ABSTRACT
S100A4 plays important roles in tumor development and metastasis, but its role in regulating inflammation and colitis-associated tumorigenesis has not been well characterized. Here, we report that S100A4 expression was increased in azoxymethane (AOM) and dextran sulfate sodium (DSS) induced colorectal cancer (CRC) in mice. After AOM/DSS treatment, both S100A4-TK mice with the selective depletion of S100A4-expressing cells and S100A4-deficient (S100A4−/−) mice developed fewer and smaller tumors than wild-type (WT) control littermates. Furthermore, S100A4−/− mice were resistant to DSS-induced colitis, reduced infiltration of macrophages, and the diminished production of proinflammatory cytokines. Further studies revealed that reduced colon inflammation and colorectal tumor development in S100A4−/− mice were partly due to the dampening of nuclear factor (NF)-κB activation in macrophages. Furthermore, the administration of a neutralizing S100A4 antibody to WT mice significantly decreased AOM/DSS-induced colon inflammation and tumorigenesis. These results indicate that S100A4 amplifies an inflammatory microenvironment that promotes colon tumorigenesis and provides a promising therapeutic strategy for treatment of inflammatory bowel disease and prevention of colitis-associated colorectal carcinogenesis.
Introduction
Chronic inflammation is a major driving force for the initiation and progression of many types of cancers, including colorectal cancer (CRC), lung cancer, and hepatocellular carcinoma (HCC).Citation1-Citation4 CRC is the third most common form of cancer and the second leading cause of cancer-related death in developed countries.Citation5 Patients with inflammatory bowel diseases (IBDs) such as ulcerative colitis (UC) and Crohn's disease (CD) are at an increased risk for the development of CRC.Citation6-Citation8 UC increases the cumulative risk of colitis-associated colorectal cancer (CAC) by up to 18–20%, while CD increases it up to 8%, after of 30 years of disease.Citation9-Citation11
It has been reported that cytokines, chemokines, matrix-degrading enzymes, and growth factors produced during chronic inflammation in IBD patients are proposed to damage DNA and/or alter cell proliferation or survival, thereby promoting cancer development.Citation7,Citation12 Immune cells, which often infiltrate tumors and preneoplastic lesions, also produce a variety of cytokines and chemokines that propagate a localized inflammatory response and enhance premalignant cell growth and survival by activating signaling pathways such as nuclear factor (NF)-κB or mitogen-activated protein kinases (MAPKs).Citation7,Citation13-Citation16 However, the underlying mechanisms that link these chronic inflammatory states to colorectal cancer development need to be further explored.
S100A4 is a member of the S100 calcium-binding protein family.Citation17,Citation18 Previous studies have reported that S100A4 is expressed in a variety of cells, such as fibroblasts, macrophages, and malignant cells, and plays a crucial role in mediating tumor-stromal interplay.Citation19-Citation23 S100A4 functions as both intracellular and extracellular forms.Citation24,Citation25 Intracellular S100A4 is involved in a wide range of biological functions, such as the regulation of angiogenesis, cell survival, motility, invasion or metastasis.Citation26,Citation27 However, the secretion of S100A4 by tumor and stromal cells is believed to serve as a key player in promoting the metastasis of cancer cells or affecting angiogenesis.Citation28-Citation30 Studies have shown that some cytokines, particularly chemokine (C-C motif) ligand 5 (CCL5), are necessary and sufficient for inducing the release of S100A4 from fibroblasts contributing to the formation of pre-metastatic niches.Citation31 Furthermore, it has been demonstrated that the ablation of S100A4 in stromal cells significantly reduced metastatic colonization by regulating matrix protein tenascin-C and growth factor vascular endothelial growth factor (VEGF)-A.Citation32 In previous studies, we found an additional mechanism of action that S100A4+ fibroblasts promote skin carcinogenesis by maintaining MCP-1-mediated macrophage infiltration and chronic inflammation.Citation20 In addition, we identified a critical role of S100A4 in promoting liver fibrosis through hepatic stellate cell activation,Citation21 and macrophage-derived S100A4 promotes liver carcinogenesis by promoting CD8+ T-cell survival (unpublished).
Previous studies have reported that the overexpression of S100A4 is closely associated with the progression of colorectal cancer.Citation33 High S100A4 levels in colorectal tumors are associated with metastasis, poor prognosis, and shortened patient survival times.Citation34 Recently, we found S100A4 promotes colitis development by enhancing host adhesion and colonization of Citrobacter rodentium through the S100A4-mediated host inflammatory responses.Citation35 Given the importance of S100A4 in tumor biology and inflammation, we questioned whether S100A4 contributes to inflammation-related colon tumorigenesis. The mouse model of colitis-associated colon cancer, which is induced by the administration of azoxymethane (AOM) followed by repeated oral administration of dextran sulfate sodium (DSS), has been highly informative.Citation36 Using the AOM/DSS mouse model, we showed here that S100A4 played key roles in the progression of IBD and CRC. We found that many S100A4+ cells infiltrated into the colon in colitis and CRC model mice. Selective depletion of S100A4+ cells and deficiency of S100A4 or blockade of S100A4 by neutralizing antibody significantly alleviated the disease severity in murine models of colitis and decreased tumor incidence in a murine model of CRC. Mechanistic study revealed that up-regulated S100A4 played an important function in inflammation via recruiting macrophages. In turn, NF-κB signaling in macrophages activated by S100A4 results in a vicious cycle of chronic inflammation, which promotes the occurrence of CRC. Our study suggests that S100A4 is an important molecule involved in inflammation and carcinogenesis, which can be a therapeutic target in the treatment of inflammatory bowel disease and prevention of CRC.
Results
S100A4 expression is upregulated in mouse model of CRC tumors
The association between S100A4 expression and CRC has been reported using tumor samples from CRC patients.Citation34,Citation37 To further investigate the kinetics of S100A4+ cells during CRC development, which could not be studied using clinical samples, C57 BL/6 mice were administered AOM/DSS () that has been used to induce a two-stage carcinogenesis model for CRC. The dynamic changes in S100A4+ cells in the colon tissue of C57 BL/6 mice before and at different times after the AOM/DSS application were examined. As shown in , there were few S100A4+ cells in the untreated colon. However, the number of S100A4+ cells was increased significantly after AOM/DSS treatment. IHC analysis revealed that S100A4 was mainly expressed in stromal cells located in the lamina propria throughout colon tissues and in the submucosal regions. In addition, S100A4 was also expressed in the lymphoid follicle (). Furthermore, as shown in , the expression of S100A4 was much higher in AOM/DSS-induced tumor-associated stroma than untreated colonic crypts.
