907), and anti-CTLA-4 (HR, 0.847; 95% CI, 0.744-0.963). A statistically significant PFS improvement was observed in both ICIs monotherapies (HR, 0.840; 95% CI, 0.794-0.889) and combination therapies of ICIs with chemotherapy (HR, 0.825; 95% CI, 0.728-0.936) (). We further performed meta-regression by the clinical variables. As shown in Supplementary Table S2, target (P = 0.879), drug (P = 0.776) and regimen (P = 0.634) did not result in the inter-study heterogeneity.
Figure 2. The pooled analyses of progression free survival of NSCLC patients who received ICIs compared to control therapies (A) based on target (B), drug (C) and regimen (D). The number of subjects with available survival information in ICIs or control arm is 3,598 and 3,321, respectively. Patients number in different subgroups was shown in Supplementary Table S2. Squares indicate study-specific HR (size of the square reflects the study-specific statistical weight); horizontal lines indicate 95% CI; diamond indicates the summary HR estimated with its 95% CI.
Overall survival
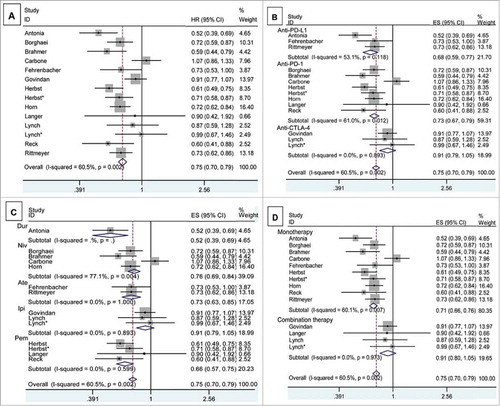
The meta-analysis of OS was based on 12 RCTs provided the required data. Between-study heterogeneity was significant (chi-squared = 32.89, P = 0.002, I-squared = 60.5%). There was significant OS improvement in ICIs arm compared with control arm (HR, 0.747, 95% CI, 0.703-0.795) (). In stratified analyses by target, significant OS benefit was found in anti-PD-1 (HR, 0.726; 95% CI, 0.670-0.786), and anti-PD-L1 (HR, 0.679; 95% CI, 0.595-0.775), not in anti-CTLA-4 (HR, 0.915; 95% CI, 0.794-1.053) (). Meta regression suggested that target (P = 0.200), drug (P = 0.451) and regimen (P = 0.057) did not alter the pooled HR significantly (Supplementary Table S2).
Figure 3. The pooled analyses of overall survival of NSCLC patients who received ICIs compared to control therapies (A) based on target (B), drug (C) and regimen (D). The number of subjects with available survival information in ICIs or control arm is 3,598 and 3,321, respectively. Patients number in different subgroups was shown in Supplementary Table S2. Squares indicate study-specific HR (size of the square reflects the study-specific statistical weight); horizontal lines indicate 95% CI; diamond indicates the summary HR estimated with its 95% CI.
Selected subgroups
Survival benefit was explored across specified subgroups, according to baseline clinicopathologic features of NSCLC patients. Notably, PFS and OS benefit of ICIs was observed irrespective of sex, age, treatment line and ECOG performance status (). Survival HRs favored ICIs compared to control therapies in NSCLC patients with former/ current smoker (PFS, P < 0.001; OS, P < 0.001), but disfavored ICIs in NSCLC patients who had never smoked (PFS, P = 0.468; OS, P = 0.317). Squamous NSCLC had slightly lower HR of death than non-squamous NSCLC (PFS, 0.715 VS. 0.786; OS, 0.694 vs. 0.805), although this comparison was not powered by statistical analysis. NSCLC patients without CNS metastases seemed to derive more survival benefit from ICIs than control therapies (PFS, P < 0.001; OS, P < 0.001). Conversely, patients with CNS metastases disease received similar survival benefit from ICIs and control therapies (PFS, P = 0.209; OS, P = 0.090). Survival improvement was also evident in patients with negative EGFR mutation (PFS, P < 0.001; OS, P < 0.001), not in those with positive EGFR mutation (PFS, P = 0.083; OS, P = 0.522).
