ABSTRACT
Recent developments in immunotherapy have prolonged overall survival in metastatic melanoma with the possibility to reach a long-term benefit. Targeted therapies based on BRAF and MEK inhibition also seem to have a long-term beneficial effect, which is more evident in patients with favorable baseline characteristics, namely normal levels of lactate dehydrogenase, without brain metastases, and low tumor burden. This long-term benefit of targeted therapies might be related to an immune-modulation: indeed BRAF and MEK inhibitors affect tumor microenvironment and immune surveillance, and it has been shown that patients with complete response to targeted treatment have a pre-existing favorable immunologic signature.
Introduction
Over the years, survival of patients with advanced melanoma has improved from the median overall survival (OS) of 6 months, and the median progression-free survival (PFS) of about 2 months reported in the metanalysis by Korn et al. in 2008.Citation1 Notably, nowadays OS can be as long as 36 months, with a 3-year survival rate of 58%, and a median PFS of 11.5 months.Citation2 This important achievement in survival follows the improvements in therapeutic strategies, mainly targeted therapies and immune-therapy: selective inhibitors of the mitogen-activated protein (MAP) kinase pathway and blockers of immune checkpoint molecules result in a major survival benefits for patients with metastatic melanoma.Citation3
Several new drugs have been developed and approved for the treatment of advanced melanoma since 2011, targeting different cellular pathways. Dabrafenib, vemurafenib, and encorafenib inhibit BRAF; trametinib, cobimetinib, and binimetinib inhibit MEK; the genetically-modified oncolytic virus talimogen laherparepvec replicates inside cancer cells causing them to die, whereas human antibodies ipilimumab and pembrolizumab/nivolumab target the anti-cytotoxic T-lymphocyte associated antigen-4 (CTLA4) and the anti-programmed cell death protein 1 (anti-PD-1), respectively. These therapies show a rapid onset of action and high response rateCitation4.
The use of combination regimens is superior over monotherapy in BRAFV600-mutated melanoma, and contributed to prolong OS; therefore, this approach now represents the standard of care in this setting.Citation4–Citation7 However, only immunotherapy was effective in patients with wild-type disease to date.Citation5 To note, the ongoing development of novel combination partners, new dosing regimens and intermittent schedules will hopefully further prolong survival in the future.
At present scant evidence exists about which treatment strategy should be attempted first, either MAP kinase inhibition or immune checkpoint blockade. Combination of BRAF plus MEK inhibition has showed higher response rates in the first-line setting during the initial 6 months following treatment institution, whereas checkpoint inhibition is more effective in prolonging long-term survival during follow-up.Citation3
This narrative review will discuss preclinical and clinical evidence for immune modulation, how it can guide treatment selection, and its relevance for clinical practice. Moreover, future development of this strategy is presented.
Effects of BRAF and MEK inhibitors on the immune system
Multiple evidence of the effects of BRAF inhibition on the immune system exists. For instance, BRAF inhibition is associated with increased CD8 + T-cell infiltrate in tumors of patients with BRAF-mutated metastatic melanoma, as observed by Wilmott et al.Citation8 In their study, tumor infiltration by CD4+ and CD8+ lymphocytes increased after treatment with BRAF inhibitors (both ρ = 0.015), with a good correlation between the degree of tumor infiltration by CD8+ and granzyme B-expressing lymphocytes in post-BRAF inhibitor-treated biopsies (r = 0.690 and ρ = 0.013).Citation8 In addition, increased expression of intra-tumor CD8+ lymphocytes correlated with reduced tumor size and enhanced necrosis in post-treatment biopsies (r = -0.793, ρ = 0.011; and r = 0.761, ρ = 0.004, respectively).
BRAF inhibition is also associated with increased expression of melanoma antigens at least in the first weeks after treatment initiation. In the study by Frederick et al., treatment with either BRAF inhibitor alone (vemurafenib) or BRAF+MEK inhibition (dabrafenib+trametinib) resulted in increased expression of melanoma antigens and increased CD8 + T-cell infiltration in patients with BRAF V600E mutation.Citation9 Furthermore, a decrease in immunosuppressive cytokines like interleukin 6 (IL6) and interleukin 8 (IL8) and an increase in markers of T-cell cytotoxicity were reported. Thus, BRAF inhibition alone may be linked to a more favorable tumor microenvironment that enhances the expression of melanoma antigens and facilitates T-cell cytotoxicity.
In their preclinical study of syngeneic BRAF V600E-driven melanoma in mice, SM1, Hu-Lieskovan et al. tested whether the addition of the MEK inhibitor trametinib would enhance the antitumor activity of combined immunotherapy with the BRAF inhibitor dabrafenib.Citation10 The combination of dabrafenib and trametinib with pmel-1 adoptive cell transfer (ACT) resulted in complete tumor regression, increased T cell infiltration, and improved in vivo cytotoxicity. T regulatory cells (Tregs) and tumor-associated macrophages were increased by dabrafenib, and decreased after the addition of trametinib. In another animal study, Schilling et al. evaluated the efficacy of the triple combination therapy with dabrafenib, trametinib, and anti-programmed cell death protein 1 (PD1) in increasing the expression of melanoma antigens and major histocompatibility complex (MHC), as well as global immune-related gene up-regulation in SM1 tumors with BRAF V600E mutation.Citation11 Noteworthy, the amount of circulating myeloid-derived suppressor cells (MDSC), which repress antitumor immunity, declined in response to vemurafenib.