Figure 1. S100A4 expression is associated with AOM/DSS-induced colitis and CRC. (A) Schematic representation of the DSS-induced colitis model. Groups of C57 BL/6 mice (n = 5 per group) were left untreated (D0) or treated with 3% DSS for 5 days for 2 cycles. Colon tissues were harvested at the indicated time points. (B) Histological characterization of colitis and S100A4+ cell accumulation. Colon sections were stained with anti-S100A4. Representative images are shown for untreated control and DSS-treated mice at each time point. (C) Number of S100A4+ cells in colon HPFs (×400) is shown. **P < 0.01. (D) AOM/DSS-induced colon sections were stained with S100A4. (E) Number of S100A4+ cells in CRC HPFs (×400) is shown. **P < 0.01.
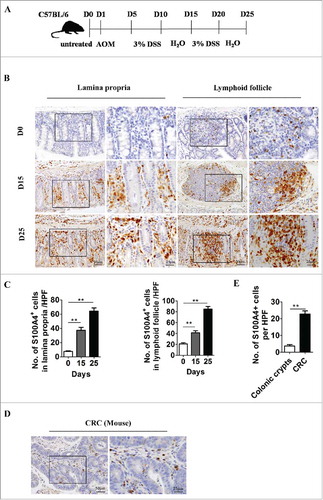
The appearance of S100A4+ cells in the process of colitis and CRC suggests that they may play important roles in local inflammation and CRC development.
S100A4 is expressed in different types of cells during colitis
Next, we characterized the cellular source of S100A4 in the colon. S100A4+/+.GFP transgenic mice expressing green fluorescent protein (GFP) under the control of the S100A4 promoterCitation38 were treated with DSS, and then cells were isolated from colon tissues, were co-stained with cellular marker antibodies for various cell types and were analyzed by flow cytometry. As shown in , , among the S100A4-GFP+ cells, approximately 97.9% were CD45+, mainly S100A4−GFP+ cells expressing myeloid cell markers, 54.3% were CD11b+, 44.2% were F4/80+, 25.7% were CD11 c+. In addition, a small number of the S100A4-GFP+ cells expressed markers of B cells, T cells and granulocytes ( and ). S100A4 was seldom expressed in epithelial cells, immunostaining of the colon tissues showed similar results (). In addition, double staining revealed that most of the S100A4+ cells were not α-SMA positive, showing that they were not fibroblasts (Fig. S1).
Figure 2. S100A4 is expressed in different types of cells in the colon. (A-B) Flow cytometry analysis of the phenotypes of S100A4+ cells in the colons of S100A4+/+.GFP mice treated with 3% DSS for 5 days for 2 cycles by staining GFP+ cells with CD45, CD11b, F4/80, CD11 c, CD4, CD8 and CD19 antibodies. (C) Double immunohistochemical staining of S100A4 with E-cadherin, CD11b, F4/80, CD4, CD8, B220 and Gr-1 in DSS-induced colon tissues. Nuclei were counter-stained with DAPI. The arrows indicate double-positive cells. Scale bar, 25 μm. (D) S100A4 concentrations in the cultured supernatants of S100A4+ CD11b+ cells or S100A4− CD11b+ cells as detected by ELISA. **P < 0.01.
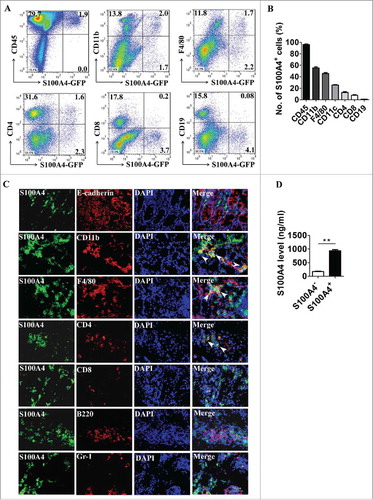
Using LPS-induce colitis model, we also found that S100A4 was upregulated significantly in colon tissues and was expressed mainly in CD11b+ myeloid cells upon stimulation with LPS (Fig. S2).
S100A4 has been reported to be secreted out of cells through unknown mechanisms and to function as an inflammatory cytokine.Citation21,Citation29,Citation30 To test whether S100A4 is secreted by S100A4+ cells in the CRC tissue, CD11b+ S100A4+ cells were isolated from DSS-treated colon tissues and were cultured in vitro, and the supernatant was analyzed for S100A4 by ELISA. CD11b+ S100A4− cells were used as control. Indeed, soluble S100A4 was detected in the supernatant of CD11b+ S100A4+ cells but not in that of CD11b+ S100A4− cells (). These results indicate that S100A4+ cells have many cellular origins in the colon during colitis and S100A4 can be secreted into the extracellular space.
Selective depletion of S100A4+ cells attenuates colon carcinogenesis
To explore the role of S100A4+ cells in the development of CRC, S100A4-thymidine kinase (TK) transgenic mice were used,Citation22,Citation39 and proliferating S100A4+ cells in these mice could be selectively depleted upon the treatment of ganciclovir (GCV) after the administration of AOM/DSS, as illustrated in . Expression of the Ki67 antigen in S100A4+ cells indicated that these S100A4+ cells were proliferating, rendering them susceptible to depletion by GCV in S100A4-TK transgenic mice (). As anticipated, the number of infiltrated S100A4+ cells in the colon, was decreased dramatically in S100A4-TK+ mice after GCV treatment for 2 weeks while this did not occur in control littermates (). This reduction of S100A4+ cells in colon correlated with an attenuated accumulation of inflammatory cells in colon tissue, as shown by H&E staining (). Importantly, GCV treatment in S100A4-TK+ mice attenuated colon tumorigenesis with a reduced tumor number and size compared with the control group (). Pathologic analysis revealed that GCV-treated S100A4-TK+ mice had smaller tumor areas than S100A4-TK− mice (). S100A4-TK− mice had much more S100A4 + cells than S100A4-TK+ mice ().
Figure 3. Selective depletion of proliferating S100A4+ cells decreases colorectal tumorigenesis. (A) Schematic illustration of S100A4+ cell depletion in S100A4-TK transgenic mice and control littermates. (B) Proliferating S100A4+ cells in DSS-treated colon. Double staining of S100A4 (green) and Ki67 (red) in DSS-treated colon tissues. The arrows indicate double-positive cells that are proliferating. Scale bar, 50 μm. (C) Groups of S100A4-TK transgenic mice (TK+) and control littermates (TK−) (n = 8–10) were treated with GCV to deplete S100A4+ cells or PBS as the control. Shown is the staining for S100A4 and H&E in colon tissue. Scale bar, 50 μm. (D) Percentages of S100A4+ cells of GCV-treated mice are shown. *P < 0.05, **P < 0.01. (E) Representative photographs of the colon from S100A4−/− and WT mice on day 120 of age. (F) Tumor incidence and incidence of tumors over 4 mm2 (G) in S100A4 Tk+ and S100A4 TK− mice are shown. (H) Colon sections were stained with H&E, S100A4 and Ki67. Scale bar, 50 μm. (I) The number of S100A4+ cells in tumors per HPF and percentages of Ki67+ cells in tumors are shown.