Table 1. Exploratory subgroup analyses of survival in the intent-to-treat population.
As shown in , survival benefit from ICIs increased with increasing PD-L1 expression on tumour cells. Survival improvement was significant in NSCLC patients with PD-L1 expression in at least 5% of cells (PFS, P = 0.003; OS, P < 0.001) and at least 50% of cells (PFS, P < 0.001; OS, P < 0.001). In the exploratory subgroup analysis involving patients with PD-L1 expression level of <5%, the HR for DFS was 0.911 (95% CI, 0.742-1.118), and HR for OS was 0.854 (95% CI, 0.730-0.998).
Overall response rate
Eleven RCTs provided information in detail about ORR. The pooled results showed ICIs significantly improved ORR (RR, 1.311; 95% CI, 1.205-1.428; P < 0.001) (Supplementary Fig. S1). However, better ORR was only found in anti-PD-1 (RR, 1.778; 95% CI, 1.535-2.059), and anti-PD-L1 (RR, 1.250; 95% CI, 1.082-1.443), not in anti-CTLA-4 (RR, 1.008; 95% CI, 0.868-1.170). In stratified analyses regarding individual drug, three ICIs (nivolumab, pembrolizumab and durvalumab) resulted in significant ORR improvement. Two agents (atezolizumab and ipilimumab) did not improve ORR (Supplementary Table S2). Subgroup analysis showed that both ICIs monotherapies and combination therapies improved ORR. Meta regression indicated that none of the examined factors were responsible for between-study heterogeneity on ORR, including target (P = 0.064), drug (P = 0.076) and regimen (P = 0.552).
Treatment related adverse events
The common AEs were summarized in . The pooled analyses showed that the risks of all grade anemia, neutropenia, leukopenia, thrombocytopenia, anorexia, stomatitis, nausea, pyrexia, asthenia, myalgia, alopecia and neuropathy were lower in patients receiving ICIs. The pooled RR indicated the risks of all-grade diarrhea (RR, 1.053; 95% CI, 0.874-1.269) were comparable between ICIs and control group. However, the risks of all-grade ALT/AST increased (P < 0.001), pruritus (P < 0.001), rash (P < 0.001) and thyroid dysfunction (P < 0.001) were higher in patients treated with ICIs than those in control group.
Table 2. Relative Risk of Treatment-related Common Adverse Events in NSCLC Patients Treated with ICIs Compared to Control Therapies.
To clarify the severity of AEs, we further analyzed the risks of ≥ 3 grade AEs. Compared with the control group, the ICIs group showed a lower incidence of ≥ 3 grade anemia, neutropenia, leukopenia, stomatitis, pyrexia, asthenia. Patients receiving ICIs experienced a comparable risk of ≥ 3 grade thrombocytopenia (P = 0.052), anorexia (P = 0.140), diarrhea (P = 0.075), nausea (P = 0.181), myalgia (P = 0.944), alopecia (P = 0.494), pruritus (P = 0.303), rash (P = 0.060), neuropathy (P = 0.439), and thyroid dysfunction (P = 0.810) (). Only the risks of ≥ 3 grade ALT/AST increased (RR, 4.451; 95% CI, 1.777-11.146; P = 0.001) were higher in ICIs arm than control arm ().
Sensitivity analyses and publication bias
We carried out sensitivity analyses to assess the stability of the results. The leave-one-out sensitivity analyses indicated that no individual study changed the pooled data qualitatively. The shapes of the funnel plots seemed symmetrical in all pooled analyses, suggesting the absence of publication bias. Z-value (continuity corrected) of Begg's test in the meta-analyses was 1.64 on PFS (P = 0.101), 0.33 on OS (P = 0.743), and 1.40 on ORR (P = 0.161). Egger's test showed that the t value (bias) of the pooled analyses was −1.67 on PFS (P = 0.121), −0.27 on OS (P = 0.793), and −2.05 on ORR (P = 0.064). As shown in Supplementary Table S2, Begg's test and Egger's test indicated no significant publication bias in subgroup analyses. We did not perform non-parametric “trim-and-fill” method, because publication bias might not have a significant influence on the results.