In a phase I-b dose escalation trial (NCT01656642) with the combination atezolizumab/cobimetinib/vemurafenib in BRAF V600 mutant melanoma (N = 30), Sullivan et al. showed an increased tumor CD8 + T-cell accumulation after about 1 month of cobimetinib + vemurafenib treatment; this effect may result in enhanced immunotherapy responsiveness. The unconfirmed response rate was 83% and CD8+ lymphocytes increased, suggesting an enhancement of immunity. No special safety signals were reported.Citation12 Another phase I, open-label study (NCT02027961) assessed the safety and efficacy of durvalumab in combination with dabrafenib-trametinib versus trametinib alone in patients with stage IIIc/IV melanoma.Citation13 Patients were enrolled by BRAF status – mutant or wild-type – into dose escalation cohorts, followed by a dose-expansion phase: BRAF mutant in Cohort A (triple therapy); BRAF wild-type in Cohort B (durvalumab+trimetinib) or Cohort C (sequential trametinib – durvalumab). In total, 50 patients were treated. Dose-limiting toxicities were observed in one patient in Cohort A and one in Cohort B. Complete response (CR) or partial responses (PR) were observed in all the 6 evaluable patients on triple therapy, thus suggesting the activity of this combination both in BRAF-mutant and BRAF wild-type melanoma. Overall similar results were reported with the triple combination with dabrafenib, trametinib and pembrolizumab, in the phase I/II KEYNOTE-022 study (NCT02130466).Citation14 This study enrolled 15 treatment-naive patients with BRAFV600E/K-mutant stage III/IV melanoma. In total, 3 patients (20%) reported dose-limiting toxicities and the overall response rate was 67% (1 CR and 9 PR).
Altogether, these findings support the testing of triple combination therapy of BRAF and MEK inhibitors with immunotherapy in BRAF-mutated melanoma. At present, a phase III study (Trilogy, NCT02908672) is ongoing, with the aim to evaluate whether the addition of atezolizumab to the BRAF/MEK inhibition can further increase anti-tumor activity in BRAF-mutated disease. Further evidence is also required on the use of BRAF and MEK inhibitors in BRAF-wild type melanoma.Citation15
Of note, BRAF V600E mutation downregulates the expression of interferon (IFN)-alpha-receptor-1 (IFNAR-1), and in turn BRAF inhibition upregulates the expression of most of the human leukocyte antigen (HLA) class I antigen-processing machinery (APM) components, and enhances the recognition of melanoma cells by cognate T-cells.Citation16 A phase I study on the triple combination vemurafenib+cobimetinib+polyethylene glycol(PEG)-IFN (VEMUPLINT, NCT01959633) in BRAF-mutated melanoma is ongoing, and in the future one additional possible option for the treatment of advanced melanoma could be to combine BRAF and MEK inhibitors with anti-CD73 and/or adenosine A2A receptor (A2AR) receptor inhibitors.Citation17 In more details, CD73 metabolizes the conversion of adenosine monophosphate (AMP) to adenosine; this latter exerts multiple effects on immune cells and tumor microenvironment.Citation17 BRAF mutation may induce CD73-dependent immune suppression in melanoma.Citation18 Moreover, a possible association between higher expression of CD73 and BRAF mutation has been reported.Citation18 Higher dosage of ipilimumab, or the addition of ipilimumab to nivolumab, may trigger antibody-dependent cell cytotoxicity, removing the activated Treg cells and enhancing the action of ipilimumab as single agent or in combination with nivolumab; at the same time, anti-PD1 agents activate T effector cells.Citation17 Overall, it has been suggested that over-expression of CD73 in BRAF-mutant melanoma may explain the benefits of higher doses of ipilimumab or the combination of ipilimumab and nivolumab in BRAF-mutant melanoma.
Treatment selection in BRAF-mutant melanoma
At the introduction of the new compounds for the treatment of advanced melanoma, there was a diffuse thought that immunotherapy would have represented the ideal front-line therapy for patients with indolent melanoma, i.e., non-bulky, asymptomatic, and with normal lactate dehydrogenase (LDH) levels. On the other hand, targeted BRAF inhibition would have been reserved to a more aggressive, bulky and symptomatic disease. For patients with intermediate features, both immunotherapy and BRAF-inhibition were considered eligible with no particular preference.Citation19 Nowadays it is known that targeted therapy may determine long-term benefit in indolent melanoma, whereas both target therapy and immune therapy present limited action in the aggressive form of the disease.
