ABSTRACT
Daratumumab (Dara), a human immunoglobulin G1 kappa (IgG1κ) monoclonal anti-CD38 antibody, has been approved by the U.S. Food and Drug Administration for the treatment of relapsed multiple myeloma (MM) as a single agent as well as in combination with immunomodulatory drugs (IMiDs) and proteasome inhibitors (PI). Although the scientific rationale behind the use of Dara in combination with IMiDs has been extensively explored, the molecular mechanisms underlying Dara-PI regimens have not yet been investigated. Here, we demonstrate that CD38 on the surface of MM cells is rapidly internalized after Dara treatment; we also show that Dara treatment impairs MM cell adhesion, an effect that can be rescued by using the endocytosis inhibitor Dynasore. Finally, we show that Dara potentiates bortezomib (BTZ) killing of MM cells in vitro and in vivo, independent of its function as an immune activator. In conclusion, our data show that Dara impairs MM cell adhesion, which results in an increased sensitivity of MM to proteasome inhibition.
Introduction
Multiple myeloma (MM) is a hematologic malignancy in which plasma cells (PCs) tend to proliferate and accumulate in the bone marrow (BM).Citation1,Citation2 MM is the second most common hematologic disease, affecting approximately 83,000 US citizens, with 30,330 new cases diagnosed per year in the country.Citation3 Although novel therapies have improved the outcome of MM patients, the existence of several malignant clones with intraclonal heterogeneityCitation4,Citation5 has now provided the scientific rationale for using combinations of novel therapies to overcome mechanisms of resistance and clonal evolution.Citation6,Citation7
Daratumumab (Dara) is a human anti-CD38 IgG1 (ĸ subclass) antibody against the receptor CD38, which is highly expressed on the surface of MM cells.Citation8 CD38 participates in a number of enzymatic activities, including breaking down extracellular nicotinamide adenine dinucleotide (NAD+)Citation9 and regulating calcium homeostasis,Citation10 which influences immune cell functions.Citation11–Citation13 It is present in large amounts in the marrow of MM patients.Citation14 In support of its function as an ectoenzyme, it has been previously reported that a fraction of the entire CD38 surface molecule can be internalized by endocytosis in a number of leukemia- and lymphoma-derived cell lines.Citation15,Citation16 CD38 also interacts with its known ligand CD31, which is expressed on microenvironmental endothelial cells, stromal cells and macrophages.Citation17 Dara and other CD38 antibodies in turn exhibit various mechanisms, including recruitment of effector cells, removal of CD38+ immune-suppressor cells, and direct apoptotic activity.Citation18
Because of its significant anti-MM activity in patients as a single agent and in combination with both immunomodulatory drugs (IMiDs) and proteasome inhibitors (PI), Dara was recently approved by the US Food and Drug Administration (FDA) for the treatment of relapsed MMCitation19–Citation23 and may soon be approved in the upfront setting.Citation24
Although the clinical benefit of IMiDs in combination with Dara can be attributed in part to improved antibody-dependent cellular cytotoxicity (ADCC), complement dependent cellular cytotoxicity (CDC), and antibody-dependent cellular phagocytosis (ADCP) activities,Citation25,Citation26 the mechanism of action in combination with a PI has not yet been investigated. It is known that adhesion of MM cells to the bone marrow (BM) microenvironment plays a pivotal role in MM cell survival and in drug resistance.Citation27,Citation28 In this work, we show that the CD38 surface receptor can be internalized in CD138+ plasma cells (MM-PCs) by antibody binding via endocytosis, leading to loss of adhesion from bone marrow stromal cells (BMSCs) thereby sensitizing MM cells to PI treatment in vitro and in vivo.
Results
CD38 is highly expressed on MM-PCs independent of the line of treatment
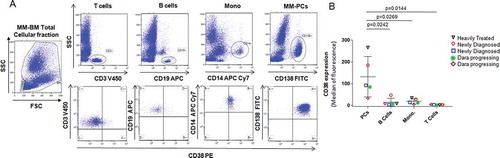
To confirm CD38 expression as a stable highly expressed surface marker for MM cells, we first analyzed different immune subsets (the CD138+ MM-PCs, CD19+ B cells, CD14+ monocyte fraction, and CD3+ T cells) in the BM cellular fraction obtained from 2 newly diagnosed untreated MM patients (ISS stage III and ISS stage I) and 3 MM patients at relapse. Out of the 3 relapsed MM patients, 1 was heavily pretreated and discontinued Dara therapy, and 2 others were not responding to Dara+IMiD combinations (,). It was observed that the MM-PCs have the highest CD38 expression compared to other immune subsets (-). The data clearly show that in all MM cases analyzed, independent of the line of treatment, CD38 is more expressed in CD138+ MM-PCs compared to its expression in CD19+ B cells (p = 0.024), CD3+ T cells (p = 0.014) and the CD14+ monocyte population (p = 0.026)().
Figure 1. MM-PCs express higher levels of CD38 compared to other immune subsets. Flow cytometric analysis showed CD38 expression in different immune subsets of the total cellular fraction isolated from BM aspirates obtained from 2 newly diagnosed and 3 relapsed MM patients. In (A) Representative gating strategy and flow analysis of total cellular BM fraction gated in T cells (CD3+), B cells (CD19+), monocytes (CD14+) and MM-PCs (CD138+); (B) Dot plot graph showing CD38 relative abundance, expressed as median of intensity of fluorescence, in MM-PCs, compared with that in the B cells, monocytes and T cells obtained from the same patient; different points linked by the same color line represent each patient. CD138+ MM-PCs have been shown to have significantly highest expression of CD38 compared to CD19+ B cells, CD3+ T cells (14 ± 4%) and the CD14+ monocyte fraction. The t-test (2 tailed, unpaired) was used to calculate CD38 expression in the different immune subsets among patients.
CD38 internalization in CD38+ MM cells
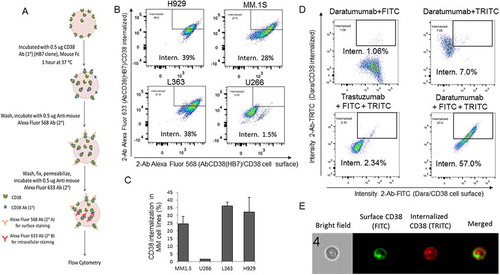
Since our data confirmed that CD38 is highly expressed in CD138+ MM-PCs, and because previously published studies have shown that the human CD38 molecule is internalized by endocytosis in a number of leukemia- and lymphoma-derived cell lines,Citation16 we decided to investigate whether CD38 on the surface of MM cells is internalized by antibody-mediated binding. MM cells were initially incubated with mouse anti-human CD38 antibody (clone HB7) and then stained with anti-mouse Fc Alexa Fluor 568 secondary antibody for extracellular staining. The cells were then washed, fixed, permeabilized and again stained with a differently colored anti-mouse Fc secondary antibody (Alexa Fluor 633) to assess the CD38 internalized fraction as schematically shown in . Cells were analyzed using imaging flow cytometry in order to visualize CD38 localization at a single cell level. Only after 1 hour of incubation, 28–39% CD38 internalization occurred in MM cells NCI-H929, MM.1S, and L363 (, and Sup. Figure 1), each cell line expressing comparably high surface levels of CD38 (Sup. Figure S2A). This phenomenon was not observed in U266 cells (,C), which express very low levels of CD38 on the surface (Sup. Figure S2B). CD38 expression in the different cell lines was also confirmed at the mRNA level (Sup. Fig. S2C); hence, the MM.1S cell line was used in the rest of the study. Interestingly, when Dara was used in the same experimental conditions in MM.1S cells, we observed almost 60% CD38/Dara complex internalization, substantially higher not only compared to a non-related clinical humanized antibody (anti-HER2, Trastuzumab, 2%) (), but also compared to the HB7 clone (28%)() which recognizes a different epitope (). Internalization was further confirmed in single cell images ().