Discussion
This study with updated data improved our understanding about the efficacy and safety of ICIs in NSCLC. Our data showed that ICIs had superior PFS, OS and ORR with improved safety profile compared with conventional therapies. Immunological checkpoints are inhibitory feedback loops of immune system to mitigate uncontrolled propagation of immune responses and maintain selftolerance. Those checkpoints contributes co-stimulatory pathways (CD28, ICOS) and co-inhibitory pathways (CTLA-4, PD-1).Citation24 ICIs restore intrinsic functions of dampening effector T cells, thus enhance anti-tumour immunity. More specifically, ICIs trigger tumor infiltrating lymphocytes (TILs) in tumor microenvironment by induction of interferon-γ and cytokines.Citation25
This meta-analysis showed that ICIs were associated with significant prolonged PFS and OS. It has been observed that target, drug or regimen did not significantly alter survival benefit of ICIs (Supplementary Table S2). Our results were strengthened by the meta-regression analyses and sensitivity analyses. In a stratified analysis based on drug, a positive effect of ICIs for PFS was not observed in atezolizumab (). However, these data were insufficient to draw definite conclusions, because only two studies performed the PFS analyses of atezolizumab. Regarding OS, subgroup analyses showed anti-CTLA-4 and ipilimumab had no obvious benefit compared with control therapies (). Those findings may reduce statistical power to get reliable results. Although meta regression suggested target, drug and regimen did not change the overall results significantly, our results regarding OS should be interpreted with very caution.
One interesting question attracted our attention: why survival improvement of anti-CTLA-4 is not consistent with anti-PD-1 and anti-PD-L1. One possible explanation is that roles of CTLA-4 are different from those of PD-1 and PD-L1. CTLA-4 is expressed mainly on T cells and provide inhibitory signals in the initial activation of T cells, typically in lymphoid tissues.Citation26,Citation27 PD-1 and PD-L1 is expressed on B, T, myeloid cells, as well as non-lymphoid organs.Citation28 The PD-1 pathway primarily inhibits effector T cells at the later stage of inflammatory responses, typically in peripheral tissues. Another possible explanation is that the mechanisms underlying the anti-tumour activity are different. CTLA-4 blockade could increase diversity of T cells pool.Citation29 Anti-tumour activity of PD-1 blockade relies on immune function restoration of peripheral T cells. Specific PD-L1 inhibition would block PD-1:PD-L1 and PD-L1:CD80 interactions, and preserve PD-1:PD-L2 interactions.Citation30 In theory, there are many differences in timing and location of immune checkpoint among CTLA-4, PD-1 and PD-L1 blockage therapies. Thus, further studies with functional analyses are needed to address this issue.
