ABSTRACT
Natural killer (NK) cell-mediated antibody-dependent toxicity is a potent mechanism of action of the anti-GD2 murine monoclonal antibody 3F8 (m3F8). Killer immunoglobulin-like receptor (KIR) and HLA genotypes modulate NK activity and are key prognostic markers in m3F8-treated patients with neuroblastoma. Endogenous NK-cells are suppressed in the setting of high tumor burden and chemotherapy. Allogeneic NK-cells however, demonstrate potent anti-neuroblastoma activity. We report on the results of a phase I clinical trial of haploidentical NK-cells plus m3F8 administered to patients with high-risk neuroblastoma after conditioning chemotherapy. The primary objective was to determine the maximum tolerated NK-cell dose (MTD). Secondary objectives included assessing anti-neuroblastoma activity and its relationship to donor-recipient KIR/HLA genotypes, NK function, and donor NK chimerism. Patients received a lymphodepleting regimen prior to infusion of haploidentical CD3-CD56+ NK-cells, followed by m3F8. Overall and progression free survival (PFS) were assessed from the time of first NK-cell dose. Univariate Cox regression assessed relationship between dose and outcomes. Thirty-five patients received NK-cells at one of five dose levels ranging from <1×106 to 50×106 CD3-CD56+cells/kg. One patient experienced grade 3 hypertension and grade 4 pneumonitis. MTD was not reached. Ten patients (29%) had complete or partial response; 17 (47%) had no response; and eight (23%) had progressive disease. No relationship was found between response and KIR/HLA genotype or between response and FcγRIII receptor polymorphisms. Patients receiving >10×106 CD56+cells/kg had improved PFS (HR: 0.36, 95%CI: 0.15–0.87, p = 0.022). Patient NK-cells displayed high NKG2A expression, leading to inhibition by HLA-E-expressing neuroblastoma cells. Adoptive NK-cell therapy in combination with m3F8 is safe and has anti-neuroblastoma activity at higher cell doses.
Introduction
Neuroblastoma (NB) comprises >8% of malignancies in children, but disproportionately accounts for 15% of all pediatric oncology deaths.Citation1 Despite aggressive multimodality therapy, long-term progression-free survival (PFS) for high-risk (HR) NB is <50%.Citation2 The prognosis for relapsed NB is much worse: a recent review reported 5-year post-relapse overall survival (OS) for stage 4 NB of 8%.Citation3 Thus, newer therapies are urgently needed for metastatic and relapsed disease. NB is the first pediatric solid tumor for which monoclonal antibody (MoAb)-based immunotherapy has been proven to be effective.Citation4 The murine anti-GD2 MoAb 3F8 (m3F8) induces complete remission (CR) in chemo-resistant osteomedullary NB,Citation5 and improves survival in stage 4 NB patients in firstCitation6 and subsequent remissions.Citation7 However, m3F8 is relatively ineffective against high disease burden: both osteomedullary and soft tissue.Citation8 Furthermore, dose-intensive chemotherapy for HR-NB depletes endogenous leucocyte stores and compromises antibody-dependent cell-mediated cytotoxicity (ADCC), a major anti-NB mechanism of m3F8.
Natural killer cells (NK-cells) can target NB via multiple pathways. They express the low-affinity FcγRIII receptor required for binding m3F8 and triggering NK-mediated ADCC; multiple other receptors bind to activating ligands expressed on NB.Citation9-Citation11 NK-cells are tolerized to cells expressing self-MHC class I molecules and are cytotoxic to cells lacking self-MHC class I molecules. Recognition of MHC class I ligands on target cells occurs through the NK-cell surface inhibitory killer immunoglobulin-like (KIR) and NKG2A receptors. In an incompletely understood process, NK-cells become educated or “licensed” for effector function (cytokine production, cytotoxicity, ADCC) based on their expression of inhibitory KIR receptors for self-MHC class I molecules.Citation12,Citation13 Expression of self-specific KIR “licenses” or endows the NK-cell with effector function, leading to killing of tumor cells lacking self-MHC class I ligands (“missing self”). The same KIR receptors signal inhibition by cells expressing cognate self-HLA class I ligands, thereby achieving tolerance to self.Citation14,Citation15 Resting NB tumors are generally deficient in MHC class I expression,Citation16 ideal for NK recognition and killing. Under mild inflammatory conditions, however, MHC class I expression can be induced on NB cell surface, thus inhibiting licensed NK-cells expressing KIR for self-MHC.