Figure 2. CD38 is internalized into CD38+ MM cells. (A) Schematic representation of surface and intracellular staining of CD38 in MM cell lines incubated with mouse anti-human CD38 Ab. Cells were stained with Anti-mouse Fc Alexa Fluor 568 for surface staining, washed, fixed, and permeabilized, and then stained with Anti-mouse Fc Alexa Fluor 633 for intracellular anti-human CD38 Ab/CD38 complex staining. (B) Flowsight cytometric analysis (using intracellular localization application wizard) showed CD38 internalization into different MM cell lines; internalization score was calculated by IDEAS Amnis software for anti-human CD38 antibody treated cells. The gating strategy to define the internalized and the surface AbCD38/CD38 populations is shown in Sup. Figure1. Internalization score was observed to be 39%, 28%, and 38% for NCI-H929, MM.1S and L363 cells, respectively, whereas internalization was only 1.5% for U266 cells, which do not express CD38. For replicates, (C) Bar diagram showing mean CD38 internalization score in MM.1S, L363, NCI-H929 cells but not in U266 cells with low CD38 expression. (D) Flowsight cytometric analysis showed CD38/Dara internalization into MM.1S cells treated with Dara. Secondary antibodies for surface and intracellular staining were FITC conjugated and TRITC conjugated anti-human IgG, respectively. Internalization score was similarly evaluated and observed to be 57% for MM.1S cells treated with Dara. Anti-HER2 humanized antibody (Trastuzumab) was used as a control and not observed to be internalized into MM.1S cells (erb2 negative). (E) Representative images of treated cells; for improved visualization, green (FITC) and red (TRITC) colors were assigned in IDEAS software to indicate surface staining and intracellular staining, respectively. Brightfield (BF), internalization (Merged) indicate single cell with internalized CD38/Dara.
To verify that the internalization process did not require secondary antibody cross linking, we incubated MM cells for 1 hour directly with primary anti-CD38 fluorescent antibody alone (clone HB7, Ab-CD38-PE), stripped with acid to remove surface bound antibodies, and still demonstrated antibody internalization (Sup. Fig. S2D). After conjugating Dara to Alexa-Fluor 647 (DARA-AF-647), and testing its specificity of binding (Sup. Fig. S3A,B), we first blocked DARA-AF-647 active internalization using ice and assessed maximum surface signal after 120 min of incubation; minimum surface signal in the same cells was instead assessed after surface acid wash (a.w.) (Sup. Fig. S3C-D). MM cells were then incubated with DARA-AF-647 at 37°C at different time points (15–120 minutes), and the internalized fluorescence left after a.w. was compared to the minimum and maximum surface signals (Sup. Fig. S3E-F).
Because the BM niche of MM is hypoxic,Citation29 often resulting in MM-mediated pro-survival acidification of the microenvironment,Citation30 we wanted to study its effect on Dara internalization. Hence, the same internalization experiments with DARA-AF-647 were next performed in MM cells cultured in hypoxic conditions. Although we observed for each time point ~20% less DARA-AF-647 internalization under hypoxia compared to normal gas conditions, we still observed increased fluorescence uptake compared to that from Dara 120ʹ on ice+a.w (Sup. Fig. S3G). Together, these results confirmed specific CD38 complex internalization both upon direct CD38 antibody binding alone or cross-linking with secondary antibody, under normal as well as hypoxic conditions in the BM niche.
Dara is internalized in primary CD38+/CD138+ MM cells in the context of the total BM microenvironment
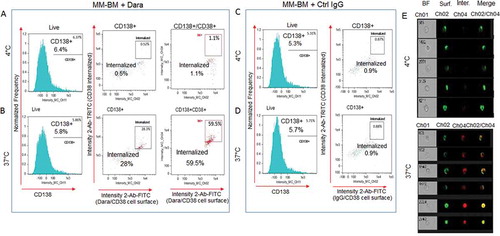
To assess whether Dara causes CD38 internalization in CD38+/CD138+ primary MM-PCs, we treated the cellular components isolated from BM aspirates of MM patients with the minimum expected concentration of Dara (100 µg/ml) that is clinically achievable in patients. Cells were then treated with FITC conjugated anti-human IgG/secondary antibody for surface staining and TRITC conjugated anti-human IgG/secondary antibody for intracellular staining. A LIVE/DEAD violet stain was used before fixation/permeabilization to include only viable cells for analyses (Sup. Fig. S4). Cells were also stained with CD138 APC to label CD138+ MM-PCs among the total cellular fraction and analyzed by imaging-flow cytometry. Our data show CD38/Dara complex internalization into the Dara-treated CD138+cell population to be almost 60% at 37°C, in contrast to 1% for those incubated at 4°C (,) or to control (Ctrl) human IgG treated cells at both temperature conditions (,). This finding was further supported by single cell flow images (). These data corroborate our findings in MM cell lines, and show that CD38 internalization may be considered an immediate consequence of Dara treatment in MM patients.
Figure 3. Dara is internalized into primary CD38+ MM cells in the context of the total bone marrow microenvironment. Total cellular fraction isolated from BM of a Dara naïve MM patient was treated with 100 µg/ml of Dara or Ctrl IgG and incubated at 37°C or at 4°C for 2 hrs. Cells were then washed with PBS1X and treated with FITC conjugated anti-human IgG for detecting surface Dara/CD38 complex, and TRITC conjugated anti-human IgG was used to evaluate Dara/CD38 or non-specific IgG/CD38 complex internalization. Cells were also stained with CD138 APC to label CD138+ MM-PCs among the total cellular fraction and analyzed by Flowsight cytometric analysis.(A-B) LIVE cells gated as in Sup. Fig. S4 were evaluated for CD138 expression. CD138+ and CD138+/CD38+ cells were evaluated for CD38 internalization after incubation with Dara at 4°C (A) and at 37°C (B); (C-D) LIVE cells gated as in Sup. Fig. S4 were evaluated for CD138 expression. CD138+ cells were evaluated for CD38 internalization after incubation with Ctrl IgG at 4°C (C) and at 37°C(D); (E) Representative images of cells gated in the B panels as Intern.+ showing bright field (CH01), Dara/CD38 complex on the membrane (CH02), internalized Dara/CD38 complex (CH04) and then merged images from both channels. For improved visualization, green (FITC) and red (TRITC) colors were assigned in IDEAS software to indicate surface staining and intracellular staining, respectively.