A retrospective, single-institution analysis of patients displaying BRAF V600 mutation revealed that sequential treatment with vemurafenib or dabrafenib followed by ipilimumab, or vice versa, is common in clinical practice.Citation20 In this analysis, of the 34 BRAF-mutation-positive patients evaluated, 6 patients received ipilimumab followed by a BRAF inhibitor, whereas 28 patients were treated with a BRAF inhibitor followed by ipilimumab. Of these latter patients, 12 (43%) rapidly progressed to death, being unable to complete ipilimumab treatment as per protocol. The median OS in this subgroup of patients was 5.7 months [95% Confidence Interval (CI) 5.0–6.3], versus 18.6 months (95% CI 3.2–41.3; p < 0.0001) in patients who completed ipilimumab treatment. Baseline factors associated with rapid progression were elevated LDH levels, a performance status of 1, and the presence of brain metastases. Positivity for two of these risk factors at baseline was linked to a higher probability of rapid disease progression (PD). This was the first time that such elements (i.e., LDH levels and presence of brain metastases) have been identified as predictive factors to treatment response. Moreover, at that time we speculated that the optimal sequence of targeted treatments in patients with BRAF-mutation-positive metastatic melanoma may be determined according to the presence of these specific risk factors. Indeed, tumor biology appears to be the most relevant factor contributing to the selection of the right treatment sequence: indolent disease may be successfully treated with BRAF/MEK inhibitors and immunotherapy, whereas a more limited action of current options is likely in case of aggressive diseases.
The best treatment sequence with anti-PD1 monotherapy (more common than ipilimumab monotherapy in clinical practice) have not been studied in prospective trial to date; only a few retrospective analysis are available on this treatment sequence.Citation21,Citation22
At present, every decision in clinical practice about which treatment should be started is based on the specific characteristics of each single patient, including his/her history (i.e., known autoimmune disease), organs function (especially cardiovascular system status), his/her wishes and lifestyle, his/her mutational status, performance status, presence of brain metastases, tumor burden, LDH levels and time from disease onset.
Blank et al. introduced the concept of ‘Cancer Immunogram’ as a tool to identify patients more likely to respond to immunotherapy.Citation23 Seven prognostic factors were included in this immunogram: tumor foreignness, general immune status, immune cell infiltration, absence of checkpoints, absence of soluble inhibitors, absence of inhibitory tumor metabolism and tumor sensitivity to immune effectors. Remarkably, some of these items are also major predictive factors for BRAF-inhibitor therapy: for instance, normal LDH levels (i.e., absence of inhibitory tumor metabolism), immune cell infiltration, absolute lymphocytes count (i.e., general immune status), MHC expression (i.e., tumor foreignness) and programmed death-ligand 1 (PD-L1) (i.e., absence of checkpoints).
To this respect, Massi et al. investigated whether the density of tumor-infiltrating mononuclear cells (TIMC), or the expression of PD-L1 can predict the occurrence of resistance, thus affecting the clinical outcome in BRAFi-treated patients.Citation24 In this study, 46 patients received vemurafenib and 34 dabrafenib. Membranous expression of PD-L1 was detected in 28/80 (35%) of patients. At multivariate analysis, the absence of tumor PD-L1 staining [odd ratio (OR) 10.8; 95% CI 2.7–43.3; p < 0.001] and the presence of TIMC (OR 6.5; 95% CI 1.7–24.3; p < 0.005) were associated with a better response to treatment. Median PFS and OS were 10 and 15 months, respectively. By multivariate assessment, PD-L1 expression [hazard ratio (HR) 4.3; 95% CI 2.1–8.7, p < 0.0001] and absence of TIMC (HR 2.5; 95% CI 1.4–4.7; p < 0.002) correlated with shorter PFS. PD-L1 overexpression (HR 6.2; 95% CI 2.8–14.2; p < 0.0001) and absence of TIMC (HR 3.1; 95% CI 1.5–6.5; p < 0.002) were independent prognostic factors for melanoma-specific survival.
Several studies have also confirmed the importance of predictive factors in identifying patients who may most benefit from BRAF-inhibitor therapy. For instance, Ascierto et al. reported the long-term OS and safety data of 92 patients with histologically-confirmed stage IV BRAF V600E/K mutation+ metastatic melanoma enrolled in the BREAK-2 study who received oral dabrafenib 150 mg twice daily until PD, death, or unacceptable adverse events (AEs).Citation25 Overall, 11 patients (12%) did not experience PD, with 9 patients who continued to receive dabrafenib. In the BRAF V600K group, median OS was 12.9 months (95% CI, 6.9–17.1), with 4 patients (25%) alive beyond 18.8 months (third quartile OS; 95% CI, 12.9-not reached). In the BRAF V600E group, median OS was 13.1 months (95% CI, 10.4–21.9), with 21 patients (28%) alive beyond 30 months (third quartile OS, not reached). To note, all these patients had normal LDH levels. These findings were recently confirmed by Chapman et al.,Citation26 according to the results of the 5-year follow-up of the BREAK-2 and BREAK-3 trials. In the BREAK-2 study, 5-year PFS was 11% in patients with V600E mutations, whereas all patients with V600K mutations had died or were censored before the 5-year landmark. Conversely, in the BREAK-3 study, 5-year PFS was 12% in the dabrafenib arm, with all patients in the dacarbazine arm showing PD or being censored before the 5-year landmark. The median follow-up for the 6 patients in the dabrafenib arm who remained progression-free at 5 years was 62.1 months (95% CI, 61.6–62.2); median PFS for crossover patients following initiation of crossover dabrafenib treatment was 4.3 months (95% CI, 4.1–6.1). Five-year OS was 20% and 13% in BREAK-2 trial patients with V600E and V600K mutations, respectively, as compared with 5-year OS of 24% in the dabrafenib arm and 22% in the dacarbazine arm in the BREAK-3 study. To note, an apparent plateau effect with dabrafenib for both PFS and OS was reported after 36 months; additionally, longer PFS was evident in patients with lower/normal LDH levels.