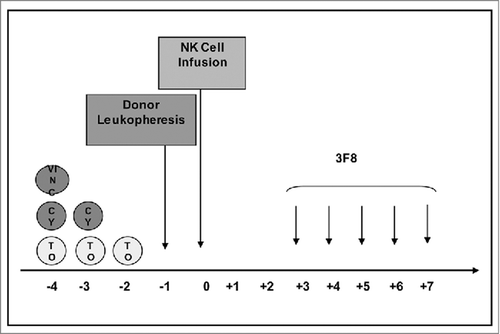
NK response, both effector and inhibitory, therefore can be predicted based on genotyping of KIR and MHC class I. We previously reported that m3F8-treated NB patients who lacked HLA ligands for their inhibitory KIR (“missing KIR ligand”) had significantly improved PFS and OS compared to those in whom all KIR ligands were present.Citation17 A similar observation was also made with the anti-GD2 immunocytokine hu14.18-IL2Citation18 though not with the anti-GD2 antibody dinutuximabCitation19 suggesting that NK cells might vary in their utilization of monoclonal antibodies for ADCC. Our preclinical findings with m3F8 indicated that endogenous unlicensed NK-cells, capable of recognizing targets with “missing ligand,” are the primary mediators of NK-mediated ADCC in patients. While endogenous NK-cells are important in controlling NB progression,Citation16 chemotherapy-treated patients and those with high tumor burden often have poorly functioning NK-cells, hampering robust ADCC.Citation20 We hypothesized that adoptively transferred allogeneic NK-cells would have potent ADCC and anti-NB cytotoxicity, especially if donor-recipient KIR/HLA genotype combinations predictive of donor NK activation due to “missing self” could be selected. This was tested in a phase I clinical trial in which adoptively transferred haploidentical NK-cells were combined with m3F8 in patients with chemoresistant or relapsed HR-NB (ClinicalTrials.gov NCT00877110). The primary objective was to determine the toxicity and maximum tolerated dose (MTD) of haploidentical NK-cells when combined with m3F8. Haploidentical NK-cells have been shown to be safe in adultsCitation21,Citation22 and are readily available from parents of young children with NB. Pilot studies have tested haploidentical NK-cells in children after autologous stem cell transplantCitation23 and with chemotherapy plus MoAb.Citation24 To create a lymphodepleted host environment for enhanced donor NK-cell survival, we treated patients with a high-dose cyclophosphamide-based chemotherapy regimenCitation25 with known anti-NB activity prior to NK-cell infusion and m3F8 therapy (). We now report the results of this first phase I trial of the combination of haploidentical NK-cells and anti-tumor MoAb in children.
Results
Patient and donor characteristics
Thirty-five patients (11 female; 24 male) with a median age of 5.6 (range 2–14.7) years received a total of 43 treatments. Six patients were re-treated: five and one with 1 and 2 additional treatments respectively. Median time from diagnosis to therapy was 15 (range 6–69) months. All patients were heavily prior treated with high-dose chemotherapy. Patients were classified into 3 groups bases on their pre-therapy disease status (a) Primary refractory (n = 13): patients who had incomplete response to induction therapy but had never had disease progression. (b) Secondary refractory (n = 13): patients who had relapse or disease progression, then received interim therapy with stable disease prior to enrolling on protocol. (c) Progressive disease (PD) (n = 9): patients received protocol therapy at time of relapse or disease progression. Mothers were NK-cell donors for 21 patients and fathers for 14. Leukapheresis was performed using peripheral venous access in all donors except one, who required insertion of a femoral central line. Clinical details and HLA, KIR and FCGR3A genotyping are presented in .
Table 1. Clinical features and results of genotyping on patients and donors.
NK-cells
Since a variable number of NK-cells were isolated, allowance was made for infusion of any number of NK-cells isolated, as long as the dose conformed to the desired or lower cell dose. This led to the final number of patients treated at each dose level to differ from the characteristic phase I 3+3 dose-escalation schema. Planned and actual dose levels and NK-cell numbers are shown in . An adequate number of NK-cells were isolated in 100% (6/6) patients at dose level 1. At dose levels 2, 3 and 4, planned numbers of cells were isolated for 75% (6/8), 62% (8/13) and 11% (1/9) patients respectively. Three infusions in two patients were considered to be unsuccessful (i.e. <1×106cells/kg were isolated, comprising dose level 0). Release criteria were met for all cell products except one, in which NK-cell viability was 61% (<70%). Mean NK-cell purity was 96.3 ± 5.1%; residual CD3+ cells 0.2 ± 0.3%; and viability 92.5 ± 7%.
Table 2. Planned and actual dosage of haploidentical NK cells administered.
Toxicities
Almost all toxicities were expected and related to m3F8, prior therapy, disease activity, or conditioning chemotherapy. These included grade 4 myelosuppression and lymphopenia, grade 3 febrile neutropenia, grade 2 pain and urticaria and grade 2 bronchospasm (entire list shown in ). Two patients, however, experienced unusual toxicities. One patient treated at dose level I developed dose-limiting toxicity (DLT): grade 3 hypertension, leading to an additional 3 patients being treated at that dose level. The same patient also had grade 4 pneumonitis in the setting of febrile neutropenia after receiving chemotherapy, NK-cells and 4 doses of m3F8. A second patient at dose level I, although asymptomatic, developed grade 2 left ventricular dysfunction (on echocardiogram). Both patients recovered completely from the toxicities. No patient experienced graft-versus-host disease (GvHD). Thirty-two (90%) patients experienced febrile neutropenia, which was uncomplicated except for the first patient described above. No other DLTs were encountered and maximal tolerated dose (MTD) for NK-cells was not reached. Nevertheless, because of the low success rate in isolating >30×106 cells/kg, further accrual was discontinued. Human anti-mouse antibody (HAMA) was noted in 3/33 (9%) of patients tested, all previously treated with anti-GD2 MoAbs. Donors did not experience adverse events.
Table 3. Numbers of patients experiencing toxicities.
Responses
Overall response rate (complete remission [CR] +partial remission [PR]) was 29%: 5 CR, 5 PR, 17 no response (NR), and 8 PD. Responses were observed in osteomedullary as well as soft tissue disease, although all responses in the latter were modest (<PR) (). Site specific responses were as follows: improvement in MIBG scores in 20/35 (57%), complete responses on bone marrow (BM) testing in 9/16 (56%) and objective responses in soft tissue NB (< PR) in 4/14 (29%). Responses were noted at all NK-cell dose levels, and no relationship was found between responses (assessed both by modified International Neuroblastoma Response Criteria [INRC] and by site-specific response) and dose level, MYCN-status, HLA/KIR interaction (“missing KIR ligand” or “missing self”), or FCGR3A polymorphism in host or donor (p > 0.2 for each) (). No correlation was found between NK dose considered as a continuous variable, and percentage change in MIBG score (rho = −0.11, 95%CI:−0.43–0.23, p = 0.51). However, all 4 patients with major reductions in MIBG scores (reduction of >10) (; response shown in a representative patient) received NK-cells at levels 2–4. Of the 6 patients who received >1 NK infusions, incremental reductions in MIBG scores were noted in 3. Patients with PD at enrollment had the worst outcomes: 0/9 CR/PR versus 10/24 for all others (p = 0.05) and lowest reduction in MIBG score (p = 0.01).