Dara impairs MM cell adhesion to bone marrow stromal cells
Besides being a highly expressed surface marker, CD38 is a glycoprotein that also functions in cell adhesion and calcium signaling.Citation31,Citation32 Little is known about the involvement of CD38 in the localization of MM-PCs in the BM environment. Adhesion experiments were performed using HS-5 BM stromal cells (BMSCs) to study the role of the CD38 molecule in PC adhesion. Quantitative binding of MM.1S GFP+/Luc+ cells to HS-5 was significantly lower when cells were treated with Dara (100 µg/ml) compared to untreated cells or cells treated with a non-related humanized antibody (anti-HER2 antibody, Trastuzumab) (,). To understand whether the endocytic machinery could play a role in CD38 internalization upon direct Dara binding, we performed immunofluorescence (IF) staining and assessed the distribution of Lysosome associated membrane protein (LAMP-1), which plays a pivotal role in the transport of extracellular cargo.Citation33,Citation34 To assess CD38 expression upon Dara binding, we used a multi-epitope anti-CD38 antibody (Cytognos) that binds to CD38 also in presence of Dara (Sup. Figure 5A,B). Our data show that LAMP-1 mobilized to the membrane upon Dara binding, an effect that was not seen when human IgG was used (,). Hence, to assess whether internalization plays a role in MM cell adhesion, we blocked endocytosis using the Dynamin I inhibitor Dynasore.Citation35 Our data show that, after 24 hrs of treatment, CD38 surface expression increased either in the presence and absence of Dara (). Moreover, when MM cells were co-cultured with BMSCs and treated with Dynasore for 24 hrs, the decreased MM adherence of MM cells caused by Dara treatment was reversed by Dynasore (). Therefore, Dara may induce loss of MM cell adhesion via CD38 internalization through the endocytic machinery.
Figure 4. Dara impairs MM cell adhesion to BMSCs. Luciferase assays showing (A) Percent increase in luciferase activity of Dara treated MM.1S GFP+/Luc+ cells in suspension at 3 and 24 hours compared to control cells treated with control human IgG (IgG) or with a clinically irrelevant antibody not targeting MM cells (Trastuzumab, Trast.). Similarly, luciferase assays showing (B) percent decrease in luciferase activity of adherent fraction of Dara treated MM.1S GFP+/Luc+ cells at 3 and 24 hours compared to control treated cells. These data indicated an increase in suspension; i.e., loss of adhesion of Dara treated MM.1S GFP+/Luc+ cells over time compared to control. (C) IF showing changes in LAMP-1 cellular localization and colocalization with CD38 in the membrane area of MM.1S cells after Dara treatment compared to control IgG. IgG and Dara treated MM.1S cells were co-stained with multi epitope anti-CD38 (green) and anti-LAMP-1 (red), and nuclei were stained with DAPI (blue); (D) Colocalization was visualized by confocal microscopy at × 40 magnification with 4X (zone1) and 6X (zone2) optical zoom. Representative co-localization signals were shown in the merged image as yellow. Scale bars, 10 μm; (E) Flow cytometric analysis showing surface expression of CD38 in MM.1S cells treated for 24 hours with Dynasore (80μM) in presence of control IgG or Dara (100μg/ml). (F) Luciferase assay showing percent decrease in luciferase activity of MM.1S GFP+/Luc+ cells adhered to HS-5 monolayer when treated for 24 hours with Dara alone compared to those treated with Dynasore+IgG or Dynasore+Dara. The t-test (2 tailed, unpaired) was used to calculate percentage of adhesion upon Dara treatment. Each experiment was performed at least in triplicate ±SD.
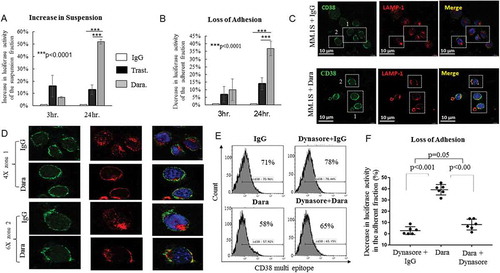
Figure 5. Dara sensitizes MM cells to bortezomib in vitro and in vivo. (A) Annexin-V/PI staining of CD138+ cells in a co-culture of MM.1S cells and HS-5. MM.1S cells were seeded alone or on top of HS-5 monolayer and treated successively with 100 µg Dara and 5 nM BTZ. The cells were then stained with CD138 BUV 737 to specifically label CD138 expressing MM.1S cells from HS-5 cells in the co-culture (top panel). In the lower panel [CD138+] PI staining shows percentage of MM.1S cell death to be 17% in the presence of BTZ, which was lowered to 7% in the presence of HS-5 monolayer. Similarly, (B) Propidium iodide (PI) staining of CD138+ gated MM.1S cells under similar conditions in an independent experiment shows 20% MM.1S cell death in the presence of 5 nM BTZ, which was reduced to 14% in the presence of HS-5, indicating HS-5 mediated protection of MM.1S to BTZ. However, the addition of Dara to the co-culture potentiated MM cell sensitivity to BTZ, as indicated by increased (25%) MM.1S cell death in the presence of both Dara and BTZ. (C) Bar diagram showing percentages of PI positive MM.1S cells under different conditions as indicated. (D) 20 × 106 MM.1S GFP+/Luc+ cells were subcutaneously injected into the right flank of athymic nude mice. Once tumors were palpable, mice were divided into 4 treatment groups: a) PBS-1+Control IgG (n = 3, Ctrl); b) PBS + Mouse Anti-Human CD38 antibody (n = 4, clone HB7, Ab-CD38); c) BTZ + Control IgG (n = 4); d) Ab-CD38 + BTZ (n = 4). Mice were treated three times a week (M-W-F) by subcutaneous injection (adjacent to the tumor site) with AbCD38 or control IgG (1 mg/kg). BTZ (0.8 mg/kg) or control vehicle (PBS) were intraperitoneally (IP) injected three times a week. Tumor volumes were measured three times weekly with calipers. Images of mice treated as indicated bearing subcutaneous tumor on their right flank (top panel) and images of the tumors isolated from respective mice (lower panel); (E) Graphical representation of sub-cutaneous tumor volume in xenograft mouse model of MM.1S GFP+/Luc+ in athymic nude mice in the different treatment groups. Data for the tumor growth between the different treatment groups were analyzed by 1 way analysis of variance (ANOVA).