Recently, Long et al. published the 5-year pooled analysis of data from the phase II BRF113220 study part C.Citation27 They reported a 5-year PFS rate of 13% in patients with BRAF-mutant melanoma treated with the combination dabrafenib-trametinib, versus 3% in patients treated with dabrafenib monotherapy. OS was approximately 30% with the combined therapy versus 21% with dabrafenib only. The Authors underline that patients with more favorable characteristics at baseline (e.g., lower LDH levels and fewer metastatic sites) showed improved PFS and OS. For instance, they found a rate of PFS of 23% in the combined treatment arm in patients with normal LHD serum levels, compared with 6% in the monotherapy arm. Rates of 45–48% OS were reported in the combination arm, versus 26–31% in the monotherapy arm in the subgroup of patients with normal LDH levels. Similar results were obtained in the subgroups of patients with a lower number of metastatic sites, with PFS rates of 25% in the combination arm versus 8% in monotherapy arm, and OS rates of 57% and 42% respectively.Citation23 Moreover, the investigators also reported on adverse effects, stating that they were more common in the combined regimen as it may easily be understood.
Of note, also the 3-year analysis of the Combi-v study confirmed the improved outcomes with the dabrafenib/trametinib combination also over vemurafenib monotherapy, despite crossover (3-year OS, 45% vs 31%; 3-year PFS, 24% vs 10%).Citation28 Again, durable benefits were observed particularly in patients with normal baseline LDH (3-year OS, 56%; 3-year PFS, 33%) and normal LDH with less than three metastatic organs (3-year OS, 70%; 3-year PFS, 39%). Remarkably, 36 of 68 patients (53%) showing CR on dabrafenib+trametinib were maintaining response at the time of the analysis, as compared with only 21/41 patients (51%) showing CR with vemurafenib monotherapy. Noteworthy, patients with favorable baseline characteristics, such as LDH levels within normality range, less than three metastatic sites, and M0, M1a, M1b status were more likely to obtain a CR.
Yan et al. compared the baseline genomic features of tumors showing CR vs PD in patients treated with cobimetinib plus vemurafenib or vemurafenib alone.Citation29 Genomic analysis were carried out with whole exome sequencing (WES) on baseline melanoma samples from 52 patients with CR and 78 patients with PD, following treatment with cobimetinib combined with vemurafenib or vemurafenib alone. The overall mutational load was not different between groups; however, samples from patients with PD showed higher rates of melanogenesis associated transcription factor (MITF) amplification and tumor protein 53 (TP53) mutation (18% and 19%, respectively) than patients with CR (4% and 5%, respectively). Thus, the genomic differences disclosed between melanomas of patients with CR vs patients with PD treated with either the combination of BRAF and MEK inhibitors or with the BRAF inhibitor alone may be responsible for the over-represented adaptive and innate immune responses, e.g. gene signatures of CD8 + T effector cells, cytolytic T-cells, antigen presentation and natural killer (NK) cells, that are all enriched in CR tumors. Authors also concluded that melanomas of patients with PD over-represented “keratin” molecular subtype melanoma, which is associated with poor prognosis.Citation29
Noteworthy, an immune signature characterized by gene expression profiling identified two patient subgroups with distinct PFS outcomes, one defined by high baseline expression of genes associated with cell cycle progression and the other characterized by high baseline expression of immune regulatory genes.Citation30 Among vemurafenib-treated patients, the cell cycle signature was associated with shortened PFS compared with the immune signature. The adverse impact of the cell cycle signature on PFS was not observed in patients treated with cobimetinib combined with vemurafenib. The subgroup with normal baseline LDH and immune signature showed the longest median PFS (9.0 months, CI 95% 7.5–13.9; 1-year PFS rate 43.2%).