Table 4. Responses.
Table 5. Relationships between response and dose and other factors.
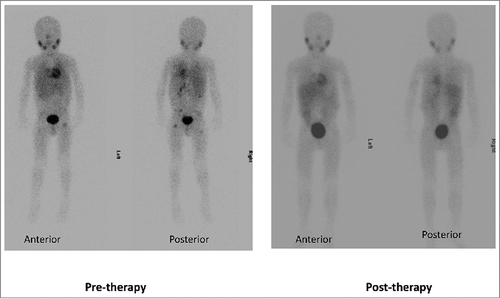
Figure 2. Complete response to protocol therapy in a 6-year old male with chemorefractory high-risk stage 4 neuroblastoma after 2 cycles of therapy at NK-cell dose level 3. Pre-therapy 123I-MIBG scan showed uptake in multiple skeletal areas including pelvis, bilateral femora and spine. Post-therapy 123I-MIBG scan showed complete resolution of skeletal uptake. Complete response was also noted on bone marrow histology.
Survival
Because most patients went on to receive other anti-NB therapies, survival rates could not solely be attributed to protocol therapy. Median PFS and overall survival (OS) for the entire group were 7.4 (95%CI: 4.6–16.3) and 30.7 (95%CI: 17.1–49.5) months respectively. In univariate analysis, patients treated at dose levels 2–4 had superior PFS compared to those treated at dose levels 0–1 (p = 0.018). Patients with PD immediately prior to treatment had significantly inferior PFS and OS. No other factors impacted survival. In a bivariate mode incorporating dose level and pre-treatment status, PFS remained inferior in PD patients (HR: 3.30, 95%CI: 1.39–7.84, p = 0.007) and marginally superior for dose-levels >1 (HR: 0.42, 95%CI: 0.17–1.02, p = 0.054).
NK-cell chimerism, phenotype and function
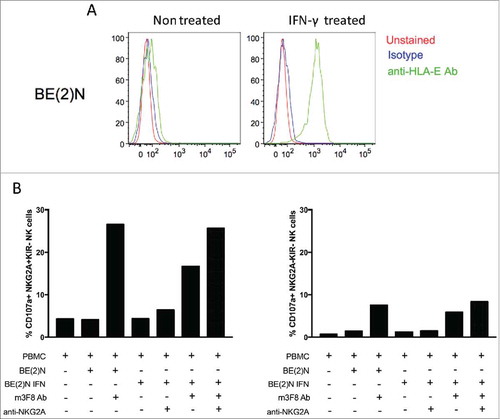
Circulating donor NK-cells could be detected in 2/15 (13%) and 0/11 patients tested 7 and 14 days post-infusion respectively. The two patients with circulating NK cells at day 7 were treated at dose level 1 and 2 respectively. Intriguingly, both patients with detectable NK-cells had durable CR. Donor NK phenotype and functional studies were performed for the first 13 products. These demonstrated normal response capacity, and confirmed that NK populations responded to m3F8-mediated ADCC of NB target cells according to NK education predicted by HLA/KIR genotyping ().Citation14,Citation15 Although previous reports have indicated that a post-treatment inflammatory environment can augment NK function,Citation26,Citation27 this was not evident in the 19 patients tested (). Nearly all patients had a sustained high frequency of cells expressing the inhibitory receptor NKG2A (median±SD 88.8±22.4%), consistent with a less mature NK repertoire.Citation28 In comparison, healthy adult individuals exhibited fewer NKG2A-expressing NK cells (54.6±15.8%; p<0.001) (). Given that NB cell lines express HLA-E,Citation29 the inhibitory ligand for NKG2A, the high frequency of NKG2A+ cells in NB patients could undermine an optimal anti-tumor NK response. Indeed, IFN-γ-induced upregulation of HLA on the neuroblastoma target cell line BE(2)N resulted in inhibition of NKG2A-expressing NK-cells, even in the setting of m3F8 activation. Specific to NKG2A-expressing NK-cells, this inhibition could be reversed by the anti-NKG2A blocking antibody ().
Table 6. Correlation of NK cytotoxicity and donor-recipient HLA/KIR phenotyping.
Figure 3. Donor and patient NK function. NK-cells were isolated from donors, the patient pre-treatment, and the patient post-treatment at indicated time points. NK response capacity was assessed by CD107a mobilization to (A) the NK-sensitive K562 cell line, or to (B) the neuroblastoma cell line LAN-1 in the presence of the m3F8 monoclonal antibody. 13 donors and 19 patients were assessed. Each bar represents the mean +/− SEM.
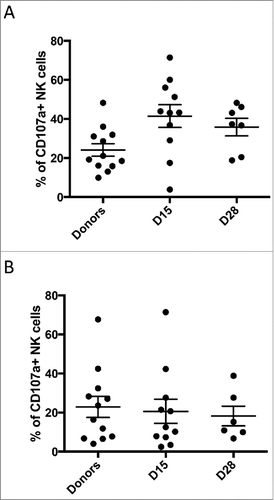
Figure 4. NKG2A expression among donors and patients. A high percentage of NKG2A expression was noted in the patient population pre- and post-infusion (A) compared to a healthy adult population (B).