As suggested by exploratory analyses, survival improvement of ICIs may be driven by certain subgroups of patients. Our data showed NSCLC patients with non-smoker or positive EGFR mutation did not acquire survival benefit from ICIs. Previous study indicated NSCLC patients who never smoking had low levels of mutational burdens and heterogeneity.Citation31 While, tumors bearing high levels of somatic mutations are related to high sensitivity of immune-checkpoint inhibitors.Citation31 EGFR activation results in suppression of anti-tumor immune response through induction of regulatory T cells or reduction of T cells chemoattractant. Akbay et al.Citation32 had demonstrated that active EGFR mutation could upregulate PD-L1 expression and facilitate evasion of tumor cells from immunity. Retrospective studies have identified several markers associated with outcomes, including TIL count, ICOS, and NY-ESO-1.Citation33 In CheckMate 026, Carbone DP et al.Citation20 reported NSCLC patients with PD-L1 expression level of 5% did not benefit from nivolumab compared with chemotherapy. However, several trials indicated nivolumab could prolong PFS or OS of NSCLC patients with PD-L1 expression level of 5%.Citation5,Citation19,Citation21 Many factors appeared to have influence on the treatment efficacy of nivolumab. In CheckMate 026, imbalances of clinicopathological features at baseline may disfavored the ICIs group, including higher ratio of metastases, higher tumor burden, and lower proportion of women in ICIs group than chemotherapy group. These disease characteristics related to worse outcomes of NSCLC patients. In addition, low tumor mutation burden also favored the chemotherapy group in CheckMate 026.Citation20 In this meta analysis of RCTs, we found that PD-L1 expression levels offered the potential for identifying patients benefited from ICIs (). Although PD-L1 negative tumors response to ICIs, improved outcomes could not been seen in many studies.Citation5,Citation7,Citation19,Citation21 Thus, these NSCLC patients received ICIs should be further evaluated with cautions. Much effort at identifying biomarkers is needed to gain utmost benefit or avoid unnecessary treatment.
ORR analyses demonstrated that patients receiving ICIs had better disease control than those in control groups. It should be noted that anti-tumour efficacy of ICIs are indirect, relied on the activities of immune effector cells. While most conventional chemotherapies directly diminish tumors. The kinetics of tumor responses therefore differ significantly. Tumor volumes could not been shrunk until ICIs restart effective anti-tumor immune. Tumor responses potentially need longer time to become clinical detectable compared with conventional chemotherapies.Citation24 In addition, ICIs induce infiltration of activated T cells into the tumor. The inflammation and edema occur in tumor tissues. So, tumour volumes would initially increase but subsequently pseudo-progression translates into tumour shrinkage. Therefore, tumor responses evaluation by RECIST may be inappropriate. Alternative immune-related response criteria (irRC) had been proposed.Citation34,Citation35 Overall tumour burden is the indicator of clinical responses compared with the baseline lesion measurements. Based on irRC, new lesions do not mean disease progression if net tumour burden is stable. Durable stable disease is considered as clinical activity of immunotherapies. The irRC make good theoretical and clinical sense. But further prospective validation is needed.
The clinical trials of ICIs have raised concerns over treatment related AEs. In the present meta-analysis, we found the spectrum of ICIs associated AEs were consistent with previous studies.Citation36-Citation38 Overall, low risks of all-grade anemia, neutropenia, leukopenia, thrombocytopenia, anorexia, stomatitis, nausea, pyrexia, asthenia, myalgia, alopecia and neuropathy were observed in ICIs compared to control therapies. However, ICIs had a higher incidence of all-grade ALT/AST increased (P < 0.001), pruritus (P < 0.001), rash (P < 0.001) and thyroid dysfunction (P < 0.001). ICIs cause AEs with potential immunologic etiologies, so-called immune-mediated AEs. These AEs include rash or pruritus, gastrointestinal disorders, and endocrinopathies. The ALT/AST increased, pruritus, and rash are natural responses to ICIs with enhanced immunity and higher cytokines. Theoretically, those reactions could be due to nonspecific activation of antigen presenting cells, rapid proliferation of T cells and reduced Treg mediated immunosuppression.Citation37 Thyroid dysfunction may be a consequence of ectopic expression of CTLA-4, PD-L2 blockage and high level of IL-2.Citation38,Citation39 Interestingly, occurrence of treatment-related dermatologic AEs is associated with better tumor response and survival benefit in cancer patients received ICIs.Citation39,Citation40 However, it is unclear whether treatment-related endocrine AEs are associated with prognostically favorable outcomes.