![Figure 5. Dara sensitizes MM cells to bortezomib in vitro and in vivo. (A) Annexin-V/PI staining of CD138+ cells in a co-culture of MM.1S cells and HS-5. MM.1S cells were seeded alone or on top of HS-5 monolayer and treated successively with 100 µg Dara and 5 nM BTZ. The cells were then stained with CD138 BUV 737 to specifically label CD138 expressing MM.1S cells from HS-5 cells in the co-culture (top panel). In the lower panel [CD138+] PI staining shows percentage of MM.1S cell death to be 17% in the presence of BTZ, which was lowered to 7% in the presence of HS-5 monolayer. Similarly, (B) Propidium iodide (PI) staining of CD138+ gated MM.1S cells under similar conditions in an independent experiment shows 20% MM.1S cell death in the presence of 5 nM BTZ, which was reduced to 14% in the presence of HS-5, indicating HS-5 mediated protection of MM.1S to BTZ. However, the addition of Dara to the co-culture potentiated MM cell sensitivity to BTZ, as indicated by increased (25%) MM.1S cell death in the presence of both Dara and BTZ. (C) Bar diagram showing percentages of PI positive MM.1S cells under different conditions as indicated. (D) 20 × 106 MM.1S GFP+/Luc+ cells were subcutaneously injected into the right flank of athymic nude mice. Once tumors were palpable, mice were divided into 4 treatment groups: a) PBS-1+Control IgG (n = 3, Ctrl); b) PBS + Mouse Anti-Human CD38 antibody (n = 4, clone HB7, Ab-CD38); c) BTZ + Control IgG (n = 4); d) Ab-CD38 + BTZ (n = 4). Mice were treated three times a week (M-W-F) by subcutaneous injection (adjacent to the tumor site) with AbCD38 or control IgG (1 mg/kg). BTZ (0.8 mg/kg) or control vehicle (PBS) were intraperitoneally (IP) injected three times a week. Tumor volumes were measured three times weekly with calipers. Images of mice treated as indicated bearing subcutaneous tumor on their right flank (top panel) and images of the tumors isolated from respective mice (lower panel); (E) Graphical representation of sub-cutaneous tumor volume in xenograft mouse model of MM.1S GFP+/Luc+ in athymic nude mice in the different treatment groups. Data for the tumor growth between the different treatment groups were analyzed by 1 way analysis of variance (ANOVA).](/cms/asset/9a04d4ac-b216-4cd9-b036-c3340fb4e190/koni_a_1486948_f0005_oc.jpg)
Dara sensitizes MM cells to a proteasome inhibitor in vitro and in vivo
Adhesion of MM cells to components of the BM microenvironment, such as BM stromal cells (BMSCs), supports MM disease progression and cell adhesion mediated drug resistance including resistance to bortezomib (BTZ).Citation36,Citation37 Therefore, we investigated whether Dara treatment could increase BTZ sensitivity when MM cells were cultured with BMSCs. Our results show that the anti-MM activity of BTZ is impaired in a co-culture experiment with HS-5 (). When cells were treated with Dara, MM cell sensitivity to BTZ was completely rescued (,). To investigate whether an intact immune system was necessary for the anti-MM activity of Dara in combination with BTZ, we tested the anti-MM activity of anti-CD38 (HB7)+BTZ in athymic nude mice, which lack T cell mediated immunity and have defective macrophage phagocytosis functions. We observed that, as expected, there was no significant difference in the tumor size of mice that were subcutaneously injected with 20 × 106 MM.1.S GFP+/Luc+ cells and then treated with anti-CD38 (1 mg/kg) compared to those treated with control human IgG (), emphasizing that the bulk of the antineoplastic activity of Dara is driven by the patient’s immune system. When mice were treated with low doses of BTZ by intraperitoneal injection (0.8 mg/kg), a significant decrease in tumor size was observed in the presence of anti-CD38 compared to that in presence of control human IgG (p = 0.0001) (), supporting the idea that the immune system may not be involved in the anti-MM activity of Dara+BTZ.
Discussion
In this work, we explored the potential of CD38 as an adhesion molecule and exploited the ability of Dara to cause CD38 internalization into MM cells and subsequent loss of adhesion to BM stroma, thereby increasing drug sensitivity. Results obtained with single agent Dara and in combination with IMiDs and PIs have been impressive.Citation19-Citation23 Although the synergistic anti-MM activity observed between Dara and IMiDs could be explained by the capacity of IMiDs to enhance immune system dependent anti-tumor activity,Citation38 such effect would not be expected when Dara was used in combination with BTZ because BTZ decreases ADCC by disrupting TRAIL expression on MM cells and impairing the activation of the NK cell receptor NKp46, among other immunosuppressive activities.Citation39,Citation40 However, our data indicate that Dara potentiates BTZ killing of MM cells, providing the scientific rationale to explore the potential benefits of Dara and BTZ combined. Here, we show that the CD38/Dara complex can be rapidly internalized in MM cells, and, although it is not certain how internalization process affects anti-neoplastic efficacy, we believe it may have contributed to positive results obtained when Dara was used for imaging studies.Citation41–Citation43
There have been several reports that end-stage MM patients are now more likely to have extramedullary disease after becoming resistant to combinations of IMiDs, proteasome inhibitors, and CD38 antibody combinations.Citation44 Clearly, clones that are more resistant to Dara combination treatments are often independent of the BM-ME,Citation45 and one could extrapolate from our study that prolonged Dara exposure may support resistance.
Our data show that MM cells lose adhesion to the BM-ME upon Dara binding to CD38, a mechanism dependent on the endocytic machinery, since Dynasore treatment alone can completely rescue Dara induced loss of adhesion, but only partially revert CD38 surface expression upon Dara treatment. The ability of Dynasore to block the endocytosis of critical surface receptors such as CXCR4 upon SDF-1 binding was also previously observed in myeloid cells (THP-1),Citation46 and because Dynasore blocks the plasma membrane pinch-off but not the early mechanisms of invagination, the surface exposure of these receptors can be still affected.Citation47,Citation48
In agreement with previously published data showing that CD38 binding to its nonsubstrate ligand CD31 is critical to mediate MM cell interactions with BM monocytic and endothelial fractions but not to BMSCs,Citation49 our data show that CD31 is expressed at low levels on HS5 (10%) and primary BMSCs (30%), in contrast to that on human umbilical vein endothelial cells, HUVEC (98%). Furthermore, CD31 knockdown in BMSCs did not significantly affect MM cell adhesion upon Dara treatment (data not shown). This observation is aligned with previous studies in chronic lymphocytic leukemia (CLL), in which CD31 expression was not associated with the ability of CLL cells to interact with the microenvironment.Citation50
CD38 participates in a number of enzymatic activities:Citation51 1) regulating calcium homeostasis within the cell through synthesis of cyclic ADP-ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP) in a pH-dependent process;Citation10 and 2) breaking down extracellular nicotinamide adenine dinucleotide (NAD+) or forming intracellular nicotinamide (NAM) or nicotinamide mononucleotide (NMN).Citation9 NAD+ hydrolysis by CD38 generates adenosine diphosphate ribose (ADPR), directly or through the cADPR intermediate, which is ultimately converted to adenosine (ADO),Citation52 a nucleotide that influences immune cell functionsCitation11-Citation13 and is present in large amounts in the MM marrowCitation14 and in microvesicles (MVs) enriched with CD38, isolated from BM plasma of MM patients.Citation53 Generation of adenosine affects the MM microenvironment but also CD38 has direct immunologic activities on surrounding cells by associating with the T-cell receptor,Citation54 the B-cell receptor complex,Citation55 and with CD16 on natural killer cells.Citation56 Although our report cannot exclude the potential for fratricide because of Dara binding on B-cell and NK cells,Citation57 we believe that further studies focusing on understanding CD38 mediated MM cell adhesion to BMSCs and Dara/CD38 internalization transduction signaling are warranted. Additionally, MVs released from myeloma cells have been shown to enhance MM cell proliferation;Citation58 therefore, the effect of Dara binding to MM cells that can be subsequently released through MVs should be noted.Citation59–Citation61 Interestingly, these MV-bearing Dara are trafficked to FcR-expressing NK cells and monocytes, raising the possibility of further modulation of immune responses.Citation59–Citation61 The wide-ranging effects of Dara are also demonstrated by its inhibition of osteoclast formation via targeting of osteoclast progenitors.Citation62
In this work, we demonstrate Dara impairs MM cell adhesion, independent of its function as an immune activator, increasing sensitivity of MM to proteasome inhibition. Anti-CD38 treatment as a single agent did not affect MM cell growth in an immunodeficient mouse model, but we did observe a substantial anti-tumor response when anti-CD38 was combined with BTZ. BTZ seems to act primarily as an immunosuppressantCitation63,Citation64 in comparison to an IMiD, but our report may provide the rationale for combining Dara with the backbone of myeloma care of an IMiD, PI, and steroid as is being studied in a recently fully accrued phase 2 trial (GRIFFIN, NCT 02874742), as Dara may potentiate IMiD and PI anti-MM activities through two independent molecular mechanisms, a hypothesis that needs further study.
