ABSTRACT
After a case report of profound clinical response in a melanoma patient following treatment with an immune checkpoint inhibitor (ICI) and RANK-ligand inhibitor denosumab, we identified similar patients from electronic health records (EHR) and described patient characteristics and outcomes.
This 2017 observational study used Flatiron Health’s EHR database from ~255 US cancer clinics. Included were advanced melanoma or non-small-cell lung cancer (NSCLC) patients who received denosumab within 30 days of CTLA-4 (ipilimumab) or PD1 (pembrolizumab, nivolumab) inhibitors start with a minimum of 6 months of follow up. Real-world tumor response (rwTR) was analyzed for scans available up to 30 days after concomitant therapy. Preclinical experiments evaluated sequencing of ICI, denosumab vs monotherapy or control.
Melanoma (n = 66) patients received concomitant denosumab/ICI for a mean 4.0 months, 3.1 months for NSCLC (n = 241). Two-thirds of patients had best rwTR evaluable (complete [CR], partial response [PR], stable disease [SD], or disease progression [PD]). Longer mean duration of concomitant exposure was associated with overall response rate (ORR; CR+PR) in melanoma (p = 0.0172), NSCLC (p < .0001), and combined cohorts (p < .0001). The disease control rate (ORR plus SD) was 56% amongst melanoma patients and 58% amongst NSCLC patients. Longer concomitant therapy was associated with increased overall survival, primarily in NSCLC (p < .0001). Preclinical data suggest that ICI initiated before or at same time as denosumab was optimal.
Results provide proof-of-concept that rwTR is associated with concomitant denosumab/ICI. Crude survival analyses supported the association of concomitant therapy and improved outcomes outside of clinical trials and warrant comparative study.
Introduction
Immune system recognition and suppression of malignant cell growth is not a new conceptCitation1, but is drawing great interest in oncology drug development. In November 2017, approximately 650 studies in Clinicaltrials.gov were enrolling patients for trials targeting the programmed death 1 (PD-1) pathway or one of its ligands (PD-L1), compared with 215 such studies listed in November 2015.Citation2 Clinical trials of monoclonal antibodies (mAbs) targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4) and PD-(L)1 have produced remarkable and durable clinical responses leading to a paradigm shift in the treatment of advanced-stage cancers, and to regulatory approvals of these agents for the treatment of several malignancies.Citation3–Citation10 Immune-checkpoint inhibitors (ICI) CTLA-4 and PD-1 are T-cell surface receptors that produce immune inhibition at discrete steps in a T-cell response that, if unregulated, can damage the host. These inhibitory processes are up-regulated in many tumors and mAbs reactive with CTLA-4 and PD-1, which block distinct immune-inhibitory receptors on activated T cells, amplify anti-tumor immunity.
Novel immunotherapy-based combinations, particularly those that impinge on non-redundant immunosuppressive mechanisms, may improve clinical responses in advanced melanoma. For example, in October 2014 Smyth et al.Citation11 reported a 39-year-old woman with metastatic melanoma (BRAF wildtype) who experienced a profound response after concurrent treatment with a CTLA-4 reactive mAb (ipilimumab) and the receptor-activator of nuclear kappaB ligand (RANKL) – specific mAb, denosumab. Another case report demonstrated complete response (CR) in a patient with metastatic melanoma using this combination.Citation12 And, a recent study in Germany reported that six of ten cases treated with ipilimumab/denosumab had CR or partial response (PR), two with a long-lasting stabilization and two with progression of their disease.Citation13 Furthermore, preclinical data has demonstrated a combination effect of RANKL blockade with anti-CTLA-4, anti-PD-(L)1 mAbs in melanoma, prostate cancer and colon adenocarcinoma.Citation14,Citation15 Mechanistically, the addition of a RANKL-specific mAb to ICI mAbs increased T-cell effector function and increased CD8+ T-cell infiltration, leading to increased anti-tumor immunity.Citation14 Denosumab is routinely prescribed for advanced solid tumors patients with bone metastases or with hypercalcemia, therefore some patients may already receive denosumab in combination with an immunotherapy agent with no reported safety concerns.Citation11–Citation13 The potential additive benefit of using RANKL inhibition in combination with PD-(L)1 or CTLA-4 blockade, however, has not been formally assessed in clinical trials.
Here, we provide real-world evidence extending beyond published case studies examining multiple ICI (ipilimumab, nivolumab, pembrolizumab) in two tumor types (advanced melanoma, metastatic non-small cell lung cancer [NSCLC]), and measuring patient outcomes. Specific objectives were: (1) to describe patient demographic, clinical, and treatment characteristics; and (2) to measure clinical response and overall survival in these patient cohorts. In addition, the combined effectiveness and sequence of RANKL blockade and PD-1 inhibition was tested in a preclinical lung cancer model. Together these data describe in greater detail how patients may benefit from ICI immunotherapy combined with denosumab. This work should be considered hypothesis generating and may be used to inform the design of future observational and randomized human studies with comparator arms to more formally evaluate the potential for using electronic health records data to evaluate real-world treatment response and to more definitively quantify the comparative effectiveness of treatment with concomitant ICI and denosumab versus ICI alone.