Preclinical studies indicated that both chemotherapy and radiotherapy induced PD-L1 expression on tumor cells and modulated the immunity against tumor cells.Citation41,Citation42 Chemotherapy and radiotherapy could kill tumor cells and create a pool of antigen for crosspresentation. Thereby, conventional therapies enhance immunogenicity and induce inflammation. Fiorica F et al.Citation43 had demonstrated that combination of radiotherapy and ICIs obtained OS and PFS benefit without an increase in toxicities in NSCLC patients. Significant survival improvement was also observed in NSCLC with combination treatment of ICIs and chemotherapy.Citation10,Citation11 Our study also showed combination of chemotherapy and ICIs could prolong survival and improve tumor response (Supplementary Table S2). Previous studies reported synergistic treatment of anti-PD-1 and anti-CTLA-4 had high response rate and durable response with tolerable safety profile.Citation44,Citation45 These exploratory investigations showed encouraging clinical activity and feasibility of combination therapies. New combinations of ICIs with classical chemotherapy and/or radiotherapy will further revolutionize the treatment of NSCLC. This meta-analysis reported our interesting preliminary findings. We will do further research and validate the clinical relevance of combination therapies in other validation sets.
In this study, several limitations need to be addressed. This meta analysis was based on study-level evidence. More reliable results could been draw from individual patient data-based study. Second, our conclusions came from the sum of 12 RCTs with between-study heterogeneity. Inconsistent HRs for different targets and drugs should be noticed. The inclusion and exclusion criteria differed from each trial. In this study, positive effects of ICIs for PFS and OS were observed in both first line treatment and ≥ second line treatment. However, patients with ≥ second line treatment of ICIs might have different clinical outcomes, compared to those with first line treatment of ICIs (). The criteria related to previous treatment (ie. chemotherapy or chemo-radiotherapy) might affect the results. Heterogeneous inclusion and exclusion criteria of treatments should be considered. In addition, confounding factors (the use of glucocorticoid and post-progression treatment) should also be incorporated into analyses. However, the effects of other drugs (ie. glucocorticoid) and post-progression treatment on outcomes were not available in including trials. We could not extract the HRs and perform pooled analyses. Due to lack of original data, we did not perform subgroup analyses based on key molecular markers (ie. ROS, ALK, and KRAS status or overall mutation loads) to identify the exact benefit population. And exploratory subgroup analyses were not conducted in different tumor stage and dosing groups. Therefore, further researches with complete information of required data from individual patient are needed to clarify the efficacy and safety of ICIs.
Taken together, this meta-analysis provided direct clinical evidence supporting the notion that ICIs had superior survival benefit over control therapies in NSCLC, especially for those patients with smoker, no CNS metastasis, wild type EGFR, and high PD-L1 expression. The outcomes appear very promising and treatment-related AEs were acceptable. Our observations support further larger scale multicenter RCTs to rigorously evaluate the long-term efficacy and safety of ICIs in NSCLC.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Author contributions
Conceptualization: Shuai Wang and Lijie Tan; Methodology and validation: Shuai Wang, Hao Wang, Yong Fang and Lijie Tan; Data analysis: Jiatao Hao and Shuai Wang; Writing: Shuai Wang; Supervision: Hao Wang, Yong Fang and Lijie Tan.
supp_data.zip
Download Zip (401.6 KB)Additional information
Funding
References
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH. Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–8. doi:10.1056/NEJMoa011954 PMID:11784875.
- Wang S, Wang Z. Meta-analysis of Epidermal Growth Factor Receptor and KRAS Gene Status between Primary and Corresponding Metastatic Tumours of Non-small Cell Lung Cancer. Clin Oncol. 2015;27(1):30–9. doi: 10.1016/j.clon.2014.09.014.
- Wang S, Wang Z. EGFR mutations in patients with non-small cell lung cancer from mainland China and their relationships with clinicopathological features: a meta- analysis. Int J Clin Exp Med. 2014;7(8):1967–78. PMID:25232377.