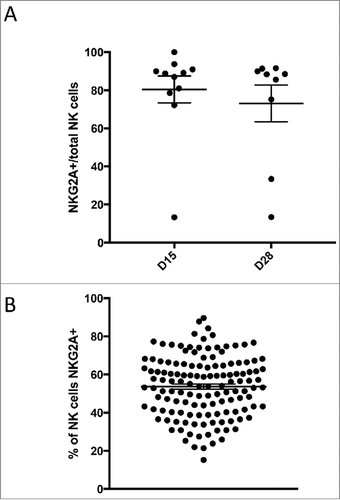
Figure 5. HLA-E expression on NB selectively inhibits NKG2A-expressing NK cells. (A) HLA-E is readily upregulated on the cell surface of the NB cell line BE(2)N upon IFN-γ treatment. (B) m3F8-induced CD107a response among NKG2A+KIR- NK cells (left panel) or NKG2A-KIR- NK cells (right panel) to the IFN-γ treated or untreated BE(2)N target cell in the presence or absence of the anti-NKG2A blocking antibody. Similar results were noted with SKNLH cell line (data not shown)
Discussion
Our results indicate that haploidentical NK-cell therapy in combination with CTV-chemotherapy and m3F8 is safe in children. The safety of adoptive NK-cell therapy has been previously reported in children with leukemia.Citation22 Two prior studies report on haploidentical NK cell infusions administered with chemotherapy and anti-GD2 antibodies in 15 patients with neuroblastoma, though neither investigated the combination in a phase I design. In one study, haploidentical NK cells were infused 2–5 days after autologous stem cell transplant along with the anti-GD2 antibody hu14.18K322A.Citation23 Encountered toxicities were primarily related to the myeloablative pre-transplant regimen and responses were not evaluable. In another pilot study, 13 patients with resistant neuroblastoma received varying doses of haploidentical NK cells plus hu14.18K322A given after various combinations of chemotherapy. The contribution of NK cells in observed toxicities and responses could not be ascertained.Citation24 In both studies, infused NK cell numbers ranged from 4–60 × 106 NK cells/kg.
We could harvest up to 30 × 106/kg pure NK-cell populations from a majority of donors. Lower dose levels were achievable in 100% of donors, but isolation of NK-cell numbers >5×106/kg was feasible only in 67%. The primary reason for the inability to isolate higher NK-cell numbers was loss of cells after overnight incubation in the presence of IL-2, possibly due to apoptosis from cytokine over-activation. This loss was especially marked when incubation media contained 1000 IU/ml of IL2 (first 10 products) and reduced when the IL2 concentration was decreased to 500 IU/ml. Future adoptive NK-cell therapy protocols may benefit from removal of ex vivo IL-2 stimulation altogether. Alternatively cytokines, such as IL-15, IL-12, and IL-18 may be more beneficial for NK survival and activation.Citation30
NK-cell infusion was not toxic; in particular, no patient developed GvHD or delayed blood count recovery. As expected with CTV-chemotherapy, almost all patients developed febrile neutropenia, requiring treatment with IV antibiotics. We had chosen this high-dose regimen not only for its anti-NB effect and to prevent the possibility of GvHD in our significantly immunosuppressed patients, but with the expectation that via its lymphodepleting effects, we would improve donor NK-cell survival. Despite severe lymphodepletion, we did not observe significant NK-cell persistence, possibly due to induced apoptosis from ex vivo IL-2 stimulation. To preserve lymphodepleting effects while minimizing myelosuppression, future adoptive NK-cell therapies could use single-agent cyclophosphamide conditioning.
This phase I study was not designed to evaluate the anti-tumor effect of NK-cells, since the regimen also included chemotherapy and anti-GD2 MoAb; and responses in patients with resistant NB have been reported with a chemoimmunotherapy regimen consisting of irinotecan, temozolomide, dinutuximab and GM-CSF.Citation31 However, in our study, a dose-response effect for NK-cells appeared to be present with robust MIBG responses and improved PFS in patients receiving ≥10×106 NK-cells/kg and durable CRs in patients with NK-cell persistence implying that adoptively transferred NK-cells contributed to the anti-NB effect of the therapeutic combination. Although direct correlations are difficult to make, the 29% CR+PR rate observed in our study was higher than the 15% response rate we had previously observed for the CTV (in which the dose of topotecan was 8 mg/m2/cycle compared to 7.2 mg/m2/cycle in our study) combination administered without any concurrent m3F8 or NK-cells in a similar patient population. These observations lend credence to our hypothesis that allogeneic NK-cells can mediate ADCC and tumor control and that higher NK-cell numbers might be more beneficial. We safely re-treated patients with additional cycles of protocol therapy and observed incremental responses in three of six patients. Alternative approaches to increase NK-cell numbers include ex vivo expansion with IL-21,Citation32 or in vivo expansion with exogenous IL-15Citation33 or IL-15 agonist complex.Citation34 The timing of therapeutic agents was primarily dictated by logistical issues (most patients received all therapy including NK-cell infusions and m3F8 on an out-patient basis). However, given our observation that circulating NK-cells could not be detected >14 days after infusion, concurrent administration of NK-cells and anti-GD2 therapy could possibly be more effective. We did not discern a clear benefit of KIR/HLA mismatch, either via the mechanisms of “missing self-HLA” or “missing KIR ligand”, although the comparison groups in this phase I study were too small and heterogeneous to yield adequate power for such an analysis. Therefore, the utility in selecting a donor based on KIR/HLA genetics for this treatment approach remains unknown.