Results
Concomitant exposure of denosumab and CTLA-4 (ipilimumab) and/or PD-1 (nivolumab, pembrolizumab) inhibitors identified 66 patients diagnosed with advanced malignant melanoma and 241 individuals with metastatic NSCLC (Supplemental Figure 1). Mean age among melanoma patients was 64.6 years, 34.8% were female, and 42 (63.6%) received ipilimumab, 22 (33.3%) nivolumab, and 13 (19.7%) pembrolizumab (9 [13.6%] received ipilimumab/nivolumab; 2 [3.0%] ipilimumab/pembrolizumab combination) concomitantly with denosumab – 71.2% as part of first-line therapy (). Among NSCLC, the mean age was 68.3 years, 48.1% female, 63.1% non-squamous histology, one patient received ipilimumab, 235 (98%) received nivolumab and 6 pembrolizumab – 21.6% as first-line therapy for advanced disease (). Over 80% of NSCLC patients had previously received chemotherapy (Supplementary Tables S1A, S1B). Nine percent of melanoma patients had bone only metastases, whereas 30.3% of NSCLC patients had metastatic disease limited to the bone. Eighty-eight percent of NSCLC patients had a history of smoking, and only 14.1% were tested for PD-L1 expression – 54.3% among those tested were deemed positive for PD-L1 expression ().
Table 1. Demographic characteristics of advanced melanoma and NSCLC cohorts (response data set).
Best response populations (i.e., patients with a response assessment no later than 30 days after the final dose of either concomitant therapy) included 44 (66.7%) melanoma and 166 NSCLC (66.9%) patients, with the following results in these populations. The mean age of patients included in the best response populations was similar to that of excluded patients, although males comprised a slightly larger proportion of excluded patients in both melanoma and NSCLC cohorts. Time since diagnosis was similar for included and excluded patients. Compared with included patients, excluded melanoma patients were less likely to have advanced (Stage 3 or 4) disease at diagnosis, while excluded NSCLC patients were more likely to have advanced disease at diagnosis compared to their included counterparts primarily due to a larger proportion of patients with Stage 3 disease. In the two disease cohorts, the proportions of patients with both bone and visceral metastases was greater among patients excluded from the best response populations than among the included patients. The mean duration (standard deviation [SD]) of concomitant exposure was 161.0 (178.3) days for melanoma patients who were included in the best response population versus 33.6 (21.5) days for excluded patients. Among NSCLC patients in the best response population, the mean duration of concomitant exposure was 126.0 (97.6) days compared with 25.8 (27.8) days in the excluded population.
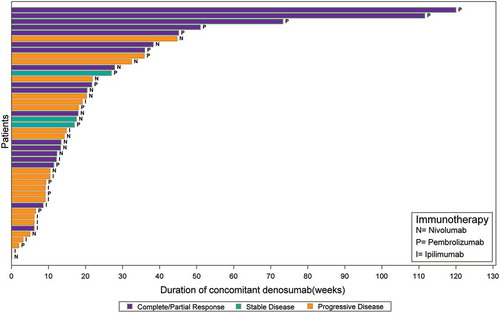
In the best response populations, the mean number (SD) of imaging assessments was 3.4 (2.6) for melanoma and 2.5 (1.6) for NSCLC patients. Seventy-three percent (32 of 44) of (best response) melanoma patients received anti-PD1 mAb and the remainder received anti-CTLA4 mAb concomitantly with denosumab (there were no instances of anti-PD1 combined with anti-CTLA4 mAbs). The median (interquartile range) follow-up for best response among melanoma patients was 6.59 (2.36;12.1) months, and 7 (16%) had 50 weeks or more of concomitant exposure (5 [71%] with PR/CR) (). Among 24 (55%) melanoma patients with 24 weeks or more of concomitant exposure, 13 (54%) had CR/PR. The overall response rate (ORR; CR plus PR) was 41% in all melanoma patients, 47% among those treated with anti-PD1, and 25% with anti-CTLA4 (). The disease control rate (DCR; ORR plus SD) was 56% among melanoma patients treated with anti-PD1. In NSCLC, all 166 best response patients had received anti-PD1 mAb concomitant with denosumab, associated with an ORR of 33% and DCR of 58% (). Fourteen (8%) patients provided more than 50 weeks of concomitant exposure (9 [64%] with PR/CR) (). Among 69 (42%) NSCLC patients with 24 weeks or more of concomitant exposure, 42 (61%) had CR/PR.
Table 2. Clinical response evaluated among melanoma and NSCLC patients treated concomitantly with denosumab and immune checkpoint inhibitors.
Figure 1. A Clinical response among advanced melanoma patients by duration of concomitant treatment with denosumab and immune checkpoint inhibitors (n = 44). B Clinical response among advanced NSCLC cohort by duration of concomitant treatment with denosumab and immune checkpoint inhibitors (n = 166).