- Pérez-Soler R, Chachoua A, Hammond LA, Rowinsky EK, Huberman M, Karp D, Rigas J, Clark GM, Santabárbara P, Bonomi P. Determinants of tumor response and survival with erlotinib in patients with non–small-cell lung cancer. J Clin Oncol. 2004;22(16):3238–47. doi: 10.1200/JCO.2004.11.057. PMID:15310767.
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(17):1627–39. doi: 10.1056/NEJMoa1507643. PMID:26412456.
- Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(1007):1540–50. doi: 10.1016/S0140-6736(15)01281-7. PMID:26712084.
- Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–46. doi: 10.1016/S0140-6736(16)00587-0. PMID:26970723.
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N Engl J Med. 2017;377(20):1919–29. doi: 10.1056/NEJMoa1709937. PMID:28885881.
- Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H, Cuillerot JM, et al. Ipilimumab in Combination With Paclitaxel and Carboplatin As First-Line Treatment in Stage IIIB/IV Non–Small-Cell Lung Cancer: Results From a Randomized, Double-Blind, Multicenter Phase II Study. J Clin Oncol. 2012;30(17):2046–54. doi: 10.1200/JCO.2011.38.4032. PMID:22547592.
- Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–508. doi: 10.1016/S1470-2045(16)30498-3. PMID:27745820.
- Govindan R, Szczesna A, Ahn MJ, Schneider CP, Gonzalez Mella PF, Barlesi F, Han B, Ganea DE, Von Pawel J, Vladimirov V, et al. Phase III Trial of Ipilimumab Combined With Paclitaxel and Carboplatin in Advanced Squamous Non-Small-Cell Lung Cancer. J Clin Oncol. 2017;35(30):3449–57. doi: 10.1200/JCO.2016.71.7629. PMID:28854067.
- Wu Y, Shi H, Jiang M, Qiu M, Jia K, Cao T, Shang Y, Shi L, Jiang K, Wu H. The clinical value of combination of immune checkpoint inhibitors in cancer patients: A meta-analysis of efficacy and safety. Int J Cancer. 2017;141(12):2562–70. doi: 10.1002/ijc.31012. PMID:28833119.
- Nishijima TF, Shachar SS, Nyrop KA, Muss HB. Safety and Tolerability of PD-1/PD-L1 Inhibitors Compared with Chemotherapy in Patients with Advanced Cancer: A Meta-Analysis. Oncologist. 2017;22(4):470–9. doi: 10.1634/theoncologist.2016-0419. PMID:28275115.
- Sheng Z, Zhu X, Sun Y, Zhang Y. The efficacy of anti-PD-1/PD-L1 therapy and its comparison with EGFR-TKIs for advanced non-small-cell lung cancer. Oncotarget. 2017;8(34):57826–35. doi: 10.18632/oncotarget.18406. PMID:28915714.
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005. PMID:19631508.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi:10.1093/jnci/92.3.205. PMID:10655437.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
- Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23(11):1663–82. doi: 10.1002/sim.1752. PMID:15160401.
- Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123–35. doi: 10.1056/NEJMoa1504627. PMID:26028407.
- Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med. 2017;376(25):2415–26. doi: 10.1056/NEJMoa1613493. PMID:28636851.
- Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, Poddubskaya E, Borghaei H, Felip E, Paz-Ares L, et al. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35(35):3924–33. doi: 10.1200/JCO.2017.74.3062. PMID:29023213.
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823–33. doi: 10.1056/NEJMoa1606774. PMID:27718847.
- Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65. doi: 10.1016/S0140-6736(16)32517-X. PMID:27979383.
- Quezada SA, Peggs KS. Exploiting CTLA-4, PD-1 and PD-L1 to reactivate the host immune response against cancer. Br J Cancer. 2013;108(8):1560–5. doi: 10.1038/bjc.2013.117. PMID:23511566.
- Kean LS, Turka LA, Blazar BR. Advances in targeting co-inhibitory and co-stimulatory pathways in transplantation settings: the Yin to the Yang of cancer immunotherapy. Immunol Rev. 2017;276(1):192–212. doi: 10.1111/imr.12523. PMID:28258702.