Donor NK phenotyping and functional studies revealed NK responsiveness predicted by KIR and HLA genetics and consistent with previous observations in normal individualsCitation14,Citation15 Our patients had a high frequency of NKG2A+ NK-cells which may reflect a repertoire that is immunologically naïve in this pediatric populationCitation35 or a derangement in NK recovery following chemotherapy.Citation27,Citation36 The high percentage of NKG2A+ NK-cells could dampen NK-ADCC, but this inhibition could be relieved by anti-NKG2A blocking antibody which might have a potential clinical role to enhance NK-cell responses in patients.Citation37 For this trial, donors were not selected on the basis of presence of an adaptive NKG2C+ FcϵR- population, an NK population found in CMV-seropositive individuals and potent mediators of ADCC.Citation38,Citation39 Future trials using this selection criterion may result in higher response to m3F8, but will continue to face challenges achieving higher cell doses and longer in vivo survival.
Results from this phase I trial haploidentical NK-cells in combination with MoAb indicate that adoptively transferred NK-cells, when activated by ADCC-eliciting MoAbs, can exhibit anti-tumor activity. Based on the lessons learned in this study with regard to NK-cell isolation and regimen toxicity, our current follow-up study (NCT02650648) combines cyclophosphamide conditioning, haploidentical NK-cells, a MoAb with superior ADCC properties (hu3F8Citation40) and in vivo administration of IL-2.
Methods
Patient selection
Patients with HR-NB (stage 4 disease diagnosed at >18 months of age or MYCN-amplified ≥ stage 3 tumor at any age) and a history of chemoresistance to high-dose induction chemotherapy), or NB patients relapsing with metastatic disease were eligible. The presence of evaluable (microscopic BM metastases, abnormal scintigraphic studies) or measurable (CT or MRI) NB ≥ 1 month after completion of systemic therapy was required for protocol eligibility. Patients with BM positivity for NB as their only evaluable disease were excluded. Prior m3F8 therapy was permitted. Patients with life-threatening infections or >grade 2 toxicity according to the National Cancer Institute's Common Toxicity Criteria version 3.0 (CTC v3.0) were excluded.
Donor selection
Prior to initiating therapy, prospective donors and patients underwent HLA and KIR genotyping by high-resolution HLA-A, B and C genotyping (Histogenetics) and PCR-SSPCitation41 respectively. The following KIRs were genotyped: KIR2DL1, KIR2DS1, KIR2DL2/S2, KIR2DL3, KIR3DL1, KIR3DS1. Related donors confirmed to be HLA-haploidentical to the patient and with negative serological testing for HIV, HTLV I and II and West Nile virus, underwent leukapheresis for a total of 10–15 liters. Donors whose class I KIR ligands were not present in the patient and who possessed the corresponding cognate inhibitory KIR receptors were prioritized. For those donor-recipient pairs where no KIR ligand incompatibility was found, donors with activating KIR were prioritized over donors who lacked all activating KIR. In this latter group, we first prioritized KIR2DS1, followed by centromeric activating KIR.Citation42
NK-cell isolation
NK-cells were isolated from a donor leukapheresis product in a two-step process using the CliniMACS System (Miltenyi Biotec). Briefly, the cell product was first depleted of T-lymphocytes by anti-CD3 antibody-coated paramagnetic particles. The CD3− effluent fraction was then enriched for NK cells with the CliniMACS CD56 reagent. Cells were incubated overnight with 500–1000 U/mL IL-2 (Chiron) at a concentration of 2×106 cells/ml, then washed before infusion into the patient the following day. Release criteria for the final product prior to patient infusion included: <2×105/kg CD3+ cells, ≥90% CD3−CD56+ purity by flow cytometry, and ≥70% viability.
Study design
The protocol was approved by the institutional review board (IRB) of Memorial Sloan Kettering Cancer Center (MSKCC). Written informed consent was obtained from patients or their guardians. Patients received cyclophosphamide 70 mg/kg intravenously (IV) on days 1 and 2, topotecan 2.4 mg/m2/day IV on days 1–3 and vincristine 0.067 mg/kg IV on day 1. Donor leukapheresis followed by NK-cell isolation was performed on day 4, and NK-cells were infused on day 5. m3F8 (20 mg/m2/day) was infused IV over 0.5–1.5 hours on days 8–12. () The study was planned with a standard 3+3 design, patients received NK cells at one of 4 escalating dosage levels: 1–4.9×106cells/kg, 5–9.9×106cells/kg, 10–30×106cells/kg and 30.1–50×106cells/kg. If DLT was encountered in 1/3 patients at a dose level, 3 additional patients were planned to be treated at that level. If the targeted NK-cell dose was not achieved but otherwise met release criteria, all isolated NK-cells were infused, and the patient counted at the actual dose level. If < 1×106cells/kg were isolated, all cells were infused (termed dose level 0). As a result, >3–6 patients were treated at each dose level. After enrollment was completed on dose level I, the protocol was amended to allow up to 2 additional NK-cell infusions if patients met all the following criteria: (a) no DLT, GvHD or HAMA response,Citation43 (b) no PD, (c) had an objective response and (d) continued to meet all eligibility criteria. In an effort to reduce severe myelosuppression, chemotherapy for subsequent treatments was restricted to cyclophosphamide 50 mg/kg/day IV on days 1 and 2. NK-cell and m3F8 doses remained unchanged for subsequent cycles.
Toxicity monitoring
Toxicities and acute GvHD were assessed by CTCv3.0 and previously described criteria.Citation44 Toxicities clearly related to chemotherapy, >grade 2 toxicities clearly related to co-interventions, prior therapy or disease activity, and grade 3 fever, rash or hypotension related to m3F8 were not DLT. All other ≥grade 3 toxicities were considered DLT. Patients were monitored for toxicity at least once weekly with physical examination, complete blood counts, liver function tests, BUN and serum creatinine.