Relevance for clinical practice
Targeted therapy is associated with long term benefit, as demonstrated by the increased OS rates reported in phase III trials:Citation26,Citation27,Citation31,Citation32 in this scenario, several new molecules have been developed. However, we already questioned whether OS should be considered the main endpoint of such trials, when several new treatment possibilities are in early phase-development and fewer patients are available for large trials.Citation4 In our opinion, PFS should be taken into account more than OS, as the latter may be influenced by immunotherapy administered in the post-progression phase.Citation4 Noteworthy, even in this case some baseline patients’ characteristics may identify those subjects who will show longer OS: again, these factors are normal LDH levels, low tumor burden, absence of brain metastases, and complete response to therapy, all features that usually pertain to indolent melanoma.
Moreover, it is important to underline that patients with a CR are also those who show a pre-existing immunologic signature, which in turn may be responsible for the long term benefit. The possibility to stop treatment in these patients is still debated. In a recent case series, Carlino et al. identified 12 patients treated with BRAF/MEK inhibitors who reached CR and stopped treatment before progression.Citation33 Median follow-up was 16 months. Six patients (50%) recurred at a median of 6.6 months after the end of treatment. One patient died after recurrence. Baseline characteristics and time to CR or to discontinuation did not influence the rate of relapse. In line with previous evidence, immunologic mechanisms might have played a role in patients without recurrence.Citation34 However, half of the patients achieving CR recurred at some stages of the follow-up, and thus it is not advisable to stop treatment even in patients who reach CR.
Recently, Long et al. assessed the efficacy of dabrafenib/trametinib combination in patients with BRAF V600E/V600K mutations and stage III melanoma in the COMBI-AD trial.Citation35 Importantly, the combination of dabrafenib plus trametinib was shown to be effective also in the adjuvant setting: a lower risk of recurrence was achieved, without excessive increase in the rate of adverse events. In this study, a high efficacy of dabrafenib/trametinib combination was reported in terms of relapse free survival (HR 0.47, p < 0.001), distant metastases free survival (HR 0.51, p < 0.001) and OS (HR 0.57, p = 0.0006) versus placebo. This effect was not so obvious, since the adjuvant setting relates to minimal residual disease, thus suggesting possible immune-related effects of target therapy.
Indeed, Amaria et al. presented their prospective neoadjuvant study in BRAF-mutant patients with resectable stage IIIB/C or oligometastatic stage IV melanoma. They aimed to investigate whether neoadjuvant treatment associated with adjuvant dabrafenib/trametinib improves PFS.Citation36,Citation37 After enrolling 21 of the expected 84 patients, an interim analysis revealed significant improvement in PFS in the dabrafenib+trametinib arm as compared to the standard-of-care arm (HR 0.016; p < 0.0001). Enrollment was thus terminated. Achievement of pathological complete response (pCR) following neoadjuvant therapy was reported in 7 out of the 13 evaluable patients with dabrafenib/trametinib combination, and this event predicted improved distant metastasis free survival. Transcription profiling of baseline tumor samples showed the high upregulation of cytotoxic CD8 + T cell genes in patients reaching pCR, not observed in non-pCR patients. Moreover, tumor profiling revealed incomplete MAPK pathway blockade and higher levels of CD8 + T cells expressing negative immunomodulators Tim-3 and Lag-3 in patients not achieving pCR. Thus, the Authors suggested that pCR and tumor analysis may reveal the ideal candidate for future targeted therapies.
We can speculate that this immune-modulating effect may also depend on the adjuvant setting in which the Combi-AD trial was performed. Moreover, additional information regarding the effectiveness and efficacy of combining target therapy and immune-therapy for the treatment of melanoma will come from the ongoing trials Trilogy, Combi-I (NCT02967692) and Keynote 022 (NCT02130466).
Conclusion
Several drugs targeting either BRAF or MEK pathways are currently available, to be administered both as monotherapy and within combination regimens. Moreover, ongoing trials may add important information on the efficacy and safety profile of these drugs.Citation38
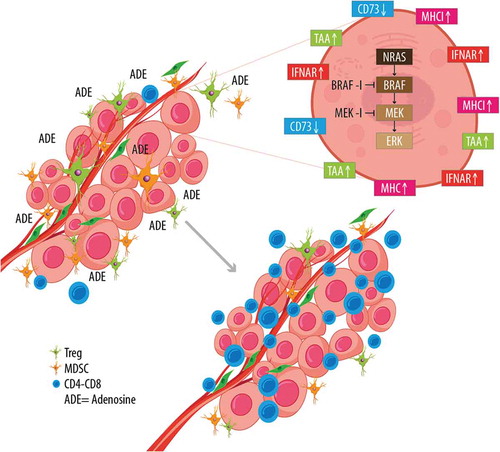
Of note, baseline patients’ characteristics like LDH levels and tumor burden may influence response to treatment, either with BRAF-inhibitors or with MEK-inhibitors, given also their effects on immune surveillance (). In turn, BRAF-MEK inhibition does have a major role on tumor microenvironment, as it markedly affects antigen display and therefore immune response ().
Figure 1. ‘Easy’ and ‘difficult’ patients. The ‘easy’ patients present some characteristics (e.g., no brain metastasis, low tumor burden, normal LDH) that result in active immune surveillance against cancer cells. Such immune surveillance may be pre-existing and responsible for reaching complete response. On the other hand, immune surveillance is impaired in difficult patients, who commonly present brain metastasis, high tumor burden, and high LDH.