- Melero I, Lasarte JJ. Genetic basis for clinical response to CTLA-4 blockade. N Engl J Med. 2015;372(8):783. doi:10.1056/NEJMc1415938#SA1. PMID:25693025.
- Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3(7):611–8. doi: 10.1038/ni0702-611. PMID:12087419.
- Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–44. doi: 10.1182/blood-2008-12-195792. PMID:19423728.
- Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–82. doi: 10.1111/j.1600-065X.2008.00662.x. PMID:18759926.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. doi: 10.1038/nrc3239. PMID:22437870.
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. Cancer immunology: mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–8. doi: 10.1126/science.aaa1348. PMID:25765070.
- Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp EM, Pugh TJ, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3(12):1355–63. doi: 10.1158/2159-8290.CD-13-0310. PMID:24078774.
- Weber JS, Kahler KC, Hauschild A. Management of immunerelated adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691–7. doi: 10.1200/JCO.2012.41.6750. PMID:22614989.
- Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immunerelated response criteria. Clin Cancer Res. 2009;15(23):7412–20. doi: 10.1158/1078-0432.CCR-09-1624. PMID:19934295.
- Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, Humphrey R, Blumenstein B, Old L, Wolchok J. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102(18):1388–97. doi: 10.1093/jnci/djq310. PMID:20826737.
- Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28(10):2377–85. doi: 10.1093/annonc/mdx286. PMID:28945858.
- Belum VR, Benhuri B, Postow MA, Hellmann MD, Lesokhin AM, Segal NH, Motzer RJ, Wu S, Busam KJ, Wolchok JD, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12–25. doi: 10.1016/j.ejca.2016.02.010. PMID:27043866.
- Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6(230):230–45. doi: 10.1126/scitranslmed.3008002.
- Phan GQ, Attia P, Steinberg SM, White DE, Rosenberg SA. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol. 2001;19(15):3477–82. doi: 10.1200/JCO.2001.19.15.3477. PMID:11481353.
- Poprach A, Pavlik T, Melichar B, Puzanov I, Dusek L, Bortlicek Z, Vyzula R, Abrahamova J, Buchler T, Czech Renal Cancer Cooperative Group. Skin toxicity and efficacy of sunitinib and sorafenib in metastatic renal cell carcinoma: a national registry-based study. Ann Oncol. 2012;23(12):3137–43. doi: 10.1093/annonc/mds145. PMID:22700990.
- Dovedi SJ, Lipowska-Bhalla G, Beers SA, Cheadle EJ, Mu L, Glennie MJ, Illidge TM, Honeychurch J. Antitumor efficacy of radiation plus immunotherapy depends upon dendritic cell activation of effector CDS+ T cells. Cancer Immunol Res. 2016;4(7):621–30. doi: 10.1158/2326-6066.CIR-15-0253. PMID:27241845.
- Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell. 2015;28(6):690–714. doi: 10.1016/j.ccell.2015.10.012. PMID:26678337.
- Fiorica F, Belluomini L, Stefanelli A, Santini A, Urbini B, Giorgi C, Frassoldati A. Immune Checkpoint Inhibitor Nivolumab and Radiotherapy in Pretreated Lung Cancer Patients: Efficacy and Safety of Combination. Am J Clin Oncol. 2018 Jan 31. doi: 10.1097/COC.0000000000000428. [Epub ahead of print].
- Xu X, Huang Z, Zheng L, Fan Y. The efficacy and safety of anti-PD-1/PD-L1 antibodies combined with chemotherapy or CTLA4 antibody as a first-line treatment for advanced lung cancer. Int J Cancer. 2018 Jan 10. doi: 10.1002/ijc.31252. [Epub ahead of print]
- Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, Ready NE, Gerber DE, Chow LQ, Juergens RA, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18(1):31–41. doi: 10.1016/S1470-2045(16)30624-6. PMID:27932067.