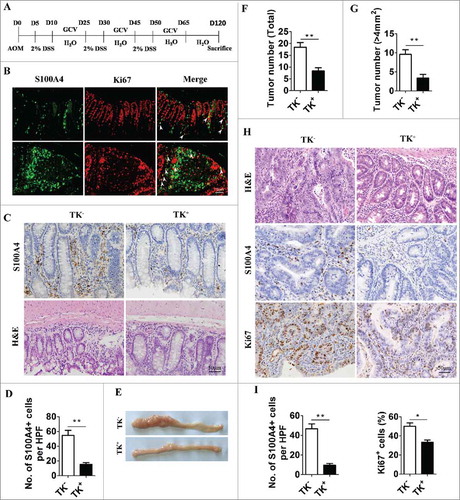
Moreover, the number of Ki67+ proliferating cells in colon tumors was also significantly reduced in GCV-treated S100A4-TK+ mice ( and ). These results suggest that the selective depletion of proliferating S100A4+ cells in colons significantly decreased colon tumorigenesis.
Genetic deletion of S100A4 prevents colitis-associated colorectal tumorigenesis
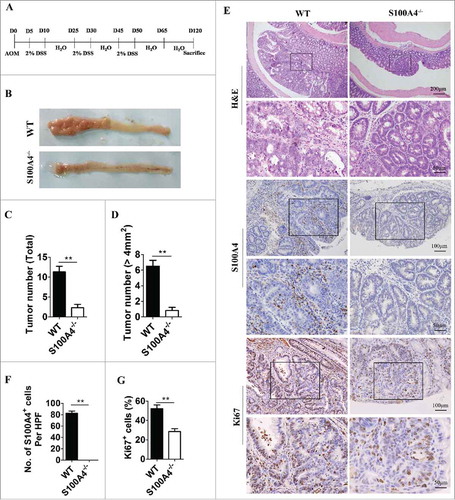
To further explore the role of S100A4 in colitis-related colorectal carcinogenesis, S100A4-deficient mice (S100A4−/−) were used.Citation38 Both S100A4–/– mice and WT control littermates were given a single intraperitoneal injection of AOM followed by DSS treatment, and then colon tumorigenesis was monitored for 120 days (). Compared with the WT control, we observed a significant decrease in the total colon tumor numbers in S100A4−/− mice, 11.3 ± 3.39 vs. 2.3 ± 1.63 ( and ). The maximal tumor diameters were notably smaller in S100A4−/− mice compared with WT controls, 6.5 ± 1.87 vs. 0.83 ± 0.68 (). Pathologic analysis confirmed the different tumor size in S100A4−/− mice compared to WT mice (). Moreover, WT mice had many S100A4+ cells. The number of proliferating cells in tumor tissues decreased significantly in S100A4–/– mice compared with WT mice (). Therefore, these results suggest a critical role of S100A4 in promoting CRC development.
Figure 4. S100A4 deficiency decreases colorectal tumorigenesis. (A) Schematic overview of the experimental CAC model using S100A4−/− and WT control littermates. (B) Representative photographs of the colon from S100A4−/− and WT mice on day 120 of age. (C) The tumor number of the entire colon was counted, and each tumor size was measured. Total tumor incidence and (D) incidence of tumors over 4 mm2 in S100A4−/− and WT mice are shown. (E) The colon sections of CAC were stained with H&E, S100A4 and Ki67. Representative images are shown. (F) The number of S100A4+ cells in tumors per HPF (×200) and (G) percentages of Ki67+ cells in tumors are shown. *P < 0.05, **P < 0.01.
To exclude the possibility that reduced tumor burden in S100A4−/− mice is not due to altered gut bacteria, the gut microbiota of S100A4−/− mice and WT control mice was assessed by detecting the counting of bacterial colonies from mice feces in LB-Medium, and there was no significant difference of the number of bacterial colonies between mice feces from WT and S100A4−/− mice (Fig. S3). This suggests that the gut bacteria were not significantly altered in S100A4−/− mice comparing with WT control littermates.
S100A4-deficient mice are resistant to DSS-induced colitis
To define the role of S100A4 in colitis-induced inflammation, S100A4−/− mice were fed with 3% DSS in drinking water for 5 days, and susceptibility was monitored by measuring body weight, assessing stool consistency and rectal bleeding, and measuring colon length during both the acute (day 5) and recovery (days 9–20) stages of the disease. The results showed that WT mice developed continued body weight loss (), diarrhea, and rectal bleeding (), whereas S100A4–/– mice started to recover once DSS was omitted from the drinking water. This reduced inflammatory phenotype was further evidenced by the gross appearance of the colon on day 10 (). The colons of S100A4−/− mice were much longer than those of WT mice (, D).
Figure 5. S100A4-deficient mice are resistant to DSS-induced colitis, and macrophage response in colon tissue was decreased. Groups of S100A4−/− and WT control littermates (n = 5 per group) were fed with 3% DSS for 5 days followed by normal drinking water until day 10. (A) The body weight change and (B) clinical score are shown. *P < 0.05; **P < 0.01. (C) Mice were sacrificed on day 10 to measure the colon length. Representative photographs and (D) length of the colon from S100A4−/− and WT mice are shown. (E) Colon sections were stained with H&E. Scale bar, 100 μm. (F) Colon tissues were collected on day 10, and colon sections were immunostained with CD11b, F4/80 and Gr-1. Scale bar, 50 μM. Number of CD11b+, F4/80+ and Gr-1+ cells per HPF (×200) are shown at right. *P < 0.05; **P < 0.01. (G) Groups of WT and S100A4−/− mice (n = 5 per group) were treated as (A), and the colons and MLN were collected on day 10. The percentages of CD11b+, F4/80+, CD11 c+, and Gr-1+ cells in colonic lamina propria cells and MLN cells were analyzed by FACS. *P < 0.05; **P < 0.01. (H) Protein levels of MCP-1, MIP2, TNF-α, IL-17 A, IL-6, INF-γ, IL-22 and GMSF in the colon tissues of WT and S100A4−/− mice were detected using the Procarta Plex TM multiplex immunoassay. *P < 0.05; **P < 0.01.
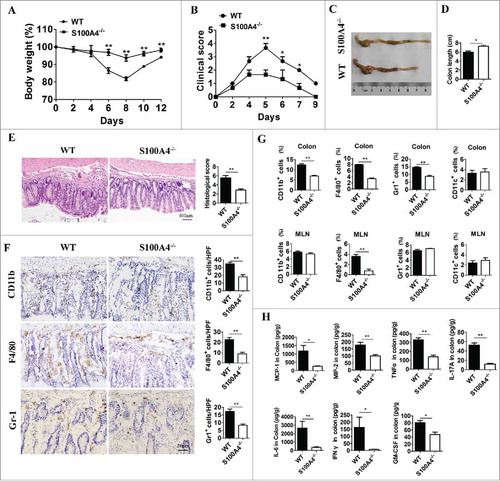
To obtain further evidence of weakened inflammation in S100A4–/– mice, the colon tissue was histologically analyzed on day 15 following AOM administration. The colons of S100A4−/− mice contained markedly less infiltrating inflammatory cells and displayed significantly less ulceration and hyperplasia ( and ).
