ABSTRACT
We report the immunological profile of a patient with upper-tract urothelial carcinoma experiencing stable disease on pembrolizumab for 20 months. The tumor exhibited extensive infiltration by CD8+ cytotoxic T lymphocytes, low-to-moderate mutational burden, no PD-L1 staining by commercially available immunohistochemical assays, but amplification of CD274 (coding for PD-L1) and/or PDCD1LG2 (encoding PD-L2) by fluorescence in situ hybridization. RNA-seq revealed multiple biomarkers of an ongoing immune response and compensatory immune evasion, including moderate PD-L1 levels coupled with robust PD-L2 expression. Pending validation in additional patients, these findings suggest that PD-L2 expression levels may constitute a biomarker of response to immune checkpoint blockade in urothelial carcinoma.
Introduction
Immunotherapy with immune checkpoint blockers (ICBs) has recently gained momentum for the treatment of urothelial carcinoma.Citation1-Citation3 In particular, two monoclonal antibodies (mAbs) targeting programmed cell death 1 (PDCD1; best known as PD-1), i.e., pembrolizumab (KEYTRUDA®) and nivolumab (OPDIVO®), and three mAbs targeting the main PD-1 ligand CD274 (best known as PD-L1), i.e., durvalumab (IMFINZI®), avelumab (BAVENCIO®), and atezolizumab (TECENTRIQ®), demonstrated robust clinical activity in subjects with urothelial carcinoma and have been approved by the US Food and Drug Administration for first-line therapy in cisplatin-ineligible patients or as second-line therapeutic strategies.Citation4-Citation14 Nonetheless, only a minority of patients with urothelial carcinoma respond to ICB-based immunotherapy, calling for the development of reliable predictive biomarkers.
In this setting, considerable attention is currently attracted by mutational burden (MuB), which is generally assessed by whole-exon DNA-seq, and PD-L1 expression levels, which are normally monitored by immunohistochemistry (IHC).Citation15-Citation17 Specifically, the IMvigor 210 trial (NCT02108652) demonstrated that urothelial carcinoma patients treated with atezolizumab exhibit an increased objective response rate (ORR) when their lesions stain positively (>5% of cells) for PD-L1.Citation10,Citation18 Nonetheless, ORR never exceeded 26%, even amongst patients with the highest IHC score for PD-L1 expression.Citation10,Citation18 In the same setting, whole-exon DNA-seq on a subset of patients demonstrated an increased likelihood for response amongst subjects with high MuB, although a considerable overlap existed between this group and individuals with low MuB.Citation10,Citation18 These findings led to the development of IHC-based companion diagnostics for the detection of PD-L1 expression levels in tumor biopsies, including the SP142 assayCitation19 from Ventana Medical Systems (Tucson, AZ, USA) and the 22C3 assayCitation20 from Dako Inc. (Santa Clara, CA, USA). Neither of these assays, however, is currently approved by the US FDA as a companion diagnostic for predicting responses to ICBs amongst urothelial carcinoma patients (22C3 is approved for predicting responses to pembrolizumab amongst non-small cell lung carcinoma and gastroesophageal carcinoma patients; source https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm301431.htm).
Here, we present a case of advanced upper-tract urothelial carcinoma experiencing prolonged disease stabilization on pembrolizumab treatment. The tumor was characterized by robust infiltration by CD8+ cytotoxic T lymphocytes (CTLs), low-to-moderate MuB, PD-L1 negativity on immunohistochemical assessment, but amplification of CD274 (coding for PD-L1) and PDCD1LG2 (encoding PD-L2), as well as by multiple biomarkers of an ongoing immune response and compensatory immune evasion (including moderate PD-L1 levels coupled with robust PD-L2 expression).
Case presentation
A 79 years-old woman with high-grade invasive urothelial carcinoma of the right kidney (T3NxMx) presented for a second opinion in 2012. Imaging over the next few months showed multiple foci of metastatic disease in the liver and urinary bladder. The patient received 6 cycles of intravenous carboplatin (AUC 5) and gemcitabine (1000 mg/m2) on a 4 week regimen with minimal tumor shrinkage consistent with stable disease until 2015. At this time, the patient experienced progressive disease (increase in size and number of metastatic lesions) and pembrolizumab treatment was initiated (i.v., every 3 weeks; 2 mg/Kg) (). Pembrolizumab enabled disease stabilization for 15 months (), but had to be discontinued for moderate (Grade 3) rash. At the latest available radiographic assessment (20 months after pembrolizumab initiation), the patient still exhibited stable disease. The patient was lost at follow-up and subsequently deceased due to other co-morbidities ().