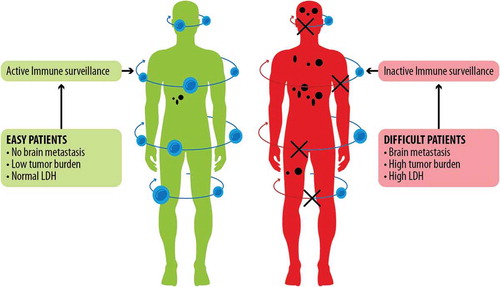
Figure 2. Effects of BRAF-MEK inhibition on melanoma cells and tumor microenvironment. Therapy with BRAF and MEK inhibitors induces profound changes in antigen display, and expression of MHC, IFNAR, and CD73 on tumor cells. These changes are also evident on tumor microenvironment: namely they result in reduction of adenosine, diminished Treg and MDSC presence, and increased activity of CD4-CD8+ lymphocytes.
Baseline patient’s characteristics may be useful both for patients’ selection and for establishing which treatment should be administered first, either target therapy or immune therapy. Noteworthy, it is important to underline that patients with complete response to treatment show a pre-existing immunologic signature, predisposing to immune activation while resistance or lack of pCR may involve suppressor/exhausted CD8. This mechanism may be account for the major results observed in the adjuvant setting.
Based on the current knowledge, it is advisable to continue treatment even in cases of CR. Importantly, the combination of BRAF and MEK inhibition was effective also in the adjuvant and neoadjuvant settings: further evidence regarding these therapies and newer possibilities are still to come.
In the future, triple combination with anti-PD-1/PD-L1, or sequencing study with target and immune therapy may give us further indications. Moreover, the emerging role of the adenosine pathwayCitation17,Citation18 or the specific targeting of AXL on dedifferentiated melanoma cellsCitation39 may be among the future news in this field.
Disclosure of potential conflicts of interest
PA received honoraria for consultancy and advisory role from BMS, Roche-Genentech, MSD, Array, Novartis, Amgen, Merck-Serono, Pierre-Fabre, Incyte, NewLink Genetics, Genmab, Medimmune, Syndax, Astra Zeneca and research funds from BMS, Roche-Genentech, Array.
RD received research funding from Novartis, Merck Sharp & Dhome (MSD), Bristol-Myers Squibb (BMS), Roche, and has an intermittent consultant or advisory board relationship with Novartis, Merck Sharp & Dhome, Bristol-Myers Squibb, Roche, Amgen, Takeda, Pierre Fabre and Sun Pharma.
Additional information
Funding
References
- Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, Moon J, Sondak VK, Atkins MB, Eisenhauer EA, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26(4):527–534. doi:10.1200/JCO.2007.12.7837.
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–1356. doi:10.1056/NEJMoa1709684.
- Ugurel S, Röhmel J, Ascierto PA, Flaherty KT, Grob JJ, Hauschild A, Larkin J, Long GV, Lorigan P, McArthur GA, et al. Survival of patients with advanced metastatic melanoma: the impact of novel therapies-update 2017. Eur J Cancer. 2017;83:247–257. doi:10.1016/j.ejca.2017.06.028.
- Ascierto PA, Long GV. Progression-free survival landmark analysis: a critical endpoint in melanoma clinical trials. Lancet Oncol. 2016;17(8):1037–1039. doi:10.1016/S1470-2045(16)30017-1.
- Da Silveira Nogueira Lima JP, Georgieva M, Haaland B, De Lima Lopes G. A systematic review and network meta-analysis of immunotherapy and targeted therapy for advanced melanoma. Cancer Med. 2017;6(6):1143–1153. doi:10.1002/cam4.1001.
- Robert C, Kaaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–39. doi:10.1056/NEJMoa1412690.
- Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, Garbe C, Schadendorf D, Krajsova I, Gutzmer R, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;pii:S1470–2045(18)30142–301426. doi:10.1016/S1470-2045(18)30142-6.
- Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, Kefford RF, Hersey P, Scolyer RA. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res. 2012;18(5):1386–1394. doi:10.1158/1078-0432.CCR-11-2479.
- Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, Mitra D, Boni A, Newton LP, Liu C, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19(5):1225–1231. doi:10.1158/1078-0432.CCR-12-1630.
- Hu-Lieskovan S, Mok S, Homet Moreno B, Tsoi J, Robert L, Goedert L, Pinheiro EM, Koya RC, Graeber TG, Comin-Anduix B, et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF (V600E) melanoma. Sci Transl Med. 2015;7(279):279ra41. doi:10.1126/scitranslmed.aaa4691.
- Schilling B, Paschen A. Immunological consequences of selective BRAF inhibitors in malignant melanoma: neutralization of myeloid-derived suppressor cells. Oncoimmunology. 2013;2(8):e25218. doi:10.4161/onci.25218.