S100A4 deficiency leads to a decreased inflammatory response in colon tissue. We then test whether S100A4 deficiency protected mice from colitis-associated tumorigenesis by dampening immune cell activation and inflammatory responses upon DSS-treatment. We examined the histopathological changes that occur during early stages of tumor induction (on day 15 after AOM injection) and found that S100A4-deficient colons showed decreased infiltration of macrophages and polumorphonuclear granulocytes (PMNs) ().
To further characterize the immune cell types associated with the induction of inflammatory responses in the colons of S100A4−/− mice, myeloid cells present in the colonic lamina propria were isolated at a later stage of colitis and were analyzed by flow cytometry. On day 15 after AOM injection, the cell percentages of all analyzed myeloid cell types (CD11b+, F4/80+, Gr-1+) in S100A4−/− mouse colons were significantly lower than those in WT colons, except CD11 c+ cells (). In addition, a dramatic decrease in the percentage of F4/80+ macrophages was evident in the mesenteric lymph nodes (MLN) of S100A4−/− mice (). However, there were no significant differences of percentages of CD4+ T cells and CD8+ T cells both in colons and MLNs between S100A4–/– and WT mice (Fig. S4). Consistent with the decreased infiltration and activation of myeloid cells in the absence of S100A4−/− mice, the production of proinflammatory cytokines such as monocyte chemotactic protein-1(MCP-1), macrophage inflammatory protein-2 (MIP-2), tumor necrosis factor (TNF)-α, IL (interleukelin)-17 A, IL-6, interferon (INF)-γ and granulocyte-macrophage colony-stimulating factor (GMSF) were all found to be decreased in the colons of S100A4−/− mice relative to the levels found in WT mice (). Taken together, these results indicate that S100A4 plays a critical role in promoting the inflammatory response in myeloid cells, especially in macrophages during DSS-induced colitis.
Nuclear factor (NF)-κB signaling in macrophage is critical for promoting colon tumorigenesis
The expression of tumorigenic and proinflammatory genes is modulated by signal transduction pathways such as NF-κB, extracellular receptor kinase (ERK), and signal transducer and activator of transcription 3 (STAT3) pathways. To understand whether these pathways and molecules were deregulated in the absence of S100A4, we examined the activation of inflammatory signaling pathways by Western blotting. Indeed, the NF-κB, ERK levels in AOM/DSS-induced colon tissues in S100A4−/− mice were clearly down-regulated compared with those in WT mice (). Consistent with the results of Western blotting, the expression of p-p65 in colon tissues in S100A4−/− mice was also down-regulated as revealed by immunofluorescence detection ().
Figure 6. NF-κB signaling in Macrophages is critical for the protection against colon tumorigenesis. Groups of S100A4−/− and WT mice (n = 5 per group) were treated as (A). (A) The protein levels of p-p65, p-β-catenin and p-ERk in colon tissues on day 10 after DSS were analyzed by Western blotting. (B) Sections from DSS-treated colon tissues of S100A4−/− and WT mice were double stained with F4/80 (green) and p-p65 (red). Scale bar, 50 μm. (C) Sections from DSS-treated colon tissues of S100A4−/− and WT mice were stained with Ki67, and the percentages of Ki67 in colons are shown. Scale bar, 50 μm. (D-E) RAW cells were cultured without or with S100A4 (200 ng/ml) or RAGE inhibitor for 2 h as indicated. The levels of p65, p-p65, IKBα, p-IKBα, ERK, p-ERK and RAGE were determined by Western blotting.
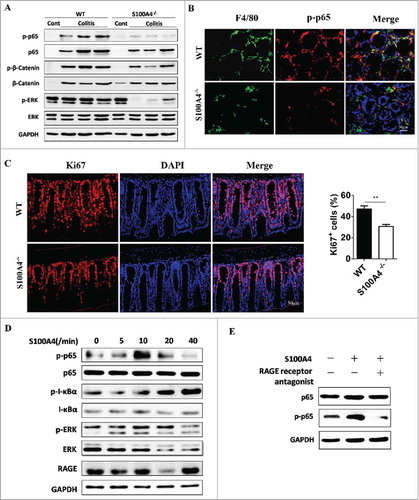
Down-regulation of the NF-κB pathways is associated with a decreased proliferation of epithelial cells in the hyperplastic colon regions of AOM/DSS-treated S100A4−/− mice (). By contrast, untreated colon tissues of both S100A4−/− and WT mice displayed similar proliferation levels as measured by Ki67 staining (data not shown).
To identify the molecular mechanisms underlying the effects of extracellular S100A4 on the induction of inflammation, macrophage cell line Raw cells were cultured with different stimuli as indicated previously and were performed for the analysis of pathway-related genes. After treatment with S100A4 for various times (), we found that phosphorylated p65 and I-κBα were up-regulated compared with those in the control group. The phosphorylation level of ERK was unaffected. Thus, these results indicate that S100A4 promotes the activation of NF-κB signaling in macrophages.
The receptor of advanced glycation end products (RAGE) is an interaction partner for S100A4.Citation40,Citation41 Next, we tested whether S100A4-induced NF-κB signaling in Raw cells was RAGE dependent. The expression of RAGE in Raw cells was validated by Western blotting (). We used a RAGE-specific inhibitor (FPS-ZM1) to prevent RAGE activation in cultured Raw cells and found that the level of phosphorylated NF-κB could not be up-regulated by S100A4 stimulation (), suggesting that S100A4-activated NF-κB signaling in Raw cells was a RAGE-dependent process.
Targeting S100A4 by S100A4-neutralizing antibody attenuates colitis and prevents CAC in a murine model
Because S100A4 plays an important role in colitis pathogenesis, we examined the therapeutic value of S100A4 neutralizing antibody in mouse models. By administering S100A4 neutralizing antibody or an isotypic control (mIgG) preventively to the DSS-treated mice (during the DSS induction process), we found that S100A4 neutralizing antibody significantly alleviated the disease severity (). Body weight loss was dramatically reduced in the neutralizing antibody-treated group compared with that in the mIgG treatment group ( and ). Consistent with the observation in the colitis model of S100A4−/− mice, we found a decrease in macrophage infiltration in neutralizing antibody-treated mice ( and ). These data indicate that neutralizing antibody could be an efficient treatment for colitis.