Materials and methods
Primary samples
Primary samples (total bone marrow aspirates) from MM patients were obtained from The Ohio State University Leukemia Tissue Bank (NCT01408225) and City of Hope liquid tissue bank (IRB#16352), conforming to the Declaration of Helsinki. Specifically, the cellular fraction of total bone marrow aspirates was isolated using Ficoll-Paque Plus (GE, Healthcare, Life Science) following the manufacturer’s instructions.
Cell culture, transfection, RNA isolation
MM cell lines (MM.1S, NCI-H929 and U266) and BM stromal cell line HS-5 were purchased from ATCC; L363 cells were purchased from German Collection of Microorganisms and Cell Cultures (GCMC, Braunschweig, Germany). MM cell lines were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (Cat.#019K8420, Sigma), 100 IU/ml penicillin and 100 µg/ml streptomycin. HS-5 cell line was cultured in DMEM supplemented with 10% fetal bovine serum, 100 IU/ml penicillin and 100 µg/ml streptomycin and transfected with LipofectamineTM2000 Transfection Reagent (Cat.#11668019, Invitrogen) following the manufacturer’s instructions. Total cellular RNA from NCI-H929, MM.1S, L363, and U266 cell lines were extracted by TRIZOL reagent (Invitrogen). cDNA was prepared using random primers. GAPDH was used as endogenous control to compare CD38 expression.
Flow cytometry
For CD38 expression analysis in MM cell lines and primary samples, cells were washed with PBS and stained for 30 minutes using CD38 PE Mouse Anti-Human (Cat.#130–092-260,Miltenyi Biotec)/CD38 FITC (Cat.#555459, BD Biosciences) alone or in combination with CD138 FITC Mouse Anti-Human (Cat.#552723, BD Pharmingen),CD14-APC Cy7 Mouse Anti-Human (Cat.#557831 BD Pharmingen), CD19-APC Mouse Anti-Human (Cat.#555415, BD Pharmingen), or CD3-v450 Mouse Anti-Human (clone ucht1, Cat.#560366, BD Biosciences) to determine median of fluorescence of CD38 expression in CD138+ MM-PCs, the CD14+ monocyte fraction, CD19+ B cells, and the CD3+ T cell population, respectively, for each patient. Cells were washed and immediately analyzed on LSRII (Becton Dickinson). Analysis was conducted using Kaluza Software (Beckman Coulter).
MM.1S cells were analyzed for apoptosis by Propidium Iodide (PI) staining according to the manufacturer’s protocol (Cat.#P1304MP, ThermoFisher Scientific). Specifically, 1x10Citation6 HS-5 cells were plated in each well of a 6 well plate in 2 ml DMEM (10% FBS) and incubated overnight. The next day, the medium was decanted, and 1 × 106 MM.1S cells were seeded on the top of the HS-5 monolayer in 4 ml RPMI 1640 (5% FBS) medium and also alone in each well of a 6 well plate.BothMM.1Scells and the co-culture were treated with 100 µg/ml of Dara or control IgG and incubated overnight for adherence. Twenty-four hours later, MM.1S cells alone or in the co-culture were treated with 5 nM BTZ. A further 24 hours later, cells were harvested and stained with CD138 BUV737 Mouse Anti-Human (Cat.#564393, BD Biosciences) to specifically label MM.1S cells. Next, cells were stained with PI. CD138+ PI+ cells were calculated to determine the percentages of MM.1S cell death.
CD38 internalization into MM cell lines
For measuring CD38/anti-CD38 internalization, MM cell lines (MM.1S, NCI-H929, L363 and U266) were treated with 0.5 µg of mouse anti-human CD38 antibody (clone HB7, Biolegend) or 0.5 µg of Dara and incubated for 1 hour at 37⁰C. After incubation with mouse anti-human CD38 antibody, cells were washed and then stained with 0.5 µg of a secondary antibody (2°A); i.e., Goat anti-mouse Alexa Fluor 568 (Goat anti-Mouse IgG [H + L]) Cross-Adsorbed Secondary Antibody, Alexa Fluor 568 (Cat.#A-11004, ThermoFisher Scientific) for 20 minutes at 4°C for surface staining. Next, cells were fixed with 10% paraformaldehyde, washed twice with 1X permeabilization buffer (Permeabilization Wash Buffer [10X], BioLegend Cat.#421002), and intracellular staining was performed with 0.5 µg of a secondary antibody (2°B); i.e., Rabbit anti-Mouse IgG Alexa Fluor 633 (Rabbit anti-Mouse IgG [H + L]) Cross-Adsorbed Secondary Antibody, Alexa Fluor 633 (Cat.#A-21063,ThermoFisher Scientific) in 1X permeabilization buffer followed by incubation at room temperature for 40 minutes. When Dara was used, cell surfaces were stained with 0.5 µg of a secondary antibody (2°A); i.e., Anti-human FITC (Fluorescein [FITC]-conjugated Affine Pure Goat Anti-Human IgG F [ab’]2 Fragment specific) (Jackson ImmunoResearch Laboratories, Inc; Code: 109–096-008), while intracellular staining was done with 0.5 µg of a second secondary antibody (2⁰B); i.e., Anti-human TRITC (Rhodamine [TRITC]AffiniPure Goat Anti-Human IgG, Fcγ fragment specific) (Jackson ImmunoResearch Laboratories, Inc; Code: 109–025-098), respectively. To further confirm specificity of internalization, MM.1S cells were also stained with Mouse Anti-Human CD38-PE (Cat.#356603, BioLegend) at 37°C for 30 minutes. Cells were then washed and incubated with acid stripping buffer (100mM glycine/100mM NaCl; pH 2.5) for 10 min to strip away surface antibodies. Cells were run on Imaging-Flow sight flow cytometer FlowSight® (Amnis®, part of EMD Millipore) as previously described,Citation65 and data were analyzed for internalization using internalization application wizard (IDEAS® Software, Millipore Sigma). CD38 internalization was also observed by conventional flow cytometry analysis. Specifically, 3 sets (per time point) of 1 × 106 MM cells (MM.1S) were incubated with Dara conjugated with Alexa-Fluor 647 for 15, 30, 60, 90 and 120 min. at 37°C in normal and hypoxic conditions (37°C 5%CO2 and 2%O2 using a hypoxic culture chamber) and at 4°C. At the end of the incubation, 5 ml of stripping buffer (100mM NaCl/100 mM glycine, pH = 2.5) was used with incubation on ice for 5min. MM cells were then spun down (1,000 rpm for 5 min.), washed with PBS (3X) at room temperature, and then analyzed on LSR Fortessa X-20 (Becton Dickinson). Analysis was conducted using Kaluza (Beckman Coulter).