Response assessment
Disease status was assessed after each treatment and then at least every three months with CT or MRI, MIBG scan, and BM aspirates and biopsies. Disease status was defined International Neuroblastoma Response CriteriaCitation45: CR: no evidence of disease; very good partial remission: >90% decrease in volume of primary tumor and no other evidence of active disease; PR: 50–90% decrease in volume of measurable disease, ≥50% reduction in modified Curie score on MIBG scanCitation46 but <CR; no new lesions, ≤1 positive BM site allowed, no response (NR): <25% increase in volume of any existing soft-tissue lesion, <50% reduction in MIBG score; PD: any new lesion, increase of any measurable lesion by >25%. In addition, for comparison between groups, objective response was defined as any improvement in modified Curie score. For purposes of statistical analyses, responses were grouped into CR/PR versus SD/PD.
Correlative studies
In addition to HLA and KIR genotyping, donor and patient NK phenotype and function, FCGR3A polymorphisms, chimerism, and HAMA were assessed. NK-cell chimerism was evaluated by quantitative PCR for DNA polymorphisms. NK phenotype was evaluated by multi-parameter flow cytometry for cell-surface expression of CD94/NKG2A and inhibitory and activating KIR, as previously described.Citation17 Functional response of NK populations was measured flow cytometrically by CD107a mobilization to the NK-sensitive line K562 and to the NB cell lines LAN-1, BE(1)N and SKNLH in the presence of m3F8.Citation17 In some studies, activation of NK cells by target cells was performed in the presence of the anti-NKG2A blocking antibody (clone Z199, Beckman Coulter). HAMA was detected using ELISACitation47 with a titer of >1000 U/ml being considered positive. FCGR3A polymorphisms were evaluated as previously described.Citation48 Allelic discrimination of FCGR3A was identified as [F/F], [V/V] or [F/V].
Statistical methods
Relationships between response and dose level (dose levels were grouped as levels 0–1 and 2–4) and other factors were assessed with Fisher's Exact Test and the Wilcoxon Rank Sum test where appropriate. The relationship between continuous dose and MIBG score percent change was assessed with Spearman's Rank Correlation. Wilcoxon Signed Rank Test was used to assess if the change in MIBG score from pre to post-treatment was significant. Kaplan-Meier estimates and plots were generated for OS and PFS in the full sample, and also stratified by dose level. The log-rank test was used to assess the relationship between dose with OS and PFS. Univariate Cox proportional hazards regression was used to assess the relationship between potential predictors and OS/PFS. Factors significant at p = 0.05 were considered for multivariate analysis with dose. Due to the hypothesis generating nature of the study, no adjustments were made for multiple hypothesis testing. All analyses were performed using SAS 9.4 (The SAS Institute, Cary, NC). OS and PFS was calculated from the time of first dose until death or PD respectively.
Disclosure of potential conflicts of interest
The authors declare the following conflicts of interest: anti-GD2 antibodies have been licensed by Memorial Sloan Kettering Cancer Center (MSK) to Ymabs Therapeutics Inc., both MSK and NK Cheung have financial interest in this company; Shakeel Modak and Kim Kramer are consultants to Ymabs Therapeutics Inc.
Acknowledgments
We thank Alison Slocum, Chandresh Undhad, and other members of the Center for Immune Cell Therapies at MSK for their work on NK cell isolation. We thank Joe Olechnowicz for editorial assistance.
Additional information
Funding
References
- Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–20. doi:10.1016/S0140-6736(07)60983-0. PMID:17586306.
- Kreissman SG, Seeger RC, Matthay KK, London WB, Sposto R, Grupp SA, Haas-Kogan DA, Laquaglia MP, Yu AL, Diller L, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial. Lancet Oncol. 2013;14:999–1008. doi:10.1016/S1470-2045(13)70309-7. PMID:23890779.
- Moreno L, Rubie H, Varo A, Le Deley MC, Amoroso L, Chevance A, Garaventa A, Gambart M, Bautista F, Valteau-Couanet D, et al. Outcome of children with relapsed or refractory neuroblastoma: A meta-analysis of ITCC/SIOPEN European phase II clinical trials. Pediatr Blood Cancer. 2017;64:25–31. doi:10.1002/pbc.26192. PMID:27555472.
- Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–34. doi:10.1056/NEJMoa0911123. PMID:20879881.
- Cheung NK, Cheung IY, Kramer K, Modak S, Kuk D, Pandit-Taskar N, Chamberlain E, Ostrovnaya I, Kushner BH. Key role for myeloid cells: phase II results of anti-G(D2) antibody 3F8 plus granulocyte-macrophage colony-stimulating factor for chemoresistant osteomedullary neuroblastoma. Int J Cancer. 2014;135:2199–205. doi:10.1002/ijc.28851. PMID:24644014.
- Cheung NK, Cheung IY, Kushner BH, Ostrovnaya I, Chamberlain E, Kramer K, Modak S. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol. 2012;30:3264–70. doi:10.1200/JCO.2011.41.3807. PMID:22869886.
- Kushner BH, Ostrovnaya I, Cheung IY, Kuk D, Kramer K, Modak S, Yataghene K, Cheung NK. Prolonged progression-free survival after consolidating second or later remissions of neuroblastoma with Anti-GD2 immunotherapy and isotretinoin: a prospective Phase II study. Oncoimmunology. 2015;4:e1016704. doi:10.1080/2162402X.2015.1016704. PMID:26140243.
- Suzuki M, Cheung NK. Disialoganglioside GD2 as a therapeutic target for human diseases. Expert opinion on therapeutic targets. 2015;19:349–62. doi:10.1517/14728222.2014.986459. PMID:25604432.