Figure 7. Treatment with S100A4 neutralizing antibody attenuates colitis and prevents colitis-associated tumorigenesis. Groups of WT mice (n = 5 per group) were treated with DSS and anti-S100A4 mAb or mIgG 4 times on days 1, 3, 7 and 10. (A) The body weight change and (B) Representative photographs and (C) length of the colon from S100A4−/− and WT mice on day 10 are shown. *P < 0.05; **P < 0.01. (D) Colon sections were immunostained with F4/80. Scale bar, 25 μm. (E) Numbers of F4/80+ cells per HPF (×400) are shown at right. *P < 0.05. (F) Representative photographs of the colons from mice with mIgG or S100A4 neutralizing antibody treatment. (G) Total tumor incidence and (H) incidence of tumors over 4 mm2 in AOM-DSS induced CRC model mice with mIgG or S100A4 neutralizing antibody treatment. *P < 0.05; **P < 0.01.
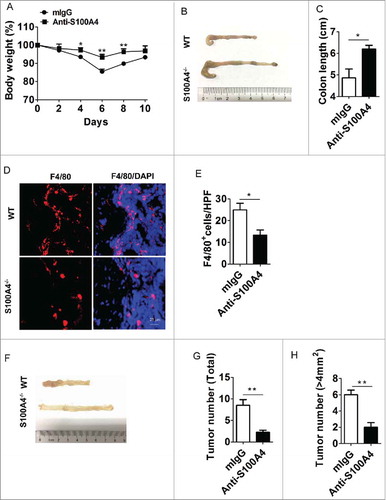
Furthermore, because S100A4 neutralizing antibody treatment appeared to ameliorate the inflammatory response in colitis, we then tested whether it could protect mice with colitis from developing tumors by treating CRC mice with either mIgG or neutralizing antibody during the DSS induction process. Interestingly, the tumor incidence and tumor volume were drastically reduced in S100A4 neutralizing antibody-treated mice (). Collectively, these data indicate the therapeutic value of anti-S100A4 antibody in treating colitis and preventing CRC.
Discussion
In this study, we have demonstrated that S100A4 promotes colitis-associated colon tumorigenesis by enhancing the inflammatory response. S100A4 is expressed in CRC both in patients and experimental mouse models. The selective depletion of S100A4-expressing cells and deficiency in S100A4 attenuated colitis and prevented colon tumorigenesis in mice using the AOM/DSS model. The colonic macrophage infiltrate and the production of cytokines in S100A4−/− mice were significantly decreased after DSS treatment. Further studies revealed that reduced colon inflammation and colorectal tumor development in S100A4−/− mice were partly due to the dampening NF-κB activation in macrophages.
A large number of reports have demonstrated that increased S100A4 is significantly correlated with tumor angiogenesis, cell survival, motility, invasion and metastasis.Citation17,Citation18 High S100A4 levels in colorectal tumors are associated with aggressive growth, metastasis, poor prognosis, and shortened patient survival times.Citation33 However, its role in colon cancer progression remains to be adequately tested. Using an AOM/DSS-induced CRC model, we also detected a high level of S100A4 expression in the colon during the process of CRC (). Further study has demonstrated that S100A4+ cells play critical roles in tumorigenesis as the depletion of S100A4-positive cells attenuates colitis and decreases the incidence of colon tumorigenesis. In addition, we found similar results to those of mice deficient in S100A4, indicating the important role of S100A4 molecules in disease progression. This work demonstrates that S100A4 plays an important and direct role in promoting CRC development in vivo.
It is becoming increasingly clear that cytokines and growth factors released during chronic inflammation contribute to tumorigenesis.Citation13 In IBD, chronic inflammation causes the release of cytokines, growth factors, proteases, and reactive oxygen species (ROS).Citation12 Cytokines released during inflammation may promote cancer development via the activation of NF-κB, which is one of the few key regulatory signaling molecules whose aberrant activation is invariably associated with inflammation and cancer.Citation42 It has been reported that IKKβ-driven NF-κB activation in intestinal epithelial cells (IECs) is essential for the development of colonic adenomas in the AOM/DSS-induced CRC model.Citation43 In addition, IKKβ-driven NF-κB activation in certain myeloid cells, most likely macrophages of the lamina propria, contributes to CRC development by the enhancing proliferation of pre-malignant IECs via stimulating the secretion of growth factors.Citation43 In this study, we found that, during the process of AOM/DSS-induced CRC, many S100A4+ cells infiltrate into the inflammatory colon. The depletion of S100A4+ cells or S100A4 deficiency significantly dampens DSS-induced colitis. Inflammatory cell infiltration, especially that of macrophages, and the levels of cytokines such as IL-6, TNF-α, IL-17 A and MCP-1, were also decreased obviously in S100A4−/− mice, a finding that is consistent with previous studies that S100A4 recruits macrophages.Citation44 Furthermore, the NF-κB levels in AOM/DSS-induced colon tissues in S100A4−/− mice were clearly down-regulated compared with those in WT mice. In vitro, S100A4 could activate the NF-κB signal pathway in macrophages, contributing to the development of CRC by enhancing the proliferation of pre-malignant IECs. Thus, we elucidated a new mechanism that bridges chronic inflammation to CRC promotion. In addition, S100A4 neutralization significantly dampened DSS-induced colitis and decreased the incidence of colon tumorigenesis (). S100A4 could be a target for CRC therapy. S100A4 blockade may be useful in reducing the risk of colitis-associated colon cancer.
S100A4 expression was initially characterized in the cells of mesenchymal origin, including stromal fibroblasts and epithelial cells undergoing epithelial-mesenchymal transition (EMT). Recently, it was found that S100A4+ cells in liver were a subtype of macrophages.Citation23 However, it seems that S100A4 is expressed in different types of cells in various tissues.Citation45,Citation46 The cellular origin of S100A4 in the colon is not clear. We found many types of stromal cells expressed S100A4 in colon tissues after DSS induction. Of the S100A4+ cells, approximately 97.9% were CD45+, 54.3% were CD11b+, 44.2% were F4/80+, and 25.7% were CD11 c+. In addition, only a small number of B cells, T cells and granulocytes expressed S100A4 (). However, the role of different type of S100A4+ cells in colon and CRC still needs further study.
S100A4 controls a variety of intra- and extracellular processes. Intracellular S100A4 influences the function of distinct targets, such as different cytoskeletal-associated molecules and the tumor suppressor p53 protein, which promotes p53 degradation.Citation47 Intracellular S100A4 is involved in a wide range of biological functions, such as the regulation of angiogenesis, cell survival, motility, invasion or metastasis.Citation26,Citation27 However, S100A4 could also serve as an extracellular cytokine, and its role in the soluble form has received increasing attention. The secretion of S100A4 by tumor and stromal cells is believed to serve as a key player in the metastasis of cancer cells or affecting angiogenesis.Citation28-Citation30 Although we did not know which form of S100A4 plays more important roles in colon tumorigenesis, our IHC staining of colon tumors suggests the weak intracellular staining, and our functional studies support a major source of myeloid cell-producing S100A4 during colon cancer development. It is certainly also important to analyze the role of intracellular S100A4 in myeloid cells during the development of colon tumorigenesis in the future.