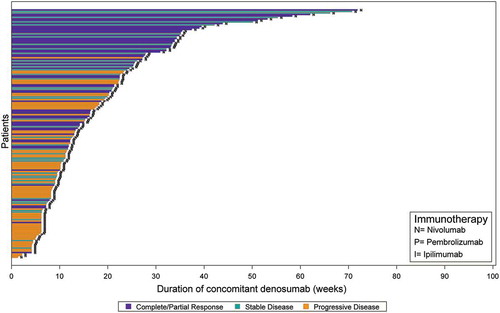
A statistically significant association was observed between concomitant therapy duration and response for melanoma (p = 0.0172), NSCLC (p < .0001) and combined cohorts (p < .0001) (ANOVA). For NSCLC and combined cohorts, better response was associated with longer duration of concomitant therapy with discrete tertile duration categories (p < .0001); statistical significance level was lost in the analysis of melanoma patients as the cell sizes were considerably reduced (p = 0.0621) (Mantel-Haenszel Chi-square). The association was consistent in analyses of first-line only; majority of best responses were observed during first- or second-line with follow up.
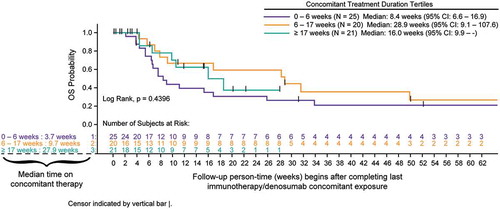
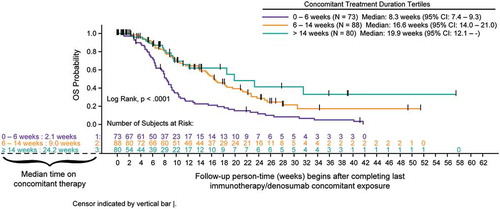
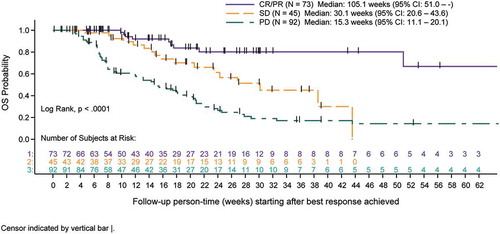
Survival analyses using Kaplan-Meier methods and tertile categories for duration of concomitant therapy indicated increased overall survival (OS) following completion of concomitant therapy with longer duration of concomitant use primarily in the NSCLC cohort (p < .0001). Any association of treatment duration with survival was not as clear in melanoma patients due to smaller sample size of each tertile ( and ). In an exploratory combined cohort analysis, categories of response show that CR/PR best response was associated with longer survival (log-rank; p < .0001) ().
Figure 2. A Overall survival for advanced melanoma cohort measured after completion of concomitant treatment duration (tertiles) of denosumab and immune checkpoint inhibitors (full analysis set). B Overall survival for advanced NSCLC cohort measured after completion of concomitant treatment duration (tertiles) of denosumab and immune checkpoint inhibitors (full analysis set). C Overall survival for advanced melanoma and NSCLC cohorts measured after completion of combined by best response category achieved with concomitant denosumab and immune checkpoint inhibitors (best response set).
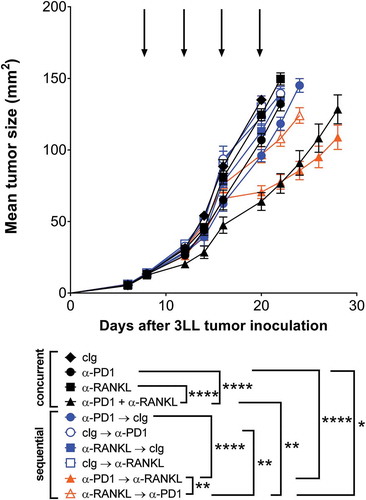
In the preclinical setting, we tested the anti-tumor efficacy of these combinations in the mouse 3LL lung adenocarcinoma model given the observations in NSCLC patients receiving denosumab and ICI. These experiments were designed to complement the observational research, and to address the optimal sequence of mAb therapy. We compared the tumor growth suppressive activity of concurrent mAb therapy with equivalent total dose of mAbs given as sequential therapy. Preclinical data show combination anti-RANKL and anti-PD-1 mAbs were superior in efficacy to either monotherapy or control Ig, irrespective of whether therapies were given concurrently or sequentially (). Sequencing anti-PD-1 therapy prior to anti-RANKL treatment led to a significantly greater reduction in tumor volume as compared with anti-RANKL treatment prior to anti-PD-1 therapy (p < 0.01) ().
Figure 3. Optimal anti-tumor efficacy of anti-PD1 and anti-RANKL is affected by sequencing of antibody administration. Groups of C57Bl/6 wild type (WT) mice (n = 10/group) were injected s.c with 5 × 10Citation5 3LL lung carcinoma cells. For concurrent treatment groups (black symbols), mice were treated i.p. on days 8, 12, 16 and 20, relative to tumor inoculation (as indicated by arrows) with cIg (1–1, 100 µg), anti-PD1 (RMP1–14, 100 µg) and/or anti-RANKL (IK22/5, 100 µg) as indicated. For sequential treatment groups (colored symbols), where treatment order is indicated in figure legend, mice were treated i.p. on days 8 and 12 (first antibody) and days 16 and 20 (second antibody) respectively (relative to tumor inoculation) with cIg (1-1, 200 µg), anti-PD1 (RMP1-14, 200 µg) and/or anti-RANKL (IK22/5, 200 µg) as indicated. Mean ± SEM tumor size is shown for each treatment group. Statistical differences between groups at day 22 were determined by one-way ANOVA with Tukey’s post-test analysis, and key comparisons are shown (*p < 0.05, **p < 0.01, ****p < 0.0001). Two independent experiments have been pooled.