- Sullivan RJ, Gonzalez R, Lewis KD, Hamid O, Infante JR, Patel MR. Safety and clinical activity of atezolizumab + cobimetinib + vemurafenib in BRAFV600-mutant metastatic melanoma. Paper presented at: Society for Melanoma Research Annual Meeting; 2016 Nov 6–9; Boston, Massachusetts.
- Ribas A, Butler M, Lutzky J, Lawrence DP, Robert C, Miller W, Linette GP, Ascierto PA, Kuzel T, Algaziet AP, et al. Phase 1 study combining anti-PD-L1 (MEDI4736) with BRAF (dabrafenib) and/or MEK (trametinib) inhibitors in advanced melanoma. J Clin Oncol. 2015;15(Suppl 15):3003. doi:10.1200/jco.2015.33.15_suppl.3003.
- Ribas A, Hodi FS, Lawrence D, Atkinson V, Agarwal S, Carlino MS, Fisher R, Long GV, Miller WH, Huang Y, et al. KEYNOTE-022 update: phase 1 study of first-line pembrolizumab (pembro) plus dabrafenib (D) and trametinib (T) for BRAF-mutant advanced melanoma. Ann Oncol. 2017;28(Suppl 5):v428–v448. doi:10.1093/annonc/mdx377.
- Johnpulle RA, Johnson DB, Sosman JA. Molecular targeted therapy approaches for BRAF wild-type melanoma. Curr Oncol Rep. 2016;18(1):6. doi:10.1007/s11912-015-0485-6.
- Sabbatino F, Wang Y, Scognamiglio G, Favoino E, Feldman SA, Villani V, Flaherty KT, Nota S, Giannarelli D, Simeone E, et al. Antitumor activity of BRAF inhibitor and IFNα combination in BRAF-mutant melanoma. JNCI J Natl Cancer Inst. 2016;108(7). doi:10.1093/jnci/djv435.
- Ascierto PA, McArthur GA. Checkpoint inhibitors in melanoma and early phase development in solid tumors: what’s the future? J Transl Med. 2017;15:173. doi:10.1186/s12967-017-1278-5.
- Young A, Ngiow SF, Madore J, Reinhardt J, Landsberg J, Chitsazan A, Rautela J, Bald T, Barkauskas DS, Ahern E, et al. Targeting adenosine in BRAF-mutant melanoma reduces tumor growth and metastasis. Cancer Res. 2017;77(17):4684–4696. doi:10.1158/0008-5472.CAN-17-0393.
- Jang S, Atkins MB. Which drug, and when, for patients with BRAF-mutant melanoma? Lancet Oncol. 2013;14(2):e60–69. doi:10.1016/S1470-2045(12)70539-9.
- Ascierto PA, Simeone E, Giannarelli D, Grimaldi AM, Romano A, Mozzillo N. Sequencing of BRAF inhibitors and ipilimumab in patients with metastatic melanoma: a possible algorithm for clinical use. J Transl Med. 2012;10:107. doi:10.1186/1479-5876-10-107.
- Simeone E, Grimaldi AM, Festino L, Giannarelli D, Vanella V, Palla M, Curvietto M, Esposito A, Palmieri G, Mozzillo N, et al. Correlation between previous treatment with BRAF inhibitors and clinical response to pembrolizumab in patients with advanced melanoma. Oncoimmunology. 2017;6(3):e1283462. doi:10.1080/2162402X.2017.1283462.
- Shoushtari AN, Munhoz RR, Kuk D, Ott PA, Johnson DB, Tsai KK, Rapisuwon S, Eroglu Z, Sullivan RJ, Luke JJ, et al. The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer. 2016;122(21):3354–3362. doi:10.1002/cncr.30259.
- Blank CU, Haanen JB, Ribas A, Schumacher TN. Cancer immunology. The “cancer immunogram”. Science. 2016;352(6286):658–660. doi:10.1126/science.aaf2834.
- Massi D, Brusa D, Merelli B, Falcone C, Xue G, Carobbio A, Nassini R, Baroni G, Tamborini E, Cattaneo L, et al. The status of PD-L1 and tumor-infiltrating immune cells predict resistance and poor prognosis in BRAFi-treated melanoma patients harboring mutant BRAFV600. Ann Oncol. 2015;26(9):1980–1987. doi:10.1093/annonc/mdv255.
- Ascierto PA, Minor DR, Ribas A, Lebbe C, O’Hagan A, Swann RS, Scheuber A, Schadendorf D, Kefford R, Grob JJ, et al. Long-term safety and overall survival update for BREAK-2, a phase 2, single-arm, open-label study of dabrafenib in previously treated metastatic melanoma (NCT01153763). J Clin Oncol. 2014;32(Suppl 15):9034. doi:10.1200/jco.2014.32.15_suppl.9034.
- Chapman PB, Ascierto PA, Schadendorf D, Grob JJ, Ribas A, Kiecke RF. Updated 5-year landmark analyses of phase 2 (BREAK-2) and phase 3 (BREAK-3) studies evaluating dabrafenib monotherapy in patients with BRAF V600–mutant melanoma. J Clin Oncol. 2017;35(Suppl 15):9526. doi:10.1200/JCO.2017.35.15_suppl.9526.