- Andre P, Castriconi R, Espeli M, Anfossi N, Juarez T, Hue S, Conway H, Romagné F, Dondero A, Nanni M, et al. Comparative analysis of human NK cell activation induced by NKG2D and natural cytotoxicity receptors. Eur J Immunol. 2004;34:961–71. doi:10.1002/eji.200324705. PMID:15048706.
- Ogbomo H, Hahn A, Geiler J, Michaelis M, Doerr HW, Cinatl J, Jr. NK sensitivity of neuroblastoma cells determined by a highly sensitive coupled luminescent method. Biochem Biophys Res Commun. 2006;339:375–9. doi:10.1016/j.bbrc.2005.11.025. PMID:16297863.
- Sivori S, Parolini S, Marcenaro E, Castriconi R, Pende D, Millo R, Moretta A. Involvement of natural cytotoxicity receptors in human natural killer cell-mediated lysis of neuroblastoma and glioblastoma cell lines. J Neuroimmuno. 2000;107:220–5. PMID:10854660.
- Yokoyama WM, Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev. 2006;214:143–54. doi:10.1111/j.1600-065X.2006.00458.x. PMID:17100882.
- Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–13. doi:10.1038/nature03847. PMID:16079848.
- Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–42. PMID:16901727.
- Yu J, Heller G, Chewning J, Kim S, Yokoyama WM, Hsu KC. Hierarchy of the human natural killer cell response is determined by class and quantity of inhibitory receptors for self-HLA-B and HLA-C ligands. J Immunol. 2007;179:5977–89. PMID:17947671.
- Venstrom JM, Zheng J, Noor N, Danis KE, Yeh AW, Cheung IY, Dupont B, O'Reilly RJ, Cheung NK, Hsu KC. KIR and HLA genotypes are associated with disease progression and survival following autologous hematopoietic stem cell transplantation for high-risk neuroblastoma. Clin Cancer Res. 2009;15:7330–4. doi:10.1158/1078-0432.CCR-09-1720. PMID:19934297.
- Tarek N, Le Luduec JB, Gallagher MM, Zheng J, Venstrom JM, Chamberlain E, Modak S, Heller G, Dupont B, Cheung NK, et al. Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment. J Clin invest. 2012;122:3260–70. doi:10.1172/JCI62749. PMID:22863621.
- Delgado DC, Hank JA, Kolesar J, Lorentzen D, Gan J, Seo S, Kim K, Shusterman S, Gillies SD, Reisfeld RA, et al. Genotypes of NK cell KIR receptors, their ligands, and Fcgamma receptors in the response of neuroblastoma patients to Hu14.18-IL2 immunotherapy. Cancer Res. 2010;70:9554–61. doi:10.1158/0008-5472.CAN-10-2211. PMID:20935224.
- Erbe AK, Wang W, Carmichael L, Kim K, Mendonca EA, Song Y, Hess D, Reville PK, London WB, Naranjo A, et al. Neuroblastoma Patients' KIR and KIR-Ligand genotypes influence clinical outcome for dinutuximab-based immunotherapy: A report from the children's oncology group. Clin Cancer Res. 2018;24:189–96. doi:10.1158/1078-0432.CCR-17-1767. PMID:28972044.
- Coudert JD, Scarpellino L, Gros F, Vivier E, Held W. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood. 2008;111:3571–8. doi:10.1182/blood-2007-07-100057. PMID:18198346.
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. doi:10.1182/blood-2004-07-2974. PMID:15632206.
- Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, Pui CH, Leung W. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955–9. doi:10.1200/JCO.2009.24.4590. PMID:20085940.
- Talleur AC, Triplett BM, Federico S, Mamcarz E, Janssen W, Wu J, Shook D, Leung W, Furman WL. Consolidation therapy for newly diagnosed pediatric patients with high-risk neuroblastoma using Busulfan/Melphalan, Autologous hematopoietic cell transplantation, Anti-GD2 Antibody, granulocyte-macrophage colony-stimulating factor, Interleukin-2, and haploidentical natural killer cells. Biol Blood Marrow Transplant. 2017;23:1910–7. doi:10.1016/j.bbmt.2017.07.011. PMID:28733263.
- Federico SM, McCarville MB, Shulkin BL, Sondel PM, Hank JA, Hutson P, Meagher M, Shafer A, Ng CY, Leung W, et al. A pilot trial of humanized Anti-GD2 monoclonal antibody (hu14.18K322A) with chemotherapy and natural killer cells in children with recurrent/refractory neuroblastoma. Clin Cancer Res. 2017;23:6441–9. doi:10.1158/1078-0432.CCR-17-0379. PMID:28939747.
- Kushner BH, Kramer K, Modak S, Cheung NK. Camptothecin analogs (irinotecan or topotecan) plus high-dose cyclophosphamide as preparative regimens for antibody-based immunotherapy in resistant neuroblastoma. Clin Cancer Res. 2004;10:84–7. PMID:14734455.
- Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–23. PMID:15728129.
- Yu J, Venstrom JM, Liu XR, Pring J, Hasan RS, O'Reilly RJ, Hsu KC. Breaking tolerance to self, circulating natural killer cells expressing inhibitory KIR for non-self HLA exhibit effector function after T cell-depleted allogeneic hematopoietic cell transplantation. Blood. 2009;113:3875–84. doi:10.1182/blood-2008-09-177055. PMID:19179302.
- Beziat V, Descours B, Parizot C, Debre P, Vieillard V. NK cell terminal differentiation: correlated stepwise decrease of NKG2A and acquisition of KIRs. PLoS One. 2010;5:e11966. doi:10.1371/journal.pone.0011966. PMID:20700504.