Discussion
Building on published case studies of melanoma patients with remarkable clinical responses when treated with anti-RANKL and anti-CTLA-4 mAbs in combination,Citation11–Citation13 we examined response in a larger series of patients with either advanced melanoma or metastatic NSCLC treated with concomitant ICI and denosumab. We observed that PR/CR were associated with longer duration of concomitant use of immunotherapy and denosumab. Crude survival analyses supported associations between concomitant therapy duration and improved response, as well as association of PR/CR with survival. Recognizing the limitations of this single-arm, descriptive, observational study, these results provide encouraging proof-of-concept that warrant further investigations.
Skeletal metastases are generally considered a negative prognostic factor in solid organ malignancies. In NSCLC and melanoma, bone metastases and/or skeletal-related events (SREs) are associated with lower overall survival.Citation16–Citation19 Taking these factors into account, the clinical responses observed in this real-world study of patients with skeletal metastases compare favorably with outcome measures (ORR and DCR) reported in pivotal trials of ICI (, ). Most strikingly, the ORR in advanced NSCLC patients treated concurrently with denosumab and anti-PD1 mAb was 33.1%. Other notable characteristics of the study cohort are a preponderance of non-squamous histology (typical of NSCLC), and a relatively high level of prior exposure to chemotherapy (>80%). Published response rates in advanced NSCLC (not selected by PD-L1 expression) to anti-PD1 mAb monotherapy range from 18–20% for second line treatment to 23–24.8% for first-line treatment ().Citation7,Citation8,Citation20,Citation21 Importantly, although tumor PD-L1 expression was infrequently assessed in this real-world cohort, the proportion staining positive was comparable to that recorded in these clinical trials; where patients were selected on the basis of PD-L1 positivity (CheckMate 026, KEYNOTE-024), response rates were strikingly higher ().
Table 3A. Comparison of objective response rates from anti-PD-1 pivotal clinical trials in NSCLC.
Table 3B. Comparison of anti-PD1 pivotal clinical trials in melanoma.
The ORR among melanoma patients treated with concurrent anti-PD1 mAb (mostly first line) and denosumab in our real-world study was 46.9%, which is encouraging when compared with ORR reported in trials utilizing anti-PD1 mAb first-line monotherapy or immunotherapy-naive patients, which range from 33.7% to 45% (). In contrast, among melanoma patients in our study who received ipilimumab concurrently with denosumab, 25% achieved ORR, while published phase 2–3 clinical trials for first line ipilimumab in melanoma reported ORR in the range of 11–19%.Citation4,Citation22,Citation23
An important caveat when interpreting these rwTR data is the use of response criteria in the EHR dataset, whereby a pragmatic real-world modification of RECIST was applied in a time-point fashion. This is likely less stringent in comparison with response assessment used in prospective clinical trials; on the other hand, assessment intervals for response in routine care were likely less structured and perhaps more infrequent than in the trial setting. A practical consequence of this is that some patients designated to have achieved PR in the EHR cohort may have achieved SD per RECIST. Reassuringly, when an alternative composite outcome measure was compared (DCR, which includes the category of SD in addition to responders), the EHR cohort similarly compared favorably to the published prospective trials (,).
Real-world studies can play a key role in identifying predictors of response, nonresponse and resistance, so that therapies are prescribed for the right patient (maximizing benefit), and avoiding the wrong patient that is unlikely to benefit from therapy (minimizing risk). These studies also have the potential to inform novel drug targets, validate efficacy (effectiveness) of a medicinal product, and possibly reduce the costs and timelines associated with drug development. With the 21st Century Cures Act, the US Food and Drug Administration (FDA) has recognized the value of real-world evidence and is developing a regulatory framework to use these data to support new indications for approved drugs or to satisfy post-approval study requirements.
While prospective clinical trial results provide informative context for observational real-world studies which analyze data obtained in the routine care setting, it is necessary to consider critical differences between these two types of research. Clinical trial populations are selected using stringent selection criteria that exclude many patients in the community who are represented in real world studies, thereby dampening magnitudes of effect with respect to clinical response and survival outcomes. This phenomenon is described in the epidemiology literature and confirmed by a recently published analysis of pembrolizumab-treated melanoma patients from community oncology clinics,Citation24 where authors specifically noted that characteristics often used as exclusion from clinical trials – brain metastases, elevated LDH, poor performance status (ECOG PS>1), and third-line or later PD-1 therapy – were associated with worse patient outcomes. Therapeutic exposures also differ, as indicated by the fact that 70% of patients in that particular study discontinued pembrolizumab during the study period. A key strength of real-world evidence is the ability to complement clinical trial data by providing insights into the performance of therapies in a more diverse (older ages, greater disease progression/burden) patient population. In fact, a recent studyCitation25 comparing characteristics of patients with NSCLC treated with PD-1 inhibitors in clinical trial cohorts to those in the real-world during the first year after US regulatory approval found that real-world patients were older at treatment initiation and were more likely to have had smoking history relative to clinical trial cohorts.