Internalization of Dara in primary myeloma cells in the context of the bone marrow microenvironment
Bone marrow (BM) aspirates were received from the Ohio State Tissue Bank and Multiple Myeloma Registry (NCT01408225). The bone marrow cellular fraction was isolated from bone marrow aspirates following informed consent according to an Institutional Review Board (IRB) approved protocol as previously mentioned.Citation66 For each assay, 1.5 x106 total cells isolated from bone marrow extracts of MM patients were incubated with 100 µg/ml Dara at 37⁰C for 1 hour; 100 µg human IgG (Human IgG Purified Immunoglobulin, Sigma 14506-50MG) was used as control. Cells were washed; Fc receptors were blocked by incubating cells with mouse serum for 15 minutes. Cells were next stained using LIVE/DEAD™ Fixable Violet Dead Cell Stain Kit (Cat:#L34955, ThermoFisher Scientific). Cell surfaces were then labeled with 0.5 µg of a secondary antibody (2⁰A); i.e., FITC conjugated anti-human IgG (Channel 2) (Fluorescein [FITC]AffiniPureF(ab’)₂ Fragment Goat Anti-Human IgG, Fcγ fragment specific)(Jackson ImmunoResearch Laboratories, Inc; Code: 109–096-008). Cells were fixed and permeabilized with permeabilization Wash Buffer (10X) (BioLegend Cat: 421002), and intracellular staining was performed using 0.5 µg of a second secondary antibody (2⁰B); i.e., TRITC conjugated anti-human (Channel 4) (Rhodamine [TRITC] and AffiniPure Goat Anti-Human IgG, Fcγ fragment specific) (Jackson-ImmunoResearch Laboratories, Inc; Code: 109–025-098). To assess Dara internalization in total cellular extracts isolated from BM of MM patients, the following conditions were tested: 1) BM cells unstained; 2) BM cells + LIVE/DEAD violet stain; 3) BM cells + APC; 4) BM cells + CD138 APC; 5) BM cells + anti-human in FITC + anti-human in TRITC; 6) BM cells + Dara/IgG isotype + anti-human in FITC (surface staining control)/anti-human in TRITC (intracellular staining control); 7) BM cells + Dara or human IgG + anti-human in FITC + anti-human in TRITC+ APC + LIVE/DEAD at 4⁰C or at 37⁰C; 8) BM cells + Dara or human IgG + anti-human in FITC + anti-human in TRITC + CD138 APC + LIVE/DEAD at 4⁰C or at 37⁰C. Cells were run on Imaging-Flow sight flow cytometer, and data were analyzed for internalization as mentioned earlier.
Dara conjugation
A 20 ml vial of Dara was purchased from the City of Hope pharmacy (400 mg/vial), and0.3 mg of antibody (2 mg/ml in PBS, 145 µL) was reacted with 0.02 mg of NHS-AlexaFluor647 (2 mg/ml in 50 mM sodium bicarbonate, pH 8.3, 10 µL) for 1 h at room temperature. The reaction was quenched by the addition of 20 µL of 1 M Tris, pH 8.0, and the product was separated from unreacted substrate on a Zeba column (7K MWCO, ThermoFisher) equilibrated with PBS.
Immunofluorescence
MM.1S cells were fixed with Histochoice (Sigma-Aldrich; St. Louis, MO) for 10 minutes at room temperature and permeabilized with 0.1% Triton X-100 in phosphate-buffered saline (PBS) for 5 minutes at room temperature. Cells were incubated for 1 hour in blocking buffer (5% fat-free dry milk in TBST). The primary antibody, mouse LAMP1 (1:20 dilution, ab25630, Abcam, Cambridge, MA) and the FITC-conjugated multi-epitope anti-CD38 antibody, ME-CD38-FITC (1:100 dilution, CYT-38F2, Cytognos S.L., Spain), were added to cells in blocking buffer and incubated overnight at 4°C. The secondary antibody (1:1000 dilution, #A-11005, Alexa Fluor® 594 Goat Anti-Mouse IgG [H + L] Antibody; Invitrogen) was added to cells in blocking buffer and incubated for 1 hour at room temperature. Cells were washed with PBS three times between each step.
Flowsight data acquisition and analysis to measure Dara/CD38 internalization
Acquisition speed was set up to low speed and at highest resolution, an automated condition provided in Flowsight. Cells were acquired on the basis of area and aspect ratio. Debris and doublets were gated out from the analysis. Bright field (420–480 nm), channel 4 for Alexa Fluor 568 (595–660 nm), channel 11 for Alexa Fluor 633 (660–740 nm), channel 2 for FITC (480–560 nm), channel 4 for TRITC (595–660 nm), and channel 7 for LIVE/DEAD violet stain (420–505 nm) were used. About 10,000 events of single cells per sample were acquired. Additional single stain labeled samples were prepared, which served as a positive control for single staining of the individual secondary antibodies. Data were analyzed in IDEAS software after compensation of single color control samples using a compensation matrix. Internalization was recorded using the internalization application Wizard in IDEAS software (IDEAS® Software, Millipore Sigma).
Adhesion assays
For adhesion assays, MM.1S GFP+/Luc+ cells were grown on bone marrow stromal cell HS-5 monolayer and treated with 100 µg/ml control human IgG, anti-HER2 antibody (Trast.) or Dara at different time points (3 hours and 24 hours). HS-5 stromal cells were trypsinized and plated in each well of 6-well tissue culture plates at a density of 0.5 x106 per well in 1 ml DMEM (10% FBS) and subsequently incubated overnight at 37°C with 5% CO2.The next day, the medium was decanted, and 0.5 x10Citation6 MM.1S GFP+/Luc+ cells were seeded in quadruplicate on the monolayer in 2 ml RPMI (5% FBS). Plasma cells were allowed to adhere to stromal cells for 3 or 24 hours at 37°C and 5% CO2 in the presence of 100 µg/ml Dara or control antibodies (human IgG or Trastuzumab). Next, the adherent fraction comprising MM.1S GFP+/Luc+ cells and HS-5 stromal cells and the supernatant fraction comprising only MM.1S GFP+/Luc+ cells of the co-culture were collected, and luciferase assays (Promega, Ref E2810) were performed. Since only CD138+ MM.1S GFP+/Luc+ cells have luciferase expression, the luciferase activity of the adherent fraction gave a measure of adherent MM.1S GFP+/Luc+ cells. To assess whether blocking endocytosis could rescue MM cell adhesion upon Dara treatment, MM cells were incubated with the Dynamin Inhibitor I, Dynasore (CAS 304448–55-3 – Calbiochem) at a concentration of 80μMCitation67 and in the presence of human IgG or Dara (100μg/ml) for 24 hrs. The luciferase activities of the adherent and supernatant fractions were compared. Dynasore treatment was done in 3 independent experiments. Results from the triplicate and quadruplicate wells were used to calculate a mean adhesion ratio. Percent binding was defined as the percentage of binding which occurs in the presence of antibodies against adhesion molecules calculated relative to binding in the presence of control antibodies set at 100%. The percentage of bound plasma cells was calculated as follows: percentage bound cells = (input cells) – (non-adherent cells)/(input cells) X 100.