- Carlson LM, Pahlman S, De Geer A, Kogner P, Levitskaya J. Differentiation induced by physiological and pharmacological stimuli leads to increased antigenicity of human neuroblastoma cells. Cell Res. 2008;18:398–411. doi:10.1038/cr.2008.27. PMID:18268541.
- Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, Leong JW, Abdel-Latif S, Schneider SE, Willey S, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med. 2016;8:357ra123. doi:10.1126/scitranslmed.aaf2341. PMID:27655849.
- Mody R, Naranjo A, Van Ryn C, Yu AL, London WB, Shulkin BL, Parisi MT, Servaes SE, Diccianni MB, Sondel PM, et al. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): an open-label, randomised, phase 2 trial. Lancet Oncol. 2017;18:946–57. doi:10.1016/S1470-2045(17)30355-8. PMID:28549783.
- Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL, Singh H, Hurton L, Maiti SN, Huls MH, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One. 2012;7:e30264. doi:10.1371/journal.pone.0030264. PMID:22279576.
- Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, Fleisher TA, Dubois SP, Perera LP, Stewart DM, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol. 2015;33:74–82. doi:10.1200/JCO.2014.57.3329. PMID:25403209.
- Felices M, Chu S, Kodal B, Bendzick L, Ryan C, Lenvik AJ, Boylan KLM, Wong HC, Skubitz APN, Miller JS, et al. IL-15 super-agonist (ALT-803) enhances natural killer (NK) cell function against ovarian cancer. Gynecol Oncol. 2017;145:453–61. doi:10.1016/j.ygyno.2017.02.028. PMID:28236454.
- Muntasell A, Vilches C, Angulo A, Lopez-Botet M. Adaptive reconfiguration of the human NK-cell compartment in response to cytomegalovirus: a different perspective of the host-pathogen interaction. Eur J Immunol. 2013;43:1133–41. doi:10.1002/eji.201243117. PMID:23552990.
- Rey J, Fauriat C, Kochbati E, Orlanducci F, Charbonnier A, D'Incan E, Andre P, Romagne F, Barbarat B, Vey N, et al. Kinetics of cytotoxic lymphocytes reconstitution after induction chemotherapy in Elderly AML patients reveals progressive recovery of normal phenotypic and functional features in NK Cells. Front Immunol. 2017;8:64. doi:10.3389/fimmu.2017.00064. PMID:28210257.
- Muntasell A, Ochoa MC, Cordeiro L, Berraondo P, Lopez-Diaz de Cerio A, Cabo M, López-Botet M, Melero I. Targeting NK-cell checkpoints for cancer immunotherapy. Curr Opin Immunol. 2017;45:73–81. doi:10.1016/j.coi.2017.01.003. PMID:28236750.
- Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD, Han H, Chiang SC, Foley B, Mattsson K, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42:443–56. doi:10.1016/j.immuni.2015.02.008. PMID:25786176.
- Lee J, Zhang T, Hwang I, Kim A, Nitschke L, Kim M, Scott JM, Kamimura Y, Lanier LL, Kim S. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity. 2015;42:431–42. doi:10.1016/j.immuni.2015.02.013. PMID:25786175.
- Cheung NK, Guo H, Hu J, Tassev DV, Cheung IY. Humanizing murine IgG3 anti-GD2 antibody m3F8 substantially improves antibody-dependent cell-mediated cytotoxicity while retaining targeting in vivo. Oncoimmunology. 2012;1:477–86. PMID:22754766.
- Hsu KC, Liu XR, Selvakumar A, Mickelson E, O'Reilly RJ, Dupont B. Killer Ig-like receptor haplotype analysis by gene content: evidence for genomic diversity with a minimum of six basic framework haplotypes, each with multiple subsets. J Immunol. 2002;169:5118–29. PMID:12391228.
- Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Le CT, Marsh SG, Geraghty D, Spellman S, Haagenson MD, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116:2411–9. doi:10.1182/blood-2010-05-283051. PMID:20581313.
- Cheung NK, Guo HF, Heller G, Cheung IY. Induction of Ab3 and Ab3′ antibody was associated with long-term survival after anti-G(D2) antibody therapy of stage 4 neuroblastoma. Clinical Can Res. 2000;6:2653–60. PMID:10914706.
- Rowlings PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Henslee-Downey PJ, Cahn JY, Calderwood S, Gratwohl A, Socié G, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–64. PMID:9217189.
- Brodeur G, Pritchard J, Berthold F, Carlen NLT, Castel V, Castleberry RP, De Bernardi B, Evans AE, Favrot M, Hedborg F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging and response to treatment. J Clin Oncol. 1993;11:1466–77. PMID:8336186.
- Naranjo A, Parisi MT, Shulkin BL, London WB, Matthay KK, Kreissman SG, Yanik GA. Comparison of (1)(2)(3)I-metaiodobenzylguanidine (MIBG) and (1)(3)(1)I-MIBG semi-quantitative scores in predicting survival in patients with stage 4 neuroblastoma: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2011;56:1041–5. doi:10.1002/pbc.22991. PMID:21328522.
- Cheung NK, Guo HF, Cheung IY. Correlation of anti-idiotype network with survival following anti-G(D2) monoclonal antibody 3F8 therapy of stage 4 neuroblastoma. Med Pediatr Oncol. 2000;35:635–7. PMID:11107135.
- Cheung IY, Hsu K, Cheung NK. Activation of peripheral-blood granulocytes is strongly correlated with patient outcome after immunotherapy with anti-GD2 monoclonal antibody and granulocyte-macrophage colony-stimulating factor. J Clin Oncol. 2012;30:426–32. doi:10.1200/JCO.2011.37.6236. PMID:22203761.