Therefore, when comparing real-world to clinical trial data, these population differences could result in bias – known as confounding by indication – such that treatments, particularly newly approved therapies, may look less effective and less safe than described in clinical trials because they are given to sicker individuals from the “real world”. Despite this potential bias – and recognizing that physicians do not record treatment responses in EHR consistent with RECIST as well as other limitations around timing, quality, and availability of tumor response assessmentsCitation26 – the observed tumor response rates in our study compare favorably to those reported in clinical trials. We note that while it is possible that real world response is underestimated our results compare well with single institution reports of real world non-concomitant use of anti-PD1 for NSCLC (Supplementary Table S2A).Citation27–Citation30 In addition, although the number of patients with advanced melanoma in this study who received ipilimumab concurrently with denosumab is limited, the ORR is markedly higher than those real-world outcomes reported with ipilimumab alone (Supplementary Table S2B).Citation31–Citation36
The precise cellular source of RANKL necessary for anti-tumor activity of RANKL blockade may include infiltrating lymphocytes, lymph nodes or tumor cells themselves, and further analysis of potential mechanisms behind the observed favorable rwTR are warranted.Citation37 In NSCLC, a population where an exploratory post-hoc analysis of phase 3 clinical trial data reported improved survival with denosumab,Citation38 RANKL is observed not only in infiltrating lymphocytes, but also in tumor epithelium of 75% of adenocarcinoma samples,Citation39 and high RANKL levels are adversely associated with survival.Citation40 Detailed analysis of RANK and RANKL expression in melanoma has not been described.Citation41 While the anti-tumor efficacy observed in preclinical models was enhanced upon combination of RANKL blockade and anti-PD-1 antibody irrespective of sequence, pre-treatment with anti-PD-1 prior to anti-RANKL was superior to other sequences. We have observed that anti-PD-1 increased RANKL expression on activated T-cells in a colon carcinoma model,Citation15 suggesting that RANKL/RANK may act as a negative feedback mechanism after checkpoint inhibition. Thus, the specific sequence of denosumab administration versus ICI may improve tumor response and should be tested in clinical trials and preclinical mechanistic studies. Altogether, these findings now prompt an examination of the impact of ICI on RANKL/RANK expression and function in the tumor microenvironment in melanoma and lung cancer.
The findings of our study were intended to provide proof-of-concept, and future research should focus on: (1) a comparative effectiveness of ICI mAbs with or without concomitant denosumab (120 mg); (2) further analysis of the optimal use (combinations, duration, and sequencing) of ICI; and (3) identifying patient characteristics or biomarkers that may predict responders and guide treatment assignments.
This real-world study of substantial cohorts of metastatic melanoma and NSCLC patients treated concurrently with denosumab and immunotherapies indicates clinical responses that compare favorably with randomized clinical trials and smaller retrospective, real-world studies of ICI given as monotherapies. These results build on several case studies to suggest that RANKL blockade may enhance the activity of ICI thus, leading to improved tumor control in patients, similar to what has been described in preclinical mechanistic studies. Moreover, 42% to 55% of patients in the best response cohorts were treated concurrently with denosumab and immunotherapy for 24 weeks or longer, demonstrating extended periods of use. These data prompt further studies to verify the potential improvement of tumor response by the addition of RANKL blockade to ICI and translational studies to determine the complementary mechanisms leading to enhanced anti-tumor immunity.
Patients and methods
Study design
This is a retrospective observational cohort study of patients with a diagnosis of advanced melanoma or metastatic NSCLC who received concomitant treatment with anti-RANKL mAb (denosumab, 120 mg) plus CTLA-4 (ipilimumab) and/or PD-1 (pembrolizumab, nivolumab) inhibitory mAbs.
Data source
Data for this study were obtained from Flatiron Health’s longitudinal, demographically and geographically diverse database derived from patient-level electronic health records (EHR), agnostic to EHR system. At the time of this project, the database drew from ~255 cancer clinics from across the US (1.7 million patients), representing mainly community-based oncology practices. Records include diagnostic and treatment details captured during routine clinical care, either as structured data (diagnostic details, laboratory values, prescribed drugs) or unstructured data (scanned reports, physician notes). Patient-level data were processed and harmonized centrally by Flatiron Health, and stored in a secure, HIPAA-compliant manner. Unstructured data processing utilized technology-enabled abstraction, described elsewhere.Citation42 Institutional Review Board approval of the study protocol was obtained prior to study conduct and informed patient consent was waived. Data provided for the study were de-identified with provisions in place to prevent re-identification to protect patients’ confidentiality.