In summary, this study provides in vivo evidence for a critical role of S100A4 in promoting colitis-related colon tumorigenesis and crosstalk between colitis, macrophages, S100A4, and CRC. It would be interesting to test further whether anti-S100A4 antagonists might be effective at targeting tumor-initiating cells as well as the tumor microenvironment, therefore providing a promising strategy for colon cancer prevention and treatment.
Materials and methods
Cell lines and mice
The murine colon cancer cell line CT26 was obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). These cells were cultured in RPMI 1640 medium containing 10% fetal calf serum at 37°C with 5% CO2.
S100A4+/+.GFP and S100A4-deficient mice (S100A4−/−) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). S100A4-TK transgenic mice were obtained from Dr. Eric G. Neilson (Northwestern University, Feinberg School of Medicine). All mice and WT control littermates were bred under specific pathogen-free conditions in the animal facilities at the Institute of Biophysics, Chinese Academy of Sciences. Male mice 8–10 weeks of age were used. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Chinese Academy of Sciences. The experiments described were approved by the Institutional Animal Care and Use Committee of the Institute of Biophysics, Chinese Academy of Sciences.
Induction of DSS-induced colitis
Acute colitis was induced with 3% (w/v) DSS (molecular mass, 36–40 kDa; MP Biologicals) dissolved in sterile, distilled water for 5 days followed by normal drinking water until the end of the experiment. The DSS solutions were made fresh on day 3.
In the antibody preventive treatment experiment, mice were injected i.p. with S100A4-neutralizing antibody or control murine IgG (mIgG) at a dose of 10 mg/kg body weight on day 1 and day 5.
Clinical scoring of colitis
Scoring for stool consistency and occult blood was performed as previously described.Citation48 Briefly, stool scores were determined as follows: 0 = well-formed pellets, 1 = semiformed stools that did not adhere to the anus, 2 = semiformed stools that adhered to the anus, and 3 = liquid stools that adhered to the anus. Bleeding scores were determined as follows: 0 = no blood by using hemoccult (Beckman Coulter), 1 = positive hemoccult, 2 = blood traces in stool visible, and 3 = gross rectal bleeding. Stool consistency scores and bleeding scores were added and presented as clinical scores.
Induction of colorectal cancer
Mice were injected intraperitoneally with 10 mg/kg AOM (Sigma). After 5 days, 2% DSS was given in drinking water over 5 days followed by regular drinking water for 2 weeks. This cycle was repeated 3 times, and mice were sacrificed 120 days after AOM injection. The S100A4 neutralizing antibody or control mIgG was injected i.p. at 10 mg/kg body weight twice during each DSS feeding period. Monoclonal anti-mouse S100A4 mAb was generated by immunizing BALB/c mice with S100A4 proteins. Using immune spleen cells and the mouse myeloma Sp2/0 cell line as fusion partner,Citation49 hybridomas were generated and selected using standard published techniques.
Depletion of S100A4-positive cells in S100A4-TK mice
Every week, S100A4-TK mice and control littermates were given 50 mg/kg ganciclovir (GCV) (HuBeiKeYi Pharmaceutic Corporation, China) dissolved in saline i.p. every other day, during the 2 weeks of the regular drinking water period in the process of colorectal cancer induction as shown in .
Isolation and subsequent analysis of murine lamina propria lymphocytes
According to the method of Weigmann et al., mouse colons were removed and cleaned. The colons were first predigested with 5 mmol/L EDTA and 1 mmol/L dithiothreitol. Next, the intraepithelial lymphocytes were discarded, and the colons were then digested with 0.05 g of collagenase D (Roche, Basel, Switzerland) and 0.05 g of DNase I (Sigma-Aldrich). The lamina propria lymphocytes were isolated using a 40/80 Percoll gradient.
Flow cytometry analysis
Single-cell suspensions prepared directly from the MLN, spleen and colon lamina propria were stained with the following directly labeled mouse-specific mAbs: Percp/Cy5.5-labeled anti-CD11b (clone M1/70), APC-labeled anti-Ly6 C (clone HK1.4), PE-labeled anti-F4/80 (clone BM8), Percp-labeled anti-CD4 (clone GK1.5), and APC-labeled anti-CD8 (clone 53–6.7). All antibodies were purchased from Biolegend and were used at a 0.2-μg/ml concentration. Cells were collected using a FACS Caliber system (BD Biosciences, San Diego, CA) and were analyzed using FlowJo software (TreeStar, Ashland, OR).
Histopathological and immunohistochemical analysis
The preparation of cryostat or paraffin tissue sections from mice was performed as described previously.Citation50 The colon was rolled as a Swiss roll and fixed in 4% PFA. Paraffin-embedded sections were cut at 5 mm and were stained with H&E solution. Histological scores were evaluated by two independent investigators, blinded to the source of treatment, as follows: epithelium scores 0, normal morphology; 1, loss of goblet cells; 2, loss of goblet cells in large areas; 3, loss of crypts; and 4, loss of crypts in large areas. Infiltration scores were evaluated as follows: 0, no infiltrate; 1, infiltrate around the crypt basis; 2, infiltrate reaching the L. muscularis mucosae; 3, extensive infiltration reaching the muscularis mucosae and thickening of the mucosa; and 4, infiltration of the L. submucosa. The total histological score represents the sum of the epithelium and infiltration scores and ranges from 0 to 8. For immunohistochemistry (IHC), paraffin sections were incubated with anti-S100A4 (Abcam, Cambridge, UK), and frozen colon sections were incubated with anti-CD8, anti-CD4, anti-F4/80, anti-Gr-1 antibodies (BD Pharmingen, San Diego, CA), followed by incubation with species-matched Alexa dye-labeled or horseradish peroxidase (HRP)-conjugated secondary antibodies. Frozen colon sections were incubated with anti-S100A4 (Abcam, Cambridge, UK), anti-CD11b (BD Pharmingen, San Diego, CA), anti-F4/80 (BD Pharmingen, San Diego, CA), anti-α-SMA (Abcam, Cambridge, UK) antibodies and Alexa Fluor 488 and 555-conjugated secondary antibodies (Invitrogen, Grand Island, NY). Sections were evaluated under the microscope (DP71, OLYMPUS) for bright-field and fluorescence microscopy.
Cytokine and serum biochemical analysis
To detect multiple cytokines in the colons, tissues were homogenized in ice-cold TE buffer. Homogenates were centrifuged at 12,000 × g for 15 minutes. The supernatant was collected, and the ProcartaPlex™ multiplex immunoassay (Luminex) (eBioscience) was used on a Bioplex-200 system with the Bioplex Manager 5.0 software. The cytokines were analyzed according to the manufacturer's protocol. S100A4 in cell culture supernatant and whole colon lysate were detected by sandwich ELISA as described previously.Citation21
Western blotting
Tissue and cell extracts were analyzed using the following primary antibodies: anti-α-SMA, anti-S100A4, anti-pro-Collagen I (Santa Cruz Biotechnology, Santa Cruz, CA), anti-c-myb (ImmunoWay, Newark, DE) and anti-β-actin (Cell Signaling, Danvers, MA). HRP-conjugated goat anti-mouse or goat anti-rabbit IgG were used as secondary antibodies.