Figure 1. Immunological characterization of unexpectedly durable disease stabilization in urothelial carcinoma patient treated with pembrolizumab. A. Clinical timeline. B. Baseline and follow-up CT scan of hepatic metastasis. C. Design of FISH probes for CD274 and PDCD1LG2 copy number evaluation. D. Representative images for CD274 and PDCD1LG2 amplification by FISH, CD3 and CD8 detection by IHC, PD-L1 expression levels as monitored by the SP142 and 22C3 assays, and hematoxylin and eosin (H&E) stain. FISH, scale bar = 20 µm. IHC, scale bar = 100 µm.
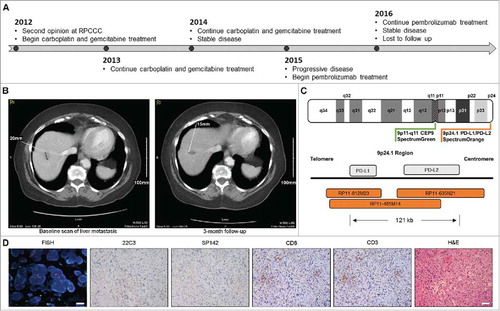
Comprehensive immunological profiling was performed on archival formalin fixed paraffin embedded (FFPE) tumor tissue collected prior to pembrolizumab initiation, in the context of standard-of-care treatment, using a New York State CLEP approved assay (Immune Report CardSM from OmniSeq® Inc., Buffalo, NY, USA).Citation21,Citation22 This assay monitors (1) MuB, by whole-exon DNA-seq on 395 cancer-related genes; (2) CD274 (coding for PD-L1) and PDCD1LG2 (encoding PD-L2) amplification, by fluorescence in situ hybridization (FISH); (3) tumor infiltration by CD3+ and CD8+ T lymphocytes, by IHC; (4) PD-L1 expression levels, by IHC; as well as (5) the abundance of 398 transcripts linked to immunological status of the tumor microenvironment, by RNA-seq.Citation21,Citation22
Mutational burden was close to the median of an internal reference population including 167 different neoplasm samples of various histological derivation (4.39 mutations/Mb), which is widely considered as low-to-moderate.Citation23,Citation24 CD274 and/or PDCD1LG2 were highly amplified (,). IHC for CD3 and CD8 revealed elevated numbers of CD8+ CTLs exhibiting a highly infiltrating pattern (). RNA-seq data confirmed high levels of CD3D, CD3E, CD3G, CD8A, CD8B, ranking in the top 10% of the abovementioned patient population (). IHC for PD-L1 expression with the SP142 and the 22C3 assays revealed infrequent cytoplasmic staining in small patches of neoplastic cells, but no membranous staining. Similarly, tumor-infiltrating cells did not stain positively for PD-L1 expression (). RNA-seq exhibited a moderate abundance of the transcript encoding PD-L1 and high levels of the PD-L2-coding transcript (). RNA-seq also revealed a relative abundance of multiple biomarkers of an ongoing immune response and compensatory immune evasion, including (but not limited to) transcripts involved in T-cell effector functions (GZMA, IFNG, PRF1), T-cell priming (CD27, CD28, CD40, CD40LG, ICOSLG), checkpoint-driven immunosuppression (PDCD1, LAG3, VSIR, TNFRSF14, BTLA), myeloid immunosuppression (CCR2, CCL2, CD68), the regulation of inflammatory responses (IL10, CXCR6, STAT1, DDX58, MX1), and immune escape (ADORA2A) ().
Table 1. Immunological profiling of an urothelial carcinoma case by RNA-seq.