- Long GV, Eroglu Z, Infante J, Patel S, Daud A, Johnson DB, Gonzalez R, Kefford R, Hamid O, Schuchter L, et al. Long-term outcomes in patients with BRAF V600–mutant metastatic melanoma who received dabrafenib combined with trametinib. J Clin Oncol. 2017. doi:10.1200/JCO.2017.74.1025.
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroyakovskiy D, Dummer R, Grange F, Mortier L, Chiarion-Sileni V, et al. Three-year estimate of overall survival in COMBI-v, a randomized phase 3 study evaluating first-line dabrafenib (D) + trametinib (T) in patients (pts) with unresectable or metastatic BRAF V600E/K–mutant cutaneous melanoma. Ann Oncol. 2016;27(Suppl 6):LBA40. doi:10.1093/annonc/mdw435.37.
- Yan Y, Robert C, Larkin J, Ascierto PA, Dreno B, Maio M, Garbe C, Chapman PB, Sosman JA, Wongchenko MJ, et al. Genomic features of complete responders (CR) versus fast progressors (PD) in patients with BRAFV600-mutated metastatic melanoma treated with cobimetinib + vemurafenib or vemurafenib alone. Ann Oncol. 2016;27(6):379–400. doi:10.1093/annonc/mdw379.
- Larkin J, Lewis KD, Ribas A, Flaherty K, Ascierto PA, Dréno B. Impact of gene expression profiles on clinical predictors of survival in patients with BRAFV600-mutated metastatic melanoma treated with vemurafenib ± cobimetinib. J Clin Oncol. 2017;35(Suppl 15):9556. doi:10.1200/JCO.2017.35.15_suppl.9556.
- Schadendorf D, Wolchok JD, Hodi FS, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Chesney J, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J Clin Oncol. 2017;35(34):3807–3814. doi:10.1200/JCO.2017.73.2289.
- Long GV, Atkinson V, Ascierto PA, Robert C, Hassel JC, Rutkowski P, Savage KJ, Taylor F, Coon C, Gilloteau I, et al. Effect of nivolumab on health-related quality of life in patients with treatment-naïve advanced melanoma: results from the phase III CheckMate 066 study. Ann Oncol. 2016;27(10):1940–1946. doi:10.1093/annonc/mdw265.
- Carlino MS, Vanella V, Girgis C, Giannarelli D, Guminski A, Festino L, Kefford RF, Menzies AM, Long GV, Ascierto PA. Cessation of targeted therapy after a complete response in BRAF-mutant advanced melanoma: a case series. Br J Cancer. 2016;115(11):1280–1284. doi:10.1038/bjc.2016.321.
- Klein O, Ribas A, Chmielowski B, Walker G, Clements A, Long GV, Kefford RF. Facial palsy as a side effect of vemurafenib treatment in patients with metastatic melanoma. J Clin Oncol. 2013;31(12):e215–217. doi:10.1200/JCO.2012.45.7028.
- Long GV, Hauschild A, Santinami M, Atkinson V, Mandalà M, Chiarion-Sileni V, Larkin J, Nyakas M, Dutriaux C, Haydon A, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377(19):1813–1823. doi:10.1056/NEJMoa1708539.
- Amaria R, Prieto PA, Tetzlaff MT, Reuben A, Andrews MC, Ross MI, Glitza IC, Cormier J, Hwu WJ, Tawbi HA, et al. Neoadjuvant plus adjuvant dabrafenib and trametinib versus standard of care in patients with high-risk, surgically resectable melanoma: a single-centre, open-label, randomised, phase 2 trial. Lancet. 2018;19(2):181–193. doi:10.1016/S1470-2045(18)30015-9.
- Wargo JA, Amaria RN, Prieto PA, Andrews MC, Tetzlaff MT, Futreal PA. Relapse-free survival and target identification to enhance response with neoadjuvant and adjuvant dabrafenib + trametinib (D+T) treatment compared to standard-of-care (SOC) surgery in patients (pts) with high-risk resectable BRAF-mutated metastatic melanoma. J Clin Oncol. 2017;35(Suppl 15):9587. doi:10.1200/JCO.2017.35.15_suppl.9587.
- Dummer R, Ramelyte E, Schindler S, Thürigen O, Levesque MP, Koelblinger P. MEK inhibition and immune responses in advanced melanoma. Oncoimmunology. 2017;6(8):e1335843. doi:10.1080/2162402X.2017.1335843.
- Boshuizen J, Koopman LA, Krijgsman O, Shahrabi A, Van Den Heuvel EG, Ligtenberg MA, Vredevoogd DW, Kemper K, Kuilman T, Song JY, et al. Cooperative targeting of melanoma heterogeneity with an AXL antibody-drug conjugate and BRAF/MEK inhibitors. Nat Med. 2018;24(2):203–212. doi:10.1038/nm.4472.