Quantitative and statistical analysis
All the data were expressed as means ± SEM and were analyzed using GraphPad Prism software. Differences between the two groups were compared using a two-tailed unpaired Student's t-test or Mann-Whitney U test analysis. Other data were analyzed using one-way or 2-way ANOVA followed by Bonferroni's multiple comparisons test. For all tests, a P value < 0.05 was considered statistically significant.
Abbreviations
| AOM | = | azoxymethane |
| CAC | = | colitis-associated colorectal cancer |
| CCL5 | = | chemokine (C-C motif) ligand 5 |
| CD | = | Crohn's disease |
| CRC | = | colorectal cancer |
| DSS | = | dextran sulfate sodium |
| EMT | = | epithelial-mesenchymal transition |
| ERK | = | extracellular receptor kinase |
| GCV | = | ganciclovir |
| GMSF | = | granulocyte-macrophage colony-stimulating factor |
| HCC | = | hepatocellular carcinoma |
| IL | = | interleukelin |
| INF | = | interferon |
| MAPK | = | mitogen-activated protein kinase |
| MCP | = | monocyte chemotactic protein |
| MIP | = | macrophage inflammatory protein |
| MLN | = | mesenteric lymph nodes |
| NF | = | nuclear factor |
| PMNs | = | polumorphonuclear granulocytes |
| RAGE | = | receptor of advanced glycation end products |
| S100A4−/− | = | S100A4-deficient |
| STAT3 | = | signal transducer and activator of transcription 3 |
| TK | = | thymidine kinase |
| TNF | = | tumor necrosis factor |
| UC | = | ulcerative colitis |
| VEGF | = | vascular endothelial growth factor |
| WT | = | wild-type |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Authors' contributions
J.Z. and Z.C. were involved in the study design. J.Z., S.H., Q.Y. and J.J. collected data. J.Z., S.H., J.G., T.T., and Z.C. analyzed and interpreted the data. J.Z., Z.Q. and Z.C. wrote the manuscript.
2017ONCOIMM0901R1-s02.doc
Download MS Word (1.9 MB)Acknowledgments
We thank Dr. Kunsong (Chinese Academy of Sciences) and Jun Wang (Yale University) for discussions and technical help.
Additional information
Funding
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi:10.1016/j.cell.2011.02.013. PMID:21376230.
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;144:420:860–7. doi:10.1038/nature01322. PMID:12490959.
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. PMID:18650914.
- Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144:512–27. doi:10.1053/j.gastro.2013.01.002. PMID:23313965.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi:10.3322/caac.21332. PMID:26742998.
- Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J. Gastroenterol. 2008;14:378–89. doi:10.3748/wjg.14.378. PMID:18200660.
- Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol. 2013;35:229–44. doi:10.1007/s00281-012-0352-6. PMCID:PMC3568220.
- Herrinton LJ, Liu L, Levin TR, Allison JE, Lewis JD, Velayos F. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology. 2012;143:382–9. doi:10.1053/j.gastro.2012.04.054. PMID:22609382.
- Rubin DC, Shaker A, Levin MS. Chronic intestinal inflammation: inflammatory bowel disease and colitis-associated colon cancer. Front Immunol. 2012;3:107. doi:10.3389/fimmu.2012.00107. PMID:22586430.
- Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–35. PMID:11247898.
- Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn's disease. Aliment Pharmacol Ther. 2006;23:1097–104. doi:10.1111/j.1365-2036.2006.02854.x. PMID:16611269.
- Klampfer L. Cytokines, inflammation and colon cancer. Curr. Cancer Drug Targets. 2011;11:451–64. PMID:21247378.
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi:10.1016/j.cell.2010.01.025. PMID:20303878.
- Nicolas-Avila JA, Adrover JM, Hidalgo A. Neutrophils in homeostasis, immunity, and cancer. Immunity. 2017;46:15–28. doi:10.1016/j.immuni.2016.12.012. PMID:28099862.
- Berraondo P, Minute L, Ajona D, Corrales L, Melero I, Pio R. Innate immune mediators in cancer: between defense and resistance. Immunol Rev. 2016;274:290–306. doi:10.1111/imr.12464. PMID:27782320.
- Lewis CE, Harney AS, Pollard JW. The Multifaceted role of perivascular macrophages in tumors. Cancer cell. 2016;30:18–25. doi:10.1016/j.ccell.2016.05.017. PMID:27411586.
- Boye K, Maelandsmo GM. S100A4 and metastasis: a small actor playing many roles. Am J Pathol. 2010;176:528–35. doi:10.2353/ajpath.2010.090526. PMID:20019188.
- Bresnick AR, Weber DJ, Zimmer DB. S100 proteins in cancer. Nat Rev Cancer. 2015;15:96–109. doi:10.1038/nrc3893. PMID:25614008.
- Zhang J, Chen L, Liu X, Kammertoens T, Blankenstein T, Qin Z. Fibroblast-specific protein 1/S100A4-positive cells prevent carcinoma through collagen production and encapsulation of carcinogens. Cancer Res. 2013;73:2770–81. doi:10.1158/0008-5472.CAN-12-3022. PMID:23539447.
- Zhang J, Chen L, Xiao M, Wang C, Qin Z. FSP1+ fibroblasts promote skin carcinogenesis by maintaining MCP-1-mediated macrophage infiltration and chronic inflammation. Am J Pathol. 2011;178:382–90. doi:10.1016/j.ajpath.2010.11.017. PMID:21224075.
- Chen L, Li J, Zhang J, Dai C, Liu X, Wang J, et al.. S100A4 promotes liver fibrosis via activation of hepatic stellate cells. J Hepatol. 2015;62:156–64. doi:10.1016/j.jhep.2014.07.035. PMID:25111176.
- Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, et al.. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393–405. PMID:7615639.
- Osterreicher CH, Penz-Osterreicher M, Grivennikov SI, Guma M, Koltsova EK, Datz C, et al.. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc Natl Acad Sci U S A. 2011;108:308–13. doi:10.1073/pnas.1017547108. PMID:21173249.
- Mishra SK, Siddique HR, Saleem M. S100A4 calcium-binding protein is key player in tumor progression and metastasis: preclinical and clinical evidence. Cancer metastasis reviews. 2012;31:163–72. doi:10.1007/s10555-011-9338-4. PMID:22109080.
- Kim EJ, Helfman DM. Characterization of the metastasis-associated protein, S100A4. roles of calcium binding and dimerization in cellular localization and interaction with myosin. J Biol Chem. 2003;278:30063–73. doi:10.1074/jbc.M304909200. PMID:12756252.