Discussion
While the 22C3 assay is approved by the US FDA as a companion diagnostic to identify non-small cell lung carcinoma patients and gastroesophageal carcinoma patients prone to respond to pembrolizumab,Citation25 the SP142 assay is currently employed as a complementary diagnostic to define the likelihood of urothelial carcinoma patients to obtain clinical benefits from atezolizumab.Citation19 Previous clinical data indicate that an improved ORR to atezolizumab amongst urothelial carcinoma patients is associated with >5% positive staining for PD-L1 (as assessed by the SP142 assay) on tumor-infiltrating immune cells.Citation10,Citation18 Conversely, PD-L1 positivity by neoplastic or immune cells (as assessed by the 22C3 assay) reportedly fails to correlate with improved objective responses to pembrolizumab in urothelial carcinoma patients.Citation7 This patient exhibited a durable disease stabilization on pembrolizumab (associated with an increase in survival as compared to expectations) despite no PD-L1 positivity in tumor-infiltrating immune cells and no membranous PD-L1 expression by malignant cells.
FISH revealed considerable amplification of CD274 (coding for PD-L1) and/or PDCD1LG2 (encoding PD-L2), and RNA-seq exhibited moderate levels of PD-L1-coding transcripts. Potentially, such a discrepancy between CD274 gene dosage, PD-L1 abundance at the RNA level, and PD-L1 protein expression may reflect transcriptional, post-transcriptional as well as post-translational layers of regulation.Citation26 Interestingly, the progressing lesion (biopsy was taken before pembrolizumab initiation) was highly infiltrated by CD8+ CTLs, but the transcripts encoding PD-1 and PD-L2 were abundant, potentially highlighting the PD-L2/PD-1 axis as the major determinants of immunosuppression in this patient. The significance of these observations remains to be validated in additional cases, but PD-L2 levels may constitute a predictive biomarker for response to ICB in at least a subset of urothelial carcinoma patients.
Disclosure of potential conflicts of interest
APS, FLL, JMC, MKN, SP, STG, BB, JA, VG, MQ, YW, and CM are all employees of OmniSeq, Inc. (Buffalo, NY) and hold restricted stock in OmniSeq, Inc. SG is an employee of Roswell Park Comprehensive Cancer Center (Buffalo, NY). Roswell Park Comprehensive Cancer Center is the majority shareholder of OmniSeq, Inc. LG provides remunerated consulting to OmniSeq, Inc.
References
- Vanpouille-Box C, Lhuillier C, Bezu L, Aranda F, Yamazaki T, Kepp O, Fucikova J, Spisek R, Demaria S, Formenti SC, Zitvogel L, et al. Trial watch: Immune checkpoint blockers for cancer therapy. Oncoimmunology. 2017;6:e1373237. doi:10.1080/2162402X.2017.1373237. PMID:29147629.
- Kang J, Galluzzi L. PD-L1 blockade for urothelial carcinoma. Oncoimmunology. 2017;6:e1334028. doi:10.1080/2162402X.2017.1334028. PMID:28811979.
- Siefker-Radtke A, Curti B. Immunotherapy in metastatic urothelial carcinoma: focus on immune checkpoint inhibition. Nat. Rev. Urol. 2017;15:112–24. doi:10.1038/nrurol.2017.190. PMID:29205200.
- Sharma P, Callahan MK, Bono P, Kim J, Spiliopoulou P, Calvo E, Pillai RN, Ott PA, de Braud F, Morse M, Le DT, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016;17:1590–8. doi:10.1016/S1470-2045(16)30496-X. PMID:27733243.
- Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, Plimack ER, Vaena D, Grimm MO, Bracarda S, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18:312–22. doi:10.1016/S1470-2045(17)30065-7. PMID:28131785.
- Plimack ER, Bellmunt J, Gupta S, Berger R, Chow LQ, Juco J, Lunceford J, Saraf S, Perini RF, O'Donnell PH, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol. 2017;18:212–20. doi:10.1016/S1470-2045(17)30007-4. PMID:28081914.
- Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017;376:1015–26. doi:10.1056/NEJMoa1613683. PMID:28212060.
- Balar AV, Castellano D, O'Donnell PH, Grivas P, Vuky J, Powles T, Plimack ER, Hahn NM, de Wit R, Pang L, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:1483–92. doi:10.1016/S1470-2045(17)30616-2. PMID:28967485.
- Petrylak DP, Powles T, Bellmunt J, Braiteh F, Loriot Y, Morales-Barrera R, Burris HA, Kim JW, Ding B, Kaiser C, et al. Atezolizumab (MPDL3280A) Monotherapy for Patients With Metastatic Urothelial Cancer: Long-term Outcomes From a Phase 1 Study. JAMA Oncol. 2018;8032:1–8.
- Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–20. doi:10.1016/S0140-6736(16)00561-4. PMID:26952546.
- Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J, Curiel TJ, Colon-Otero G, Hamid O, Sanborn RE, et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J. Clin. Oncol. 2016;34:3119–25. doi:10.1200/JCO.2016.67.9761. PMID:27269937.
- Powles T, O'Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, Lee JL, Ong M, Sridhar SS, Vogelzang NJ, et al. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study. JAMA Oncol. 2017;3:e172411. doi:10.1001/jamaoncol.2017.2411. PMID:28817753.
- Patel MR, Ellerton J, Infante JR, Agrawal M, Gordon M, Aljumaily R, Britten CD, Dirix L, Lee KW, Taylor M, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19:51–64. doi:10.1016/S1470-2045(17)30900-2. PMID:29217288.
- Apolo AB, Infante JR, Balmanoukian A, Patel MR, Wang D, Kelly K, Mega AE, Britten CD, Ravaud A, Mita AC, et al. Avelumab, an Anti-Programmed Death-Ligand 1 Antibody, In Patients With Refractory Metastatic Urothelial Carcinoma: Results From a Multicenter, Phase Ib Study. J. Clin. Oncol. 2017;35:2117–24. doi:10.1200/JCO.2016.71.6795. PMID:28375787.
- Lhuillier C, Vanpouille-Box C, Galluzzi L, Formenti SC, Demaria S. Emerging biomarkers for the combination of radiotherapy and immune checkpoint blockers. Semin. Cancer Biol. 2017;0–1. doi:10.1016/j.semcancer.2017.12.007.
- Fridman WH, Zitvogel L, Sautès-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nature Reviews Clinical Oncology. 2017;14:717–34. doi:10.1038/nrclinonc.2017.101. PMID:28741618.
- Lesterhuis WJ, Bosco A, Millward MJ, Small M, Nowak AK, Lake RA. Dynamic versus static biomarkers in cancer immune checkpoint blockade: unravelling complexity. Nat. Rev. Drug Discov. 2017;16:264–72. doi:10.1038/nrd.2016.233. PMID:28057932.
- Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J, Perez-Gracia JL, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389:67–76. doi:10.1016/S0140-6736(16)32455-2. PMID:27939400.
- Vennapusa B, Baker B, Kowanetz M, Boone J, Menzl I, Bruey JM, Fine G, Mariathasan S, McCaffery I, Mocci S, et al. Development of a PD-L1 Complementary Diagnostic Immunohistochemistry Assay (SP142) for Atezolizumab. Appl. Immunohistochem. Mol. Morphol. 2018;0:1.
- Tretiakova M, Fulton R, Kocherginsky M, Long T, Ussakli C, Antic T, Gown A. Concordance study of PD-L1 expression in primary and metastatic bladder carcinomas: comparison of four commonly used antibodies and RNA expression. Mod. Pathol. 2017;31:1–10. doi:10.1038/modpathol.2017.188.
- Paluch BE, Glenn ST, Conroy JM, Papanicolau-Sengos A, Bshara W, Omilian AR, Brese E, Nesline M, Burgher B, Andreas J, et al. Robust detection of immune transcripts in FFPE samples using targeted RNA sequencing. Oncotarget. 2016;8:3197–205.
- Conroy JM, Pabla S, Glenn ST, Burgher B, Nesline M, Papanicolau-Sengos A, Andreas J, Giamo V, Lenzo FL, Hyland FCL, et al. Analytical Validation of a Next-Generation Sequencing Assay to Monitor Immune Responses in Solid Tumors. J. Mol. Diagnostics. 2018;20:95–109. doi:10.1016/j.jmoldx.2017.10.001.
- Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–81. doi:10.1038/nature13988. PMID:25428507.
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (80-.). 2015;348:124–8. doi:10.1126/science.aaa1348.
- Hersom M, Jørgensen JT. Companion and Complementary Diagnostics–Focus on PD-L1 Expression Assays for PD-1/PD-L1 Checkpoint Inhibitors in NSCLC. Ther. Drug Monit. 2017;40:1. doi:10.1097/FTD.0000000000000460.
- Mezzadra R, Sun C, Jae LT, Gomez-Eerland R, de Vries E, Wu W, Logtenberg MEW, Slagter M, Rozeman EA, Hofland I, et al. Identification of CMTM6 and CMTM4 as PD-L1 protein regulators. Nature. 2017;549:106–10. doi:10.1038/nature23669. PMID:28813410.