Patients
Two distinct cohorts were selected: advanced melanoma or metastatic NSCLC (Supplemental Figure 1). Inclusion criteria from structured data were: confirmed diagnosis via ICD codes (melanoma ICD-9 172.x or ICD-10 C43.x or D03.x; and NSCLC ICD-9 162.x or ICD-10 C34.x or C39.9), two visits on or after January 1, 2011, and co-administration of immunotherapy (ipilimumab, nivolumab, or pembrolizumab) and denosumab. Concomitant therapy was defined as receipt of denosumab at any point prior to ICI initiation, or no later than 30 days following ICI initiated at least 6 months before the data cutoff (November 30, 2016), and no later than second line of therapy. Clinical abstractors confirmed cutaneous melanoma or NSCLC (and advanced disease), defined as stage III or IV melanoma or stage IIIB or IV NSCLC at initial diagnosis, or recurrent disease following an earlier stage diagnosis.
“Real-world” endpoints
Tumor response was extracted from documents in the EHR from routine clinical care. This “real world” tumor response (rwTR) variable was generated by trained abstractors using a predefined process to identify imaging assessments, review associated clinical documentation, and report the treating clinician’s interpretation of the imaging. RECIST criteria were adapted for real-world application, with each imaging assessment mapped to one of the following categories: CR, PR, stable disease (SD), progressive disease (PD), pseudo-progression, indeterminate response, and not documented. In contrast to RECIST criteria, PR was defined as any reduction in visible disease on imaging short of a CR.
Mortality data was generated by supplementing structured EHR death data with unstructured EHR and external commercial data. The quality of this derived mortality data has been assessed for a large cohort by measuring sensitivity, specificity, positive and negative predictive values, and date agreement, against the highest-completeness US mortality data, the National Death Index (NDI); sensitivity ranged from 76–83% and specificity ranged from 96–99% (unpublished data).
Preclinical examination of anti-pd1 and anti-RANKL
C57BL/6 wild-type (WT) mice were bred-in-house at the Queensland Institute of Medical Research Berghofer (QIMRB). All experiments were approved by the QIMRB Animal Ethics Committee. The mouse lung carcinoma cell line 3LL (kindly provided by Dr. Robert Wiltrout, NCI Frederick, MD), was injected, maintained and monitored as previously described.Citation43 Purified anti-mouse anti-RANKL (IK22-5; rat IgG2a) and control mAbs (1–1, rat IgG2a) were purchased from BioXcell (West Lebanon, NH). Anti-PD1 (RMP1-14, rat IgG2a) was purchased from Leinco (St Louis, MO). Digital calipers were used to measure the perpendicular diameters of the tumors. The tumor size was calculated as the product of the two measurements and is presented as mean ± SEM.
Analysis
Descriptive analyses are provided. Significance testing was limited to comparisons between discrete groups using one-way ANOVA for continuous variables, or Chi-square test for binary variables and Mantel-Haenszel Chi-Square for multiple categories. Analyses performed on rwTR data used response assessments no later than 30 days after the final dose of either concomitant therapy. These resulting data were analyzed to derive the mean number of imaging assessments and best response. Overall response rate was calculated based on the number of patients with either a CR or PR as their best response.
Survival analyses were based on Kaplan-Meier methods with log-rank for significance testing. For comparison of treatment groups in preclinical sequencing experiment, one-way ANOVA was performed, with Tukey’s post-test for multiple comparisons.
Disclosure of interest
A. Liede and R.K. Hernandez are employees and stockholders of Amgen Inc. S. Wade is employed by Wade Outcomes Research and Consulting which has received remuneration from Amgen Inc. R. Bo has no financial interests to disclose. N.C. Nussbaum is an employee and stockholder of Flatiron Health, which is an independent subsidiary of the Roche Group. E. Ahern has received a speaker’s honorarium from Amgen Australia and travel accommodations/expenses from MSD Australia. W.C. Dougall has received a speaker’s honorarium from Amgen and research funding from Bristol Myers Squibb. M.J. Smyth has research agreements with and has consulted to Bristol Myers Squibb, Aduro Biotech, Corvus Pharmaceuticals, and Tizona Therapeutics, consulted to Arcus Biosciences, provided expert testimony to Corvus Pharmaceuticals, and holds patents or intellectual property rights with QIMR Berghofer and Bristol Myers Squibb.
Supplemental Material
Download PDF (514.8 KB)Supplemental Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Schreiber RD, Old LJ, Smyth MJ. 2011. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331:1565–1570. doi:10.1126/science.1203486.
- ClinicalTrials.gov. [Internet]. Bethesda (MD): National Library of Medicine (US). Search terms “PD-1” “PD-L1” combined; recruiting and enrolling studies. http://clinicaltrials.gov/ ( Accessed 27 Nov 2017)
- Schachter J, Ribas A, Long GV, Arane A, Grob J-J, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al. 2017. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 390:1853–1862. doi:10.1016/S0140-6736(17)31601-X.
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey L, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. 2015. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:23–34. doi:10.1056/NEJMoa1504030.
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al. 2015. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372:320–330. doi:10.1056/NEJMoa1412082.
- Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, Poddubskaya E, Borghaei H, Felip E, Paz-Ares L, et al. 2017. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol JCO2017743062. doi:10.1200/JCO.2017.74.3062.
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. 2015. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639. doi:10.1056/NEJMoa1507643.
- Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, Antonia S, Pluzanski A, M.D., Vokes EE, Holgado E, et al. 2015. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123–135. doi:10.1056/NEJMoa1504627.
- Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, et al. 2016. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 17:1497–1508. doi:10.1016/S1470-2045(16)30498-3.
- Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al. 2016. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387:1540–1550. doi:10.1016/S0140-6736(15)01281-7.
- Smyth MJ, Yagita H. 2016. McArthur GA: combination anti-CTLA-4 and anti-RANKL in metastatic melanoma. J Clin Oncol 34:e104–e106. doi:10.1200/JCO.2013.51.3572.
- Bostwick AD, Salama AK, Hanks BA. 2015.Rapid complete response of metastatic melanoma in a patient undergoing ipilimumab immunotherapy in the setting of active ulcerative colitis. J Immunother Cancer 3:19.
- Angela Y, Tolk H, Oberndörfer F, et al.: Kombination von denosumab und checkpoin inhibitoren - eine retrospektive analyse von 10 patienten mit metastasierenden melanom und knochenmetastasen. Presented at the Deutscher Hautkrebskongress (German Skin Cancer Congress) - Arbeitsgemeinschaft Dermatologische Onkologie (ADO), Mainz, Germany, 2017
- Ahern E, Harjunpaa H, Barkauskas D, Allen S, Takeda K, Yagita H, Wyld D, Dougall WC, Teng MWL, Smyth MJ. 2017. Co-administration of RANKL and CTLA4 antibodies enhances lymphocyte-mediated antitumor immunity in mice. Clin Cancer Res 23:5789–5801. doi:10.1158/1078-0432.CCR-17-0606.
- Ahern E, Harjunpaa H, O’Donnell JS, Allen S, Dougall WC, Teng MWL, Smyth MJ. 2018. RANKL blockade improves efficacy of PD1-PD-L1 blockade or dual PD1-PD-L1 and CTLA4 blockade in mouse models of cancer. OncoImmunology. doi:10.1080/2162402X.2018.1431088.
- Barth A, Wanek LA, Morton DL. 1995.Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg 181:193–201.
- Hsu F, De Caluwe A, Anderson D, Nichol A, Toriumi T, Ho C. 2017. Patterns of spread and prognostic implications of lung cancer metastasis in an era of driver mutations. Curr Oncol 24:228–233. doi:10.3747/co.24.3496.
- Kuchuk M, Addison CL, Clemons M, Kuchuk I, Wheatley-Price P. 2013. Incidence and consequences of bone metastases in lung cancer patients. J Bone Oncol 2:22–29. doi:10.1016/j.jbo.2012.12.004.
- Tsuya A, Kurata T, Tamura K, Fukuoka M. 2007. Skeletal metastases in non-small cell lung cancer: a retrospective study. Lung Cancer 57:229–232. doi:10.1016/j.lungcan.2007.03.013.
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L. 2015. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372:2018–2028. doi:10.1056/NEJMoa1501824.
- Gettinger S, Rizvi NA, Chow LQ, Borghaei H, Brahmer J, Ready N, Gerber DE, Shepherd FA, Antonia S, Goldman JW, et al. 2016. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 34:2980–2987. doi:10.1200/JCO.2016.66.9929.
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. 2010. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723. doi:10.1056/NEJMoa1003466.
- Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, M.D., McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, et al. 2015. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 372:2006–2017. doi:10.1056/NEJMoa1414428.
- Cowey CL, Liu FX, Black-Shinn J, Stevinson K, Boyd M, Frytak JR, Ebbinghaus SW. 2018. Pembrolizumab utilization and outcomes for advanced melanoma in US community oncology practices. J Immunother 41:86–95.
- Khozin S, Abernethy AP, Nussbaum NC, Zhi J, Curtis MD, Tucker M, Lee SE, Light DE, Gossai A, Sorg RA, et al. 2018. Characteristics of real-world metastatic non-small cell lung cancer patients treated with nivolumab and pembrolizumab during the year following approval. Oncologist doi:10.1634/theoncologist.2017-0353.
- Cowey CL, Mahnke L, Espirito J, Helwig C, Oksen D, Bharmal M. 2017. Real-world treatment outcomes in patients with metastatic Merkel cell carcinoma treated with chemotherapy in the USA. Future Oncol 13:1699–1710. doi:10.2217/fon-2017-0187.
- Hida T, Nishio M, Nogami N, Ohe Y, Nokihara H, Sakai H, Satouchi M, Nakagawa K, Takenoyama M, Isobe H 2017. Efficacy and safety of nivolumab in Japanese patients with advanced or recurrent squamous non-small cell lung cancer. Cancer Sci 108:1000–1006. doi:10.1111/cas.13225.
- Tournoy KG, Thomeer M, Germonpre P, Derijcke S, De Pauw R, Galdermans D, Govaert K, Govaerts E, Schildermans R, Declercq I, et al. 2018. Does nivolumab for progressed metastatic lung cancer fulfill its promises? An efficacy and safety analysis in 20 general hospitals. Lung Cancer 115:49–55. doi:10.1016/j.lungcan.2017.11.008.