- Liang J, Piao Y, Holmes L, Fuller GN, Henry V, Tiao N, et al.. Neutrophils promote the malignant glioma phenotype through S100A4. Clin Cancer Res. 2014;20:187–98. doi:10.1158/1078-0432.CCR-13-1279. PMID:24240114.
- Fabris L, Cadamuro M, Moserle L, Dziura J, Cong X, Sambado L, et al.. Nuclear expression of S100A4 calcium-binding protein increases cholangiocarcinoma invasiveness and metastasization. Hepatology. 2011;54:890–9. doi:10.1002/hep.24466. PMID:21618579.
- Schmidt-Hansen B, Ornas D, Grigorian M, Klingelhofer J, Tulchinsky E, Lukanidin E, et al.. Extracellular S100A4(mts1) stimulates invasive growth of mouse endothelial cells and modulates MMP-13 matrix metalloproteinase activity. Oncogene. 2004;23:5487–95. doi:10.1038/sj.onc.1207720. PMID:15122322.
- Grum-Schwensen B, Klingelhofer J, Grigorian M, Almholt K, Nielsen BS, Lukanidin E, et al.. Lung metastasis fails in MMTV-PyMT oncomice lacking S100A4 due to a T-cell deficiency in primary tumors. Cancer Res. 2010;70:936–47. doi:10.1158/0008-5472.CAN-09-3220. PMID:20103644.
- Hansen MT, Forst B, Cremers N, Quagliata L, Ambartsumian N, Grum-Schwensen B, et al.. A link between inflammation and metastasis: serum amyloid A1 and A3 induce metastasis, and are targets of metastasis-inducing S100A4. Oncogene. 2015;34:424–35. doi:10.1038/onc.2013.568. PMID:24469032.
- Forst B, Hansen MT, Klingelhofer J, Moller HD, Nielsen GH, Grum-Schwensen B, et al.. Metastasis-inducing S100A4 and RANTES cooperate in promoting tumor progression in mice. PloS one. 2010;5:e10374. doi:10.1371/journal.pone.0010374. PMID:20442771.
- O'Connell JT, Sugimoto H, Cooke VG, MacDonald BA, Mehta AI, LeBleu VS, et al.. VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc Natl Acad Sci U S A. 2011;108:16002–7. doi:10.1073/pnas.1109493108. PMID:21911392.
- Cho YG, Kim CJ, Nam SW, Yoon SH, Lee SH, Yoo NJ, et al.. Overexpression of S100A4 is closely associated with progression of colorectal cancer. World J Gastroenterol. 2005;11:4852–6. PMID:16097057.
- Liu Y, Tang W, Wang J, Xie L, Li T, He Y, et al.. Clinicopathological and prognostic significance of S100A4 overexpression in colorectal cancer: a meta-analysis. Diagn Pathol. 2013;8:181. doi:10.1186/1746-1596-8-181. PMID:24188373.
- Zhang J, Jiao Y, Hou S, Tian T, Yuan Q, Hao H, et al.. S100A4 contributes to colitis development by increasing the adherence of citrobacter rodentium in intestinal epithelial cells. Sci Rep. 2017;7:12099. doi:10.1038/s41598-017-12256-z. PMID:28935867.
- Clapper ML, Cooper HS, Chang WC. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta Pharmacol Sin. 2007;28:1450–9. doi:10.1111/j.1745-7254.2007.00695.x. PMID:17723178.
- Flatmark K, Pedersen KB, Nesland JM, Rasmussen H, Aamodt G, Mikalsen SO, et al.. Nuclear localization of the metastasis-related protein S100A4 correlates with tumour stage in colorectal cancer. J. Pathol. 2003;200:589–95. doi:10.1002/path.1381. PMID:12898594.
- Xue C, Plieth D, Venkov C, Xu C, Neilson EG. The gatekeeper effect of epithelial-mesenchymal transition regulates the frequency of breast cancer metastasis. Cancer Res. 2003;63:3386–94. PMID:12810675.
- Iwano M, Fischer A, Okada H, Plieth D, Xue C, Danoff TM, et al.. Conditional abatement of tissue fibrosis using nucleoside analogs to selectively corrupt DNA replication in transgenic fibroblasts. Mol Ther. 2001;3:149–59. doi:10.1006/mthe.2000.0251. PMID:11237671.
- Yammani RR, Carlson CS, Bresnick AR, Loeser RF. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100A4: role of the receptor for advanced glycation end products. Arthritis Rheum. 2006;54:2901–11. doi:10.1002/art.22042. PMID:16948116.
- Dahlmann M, Okhrimenko A, Marcinkowski P, Osterland M, Herrmann P, Smith J, et al.. RAGE mediates S100A4-induced cell motility via MAPK/ERK and hypoxia signaling and is a prognostic biomarker for human colorectal cancer metastasis. Oncotarget. 2014;5:3220–33. doi:10.18632/oncotarget.1908. PMID:24952599.
- Viennois E, Chen F, Merlin D. NF-kappaB pathway in colitis-associated cancers. Transl Gastrointest Cancer. 2013;2:21–9. doi:10.3978/j.issn.2224-4778.2012.11.01. PMID:23626930.
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al.. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi:10.1016/j.cell.2004.07.013. PMID:15294155.
- Li ZH, Dulyaninova NG, House RP, Almo SC, Bresnick AR. S100A4 regulates macrophage chemotaxis. Mol Biol Cell. 2010;21:2598–610. doi:10.1091/mbc.E09-07-0609. PMID:20519440.
- Cabezon T, Celis JE, Skibshoj I, Klingelhofer J, Grigorian M, Gromov P, et al.. Expression of S100A4 by a variety of cell types present in the tumor microenvironment of human breast cancer. Int. J. Cancer. 2007;121:1433–44. doi:10.1002/ijc.22850. PMID:17565747.
- Kong P, Christia P, Saxena A, Su Y, Frangogiannis NG. Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. Am J Physiol Heart Circ Physiol. 2013;305:H1363–72. doi:10.1152/ajpheart.00395.2013. PMID:23997102.
- Orre LM, Panizza E, Kaminskyy VO, Vernet E, Graslund T, Zhivotovsky B, et al.. S100A4 interacts with p53 in the nucleus and promotes p53 degradation. Oncogene. 2013;32:5531–40. doi:10.1038/onc.2013.213. PMID:23752197.
- Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–91. doi:10.1016/j.immuni.2010.03.003. PMID:20303296.
- McEver RP, Baenziger NL, Majerus PW. Isolation and quantitation of the platelet membrane glycoprotein deficient in thrombasthenia using a monoclonal hybridoma antibody. J Clin Invest. 1980;66:1311–8. doi:10.1172/JCI109983. PMID:6449521.
- Moolenbeek C, Ruitenberg EJ. The “Swiss roll”: a simple technique for histological studies of the rodent intestine. Lab Anim. 1981;15:57–9. PMID:7022018.