Mice experiments
All animal studies were approved by the City of Hope Institutional Laboratory Animal Care and Use Committee (IACUC). Mice were housed under a 12 hour light-dark cycle with food and water ad libitum. A cohort of nude mice (nu/nu) was injected subcutaneously with 20 × 10Citation6 MM.1S GFP+/Luc+ cells in their right flank. At the time of presence of palpable tumors, the mice were randomly distributed into 4 experimental groups: a) PBS + Control IgG (n = 3); b) PBS + Mouse Anti-Human CD38 antibody (clone HB7, Ab-CD38) (n = 4); c) BTZ + Control IgG (n = 4); d) Ab-CD38 + BTZ (n = 4). Mice were treated three times a week (M-W-F) by subcutaneous injection (adjacent to the tumor site) with Ab-CD38 or control IgG (1 mg/kg). BTZ (0.8 mg/kg) or control vehicle (PBS) were intraperitoneally (IP) injected three times a week. Tumor measurements (by caliper) were taken 3 times before some of the tumors reached the endpoint volume (8 days), and mice were humanely euthanized. Because of the characteristic irregular tumor shape using the MM.1.S GFP+/Luc+ cell line, tumor growth was monitored taking the length (mm) for the long side, short side and the height of the tumor. The total volume (in mmCitation3) was estimated by measuring the long side (mm) times the short side (mm) times the height (mm).
Statistics
For in vitro experiments, data are reported as mean ± SD of three to four experiments. The Student t test (two tailed, unpaired) was used to determine significant differences in experiments within two groups. Animal data were analyzed by analysis of variance (ANOVA). A p value <0.05 was considered statistically significant.
Abbreviations
| MM | = | Multiple Myeloma |
| MM-PCs | = | CD138+ myeloma plasma cells |
| BM | = | bone marrow |
| BMSCs | = | bone marrow stromal cells |
| Dara | = | Daratumumab |
| BTZ | = | bortezomib |
| MVs | = | microvesicles |
Conflict of Interest
AK is a consultant and serves on the speakers’ bureau for Janssen Pharmaceuticals. AK and CCH have received research funding for clinical trials supported by Janssen Pharmaceuticals.
Supplemental Material
Download MS Word (1.3 MB)Acknowledgments
We thank Drs. John Charles Williams and Krzysztof P. Bzymek for the Dara conjugation with NHS-AlexaFluor647. We thank Dori Triplett, Evelyn Flores, and Debbie Flood for administrative support. Research was in part supported by the National Institutes of Health under grant number NIH-2-R01-CA201382 (CCH, FP) and in part by the Steven Gordon & Briskin Family Innovation Grant Program (F. Pichiorri, D. Colcher and J. Shively). Research reported in this publication included work performed at the Liquid Tissue Bank and X-ray Crystallography City of Hope Shared Resources supported by the National Cancer Institute of the National Institutes of Health under award number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary material
Supplemental data can be accessed here.
Additional information
Funding
References
- Fonseca R, San Miguel J. Prognostic factors and staging in multiple myeloma. Hematol Oncol Clin North Am. 2007;21:1115–1140. ix. doi:10.1016/j.hoc.2007.08.010.
- Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK, et al. International myeloma working group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23:2210–2221. doi:10.1038/leu.2009.174.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi:10.3322/caac.21332.
- Keats JJ, Chesi M, Egan JB, Garbitt VM, Palmer SE, Braggio E, et al. Clonal competition with alternating dominance in multiple myeloma. Blood. 2012;120:1067–1076. doi:10.1182/blood-2012-01-405985.
- Rasche L, Chavan SS, Stephens OW, Patel PH, Tytarenko R, Ashby C, et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat. 2017;8:268. doi:10.1038/s41467-017-00296-y.
- Richardson P, Mitsiades C, Schlossman R, Ghobrial I, Hideshima T, Chauhan D, et al. The treatment of relapsed and refractory multiple myeloma. Hematology Am Soc Hematol Educ Program. 2007;317–323.
- Anderson KC, Kyle RA, Rajkumar SV, Stewart AK, Weber D, Richardson P, et al. Clinically relevant end points and new drug approvals for myeloma. Leukemia. 2008;22:231–239. doi:10.1038/sj.leu.2405016.
- Lin P, Owens R, Tricot G, Wilson CS. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol. 2004;121:482–488. doi:10.1309/74R4-TB90-BUWH-27JX.
- Zhao YJ, Lam CM, Lee HC. The membrane-bound enzyme CD38 exists in two opposing orientations. Sci Signal. 2012;5:ra67. doi:10.1126/scisignal.2003289.
- Lee HC. Structure and enzymatic functions of human CD38. Mol Med. 2006;12:317–323. doi:10.2119/2006–00086.Lee.
- Hubert S, Rissiek B, Klages K, Huehn J, Sparwasser T, Haag F, et al. Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2-P2X7 pathway. J Exp Med. 2010;207:2561–2568. doi:10.1084/jem.20091154.
- Karakasheva TA, Waldron TJ, Eruslanov E, Kim SB, Lee JS, O’Brien S, et al. CD38-expressing myeloid-derived suppressor cells promote tumor growth in a murine model of esophageal cancer. Cancer Res. 2015;75:4074–4085. doi:10.1158/0008-5472.CAN-14-3639.
- Rissiek B, Haag F, Boyer O, Koch-Nolte F, Adriouch S. ADP-ribosylation of P2X7: a matter of life and death for regulatory T cells and natural killer T cells. Curr Top Microbiol Immunol. 2015;384:107–126. doi:10.1007/82_2014_420.
- Quarona V, Ferri V, Chillemi A, Bolzoni M, Mancini C, Zaccarello G, et al. Unraveling the contribution of ectoenzymes to myeloma life and survival in the bone marrow niche. Ann N Y Acad Sci. 2015;1335:10–22. doi:10.1111/nyas.12485.
- Ghose J, Terrazas C, Viola D, Caserta E, Krishnan A, Hofmeister CC, et al. Daratumumab impairs myeloma cell adhesion mediated drug resistance through CD38 internalization. Blood. 2016;128:abstract#4479. doi:10.1182/blood-2016-06-724161.
- Funaro A, Reinis M, Trubiani O, Santi S, Di Primio R, Malavasi F. CD38 functions are regulated through an internalization step. J Immunol. 1998;160:2238–2247.
- Deaglio S, Mallone R, Baj G, Arnulfo A, Surico N, Dianzani U, et al. CD38/CD31, a receptor/ligand system ruling adhesion and signaling in human leukocytes. Chem Immunol. 2000;75:99–120.
- Van de Donk N, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood. 2018;131:13–29. doi:10.1182/blood-2017-06-740944.
- Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–1331. doi:10.1056/NEJMoa1607751.
- Plesner T, Arkenau HT, Gimsing P, Krejcik J, Lemech C, Minnema MC, et al. Phase 1/2 study of daratumumab, lenalidomide, and dexamethasone for relapsed multiple myeloma. Blood. 2016;128:1821–1828. doi:10.1182/blood-2016-07-726729.
- Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754–766. doi:10.1056/NEJMoa1606038.
- Rajan AM, Kumar S. New investigational drugs with single-agent activity in multiple myeloma. Blood Cancer J. 2016;6:e451. doi:10.1038/bcj.2016.53.
- Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, et al. Targeting CD38 with Daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373:1207–1219. doi:10.1056/NEJMoa1506348.
- Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018;378:518–528. doi:10.1056/NEJMoa1714678.
- Phipps C, Chen Y, Gopalakrishnan S, Tan D. Daratumumab and its potential in the treatment of multiple myeloma: overview of the preclinical and clinical development. Ther Adv Hematol. 2015;6:120–127. doi:10.1177/2040620715572295.
- Overdijk MB, Verploegen S, Bogels M, Van Egmond M, Lammerts Van Bueren JJ, Mutis T, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. mAbs. 2015;7:311–321. doi:10.1080/19420862.2015.1007813.