- Kobayashi K, Nakachi I, Naoki K, Satomi R, Nakamura M, Inoue T, Tateno H, Sakamaki F, Sayama K, Terashima T, et al. 2018. Real-world efficacy and safety of nivolumab for advanced non-small-cell lung cancer: a retrospective multicenter analysis. Clin Lung Cancer 19:e349–e358. doi:10.1016/j.cllc.2018.01.001.
- Dudnik E, Moskovitz M, Wollner M, Zer A, Bar J, Agbarya A, Idan T, Shechtman Y, Amna MA, Peled N. 2016. Anti-PD-1 antibodies in non-small cell lung cancer (NSCLC): the real-life setting experience (181P). J Thorac Oncol 11:S57–S166. doi:10.1016/S1556-0864(16)30291-X.
- Mohr P, Ascierto P, Arance A, Ascierto PA, Banos A, Kaskel P, Shinde R, Stevinson K. 2017. Real-world treatment patterns and outcomes among metastatic cutaneous melanoma patients treated with ipilimumab. J Eur Acad Dermatol Venereol
- Middleton MR, Dalle S, Claveau J, Mut P, Hallmeyer S, Plantin P, Highley M, Kotapati S, Kim T, Brokaw LJ, et al. 2016. Real-world treatment practice in patients with advanced melanoma in the era before ipilimumab: results from the IMAGE study. Cancer Med 5:1436–1443. doi:10.1002/cam4.717.
- Ahmad SS, Qian W, Ellis S, Mason E, Khattak M, Gupta A, Shaw H, Quinton A, Kovarikova J, Thillai K, et al. 2015. Ipilimumab in the real world: the UK expanded access programme experience in previously treated advanced melanoma patients. Melanoma Res 25:432–442. doi:10.1097/CMR.0000000000000185.
- Gallagher PF, Stewart J, Carser JE, et al. 2017. Ipilimumab for advanced melanoma in the real world: the Northern Ireland experience (22P). ESMO immuno-oncology congress. Ann Oncol 28:xi6–xi29. doi:10.1093/annonc/mdx711/2017.
- Flynn AE, Day FL, Geyde CA, et al. ‘Real world’ pembrolizumab in metastatic melanoma (#226), Joint 2016 COSA and ANZBCTG Annual Scientific Meeting. Queensland, Australia, 2016
- Jansen Y, Rozeman EA, Foppen MG, Bastholt L, Schmidt H, Van Thienen JV, Haanen JBAG, Tiainen L, Svane IM, Mäkelä SP, et al. 2017. Real life outcome of advanced melanoma patients who discontinue pembrolizumab (PEMBRO) in the absence of disease progression. J Clin Oncol 35:9539.
- Dougall WC. 2012. Molecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin Cancer Res 18:326–335. doi:10.1158/1078-0432.CCR-10-2507.
- Scagliotti GV, Hirsh V, Siena S, Henry DH, Woll PJ, Manegold C, Solal-Celigny P, Rodriguez G, Krzakowski M, Mehta ND, et al. 2012. Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: subgroup analysis from a randomized phase 3 study. J Thorac Oncol 7:1823–1829. doi:10.1097/JTO.0b013e31826aec2b.
- Branstetter D RANK and RANK Ligand (RANKL) expression in primary human lung cancer (ID 870). Presented at the 15th World Conference on Lung Cancer, Sydney, Australia, 2013
- Rao S, Sigl V, Wimmer RA, Novatchkova M, Jais A, Wagner G, Handschuh S, Uribesalgo I, Hagelkruys A, Kozieradzki I, et al. 2017. RANK rewires energy homeostasis in lung cancer cells and drives primary lung cancer. Genes Dev 31:2099–2112. doi:10.1101/gad.304162.117.
- Kupas V, Weishaupt C, Siepmann D, Kaserer ML, Eickelmann M, Metze D, Luger TA, Beissert S, Loser K. 2011. RANK is expressed in metastatic melanoma and highly upregulated on melanoma-initiating cells. J Invest Dermatol 131:944–955. doi:10.1038/jid.2010.377.
- Liede A, Hernandez RK, Roth M, Calkins G, Larrabee K, Nicacio L. 2015. Validation of international classification of diseases coding for bone metastases in electronic health records using technology-enabled abstraction. Clin Epidemiol 7:441–448. doi:10.2147/CLEP.S92209.
- Takaki R, Hayakawa Y, Nelson A, Sivakumar PV, Hughes S, Smyth MJ, Lanier LL. 2005. IL-21 enhances tumor rejection through a NKG2D-dependent mechanism. J Immunol 175:2167–2173. doi:10.4049/jimmunol.175.4.2167.
- Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu T-E, Badin F, et al. 2017. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 376:2415–2426. doi:10.1056/NEJMoa1613493.
- Reck M, Rodriguez-Abreu D, Robinson AG, Hui R1, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al.2016. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823–1833.
- Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, Joshua AM, Patnaik A, Hwu WJ, Weber JS, et al. 2016. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 315:1600–1609. doi:10.1001/jama.2016.4059.
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al. 2015. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372:2521–2532. doi:10.1056/NEJMoa1503093.