- Markovina S, Callander NS, O’Connor SL, Xu G, Shi Y, Leith CP, et al. Bone marrow stromal cells from multiple myeloma patients uniquely induce bortezomib resistant NF-kappaB activity in myeloma cells. Mol Cancer. 2010;9:176. doi:10.1186/1476-4598-9-254.
- Wang LH, Yang XY, Zhang X, Farrar WL. Inhibition of adhesive interaction between multiple myeloma and bone marrow stromal cells by PPARgamma cross talk with NF-kappaB and C/EBP. Blood. 2007;110:4373–4384. doi:10.1182/blood-2006-07-038026.
- Hu J, Van Valckenborgh E, Menu E, De Bruyne E, Vanderkerken K. Understanding the hypoxic niche of multiple myeloma: therapeutic implications and contributions of mouse models. Dis Model Mech. 2012;5:763–771. doi:10.1242/dmm.008961.
- Gastelum G, Kraut J, Poteshkina A, Artiga E, Weckstein G, Frost P. Targeting of the hypoxia-induced acid microenvironment of multiple myeloma cells increases hypoxia-mediated apoptosis. Blood. 2017;130:abstract#4376.
- Orciani M, Trubiani O, Guarnieri S, Ferrero E, Di Primio R. CD38 is constitutively expressed in the nucleus of human hematopoietic cells. J Cell Biochem. 2008;105:905–912. doi:10.1002/jcb.21887.
- Jackson DG, Bell JI. Isolation of a cDNA encoding the human CD38 (T10) molecule, a cell surface glycoprotein with an unusual discontinuous pattern of expression during lymphocyte differentiation. J Immunol. 1990;144:2811–2815.
- Lee EJ, Park KS, Jeon IS, Choi JW, Lee SJ, Choy HE, et al. LAMP-3 (Lysosome-Associated Membrane Protein 3) promotes the intracellular proliferation of Salmonella typhimurium. Mol Cells. 2016;39:566–572. doi:10.14348/molcells.2016.0112.
- WHt H, Szymanski CJ, Payne CK. Endo-lysosomal vesicles positive for Rab7 and LAMP1 are terminal vesicles for the transport of dextran. PloS One. 2011;6:e26626. doi:10.1371/journal.pone.0026626.
- Kirchhausen T, Macia E, Pelish HE. Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis. Methods Enzymol. 2008;438:77–93. doi:10.1016/S0076-6879(07)38006-3.
- Abdi J, Chen G, Chang H. Drug resistance in multiple myeloma: latest findings and new concepts on molecular mechanisms. Oncotarget; 2013. 2186–2207.
- Di Marzo L, Desantis V, Solimando AG, Ruggieri S, Annese T, Nico B, et al. Microenvironment drug resistance in multiple myeloma: emerging new players. Oncotarget. 2016;7:60698–60711. doi:10.18632/oncotarget.10849.
- Van der Veer MS, De Weers M, van Kessel B, Bakker JM, Wittebol S, Parren PW, et al. Towards effective immunotherapy of myeloma: enhanced elimination of myeloma cells by combination of lenalidomide with the human CD38 monoclonal antibody daratumumab. Haematologica. 2011;96:284–290. doi:10.3324/haematol.2010.030759.
- Wang X, Ottosson A, Ji C, Feng X, Nordenskjold M, Henter JI, et al. Proteasome inhibition induces apoptosis in primary human natural killer cells and suppresses NKp46-mediated cytotoxicity. Haematologica. 2009;94:470–478. doi:10.3324/haematol.13783.
- Feng X, Yan J, Wang Y, Zierath JR, Nordenskjold M, Henter JI, et al. The proteasome inhibitor bortezomib disrupts tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression and natural killer (NK) cell killing of TRAIL receptor-positive multiple myeloma cells. Mol Immunol. 2010;47:2388–2396. doi:10.1016/j.molimm.2010.05.003.
- Ghai A, Maji D, Cho N, Chanswangphuwana C, Rettig M, DiPersio J, et al. 2017. Preclinical development of CD38-targeted [(89)Zr]Zr-DFO-daratumumab for imaging multiple myeloma. J Nucl Med.
- Caserta E, Chea J, Minnix M, Viola D, Vonderfecht S, Yazaki P, et al. Copper-64-labeled daratumumab as a PET/CT imaging tracer for multiple myeloma. Blood. 2018. doi:10.1182/blood-2017-09-807263.
- Green DJ, O’Steen S, Lin Y, Comstock ML, Kenoyer AL, Hamlin DK, et al. CD38-bispecific antibody pretargeted radioimmunotherapy for multiple myeloma and other B-cell malignancies. Blood. 2018;131:611–620. doi:10.1182/blood-2017-09-807610.
- Usmani SZ, Heuck C, Mitchell A, Szymonifka J, Nair B, Hoering A, et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over-represented in high-risk disease even in the era of novel agents. Haematologica. 2012;97:1761–1767. doi:10.3324/haematol.2012.065698.
- Wirk B, Wingard JR, Moreb JS. Extramedullary disease in plasma cell myeloma: the iceberg phenomenon. Bone Marrow Transplant. 2013;48:10–18. doi:10.1038/bmt.2012.26.
- Pelekanos Ra, Ting Mj, Sardesai Vs, Ryan Jm, Lim Yc, Chan Jk, et al. Intracellular trafficking and endocytosis of CXCR4 in fetal mesenchymal stem/stromal cells. BMC Cell Biol. 2014;15:15. doi:10.1186/1471-2121-15-15.
- Chaffey N, Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular biology of the cell. 4thedn. Annals of Botany. 2003;91:401. doi:10.1093/aob/mcg023.
- Alberts B. Molecular biology of the cell; 2002. New York: Garland Science.
- An G, Jiang H, Acharya C, Zhong MY, Cai T, Yang G, et al. SAR 650984, a therapeutic anti-CD38 monoclonal antibody, blocks CD38-CD31 interaction in multiple myeloma. Blood. 2014;124:4729.
- Tonino SH, Spijker R, Luijks DM, van Oers MH, Kater AP. No convincing evidence for a role of CD31-CD38 interactions in the pathogenesis of chronic lymphocytic leukemia. Blood. 2008;112:840–843. doi:10.1182/blood-2008-03-144576.
- Chillemi A, Zaccarello G, Quarona V, Lazzaretti M, Martella E, Giuliani N, et al. CD38 and bone marrow microenvironment. Front Biosci (Landmark Ed). 2014;19:152–162.
- Horenstein AL, Chillemi A, Zaccarello G, Bruzzone S, Quarona V, Zito A, et al. A CD38/CD203a/CD73 ectoenzymatic pathway independent of CD39 drives a novel adenosinergic loop in human T lymphocytes. Oncoimmunology. 2013;2:e26246. doi:10.4161/onci.26246.
- Morandi F, Marimpietri D, Horenstein AL, Bolzoni M, Toscani D, Costa F, et al. Microvesicles released from multiple myeloma cells are equipped with ectoenzymes belonging to canonical and non-canonical adenosinergic pathways and produce adenosine from ATP and NAD+. Oncoimmunology; 2018. e1458809.
- Funaro A, De Monte LB, Dianzani U, Forni M, Human MF. CD38 is associated to distinct molecules which mediate transmembrane signaling in different lineages. Eur J Immunol. 1993;23:2407–2411. doi:10.1002/eji.1830231005.
- Kuemmerle JF, Makhlouf GM. Agonist-stimulated cyclic ADP ribose. Endogenous modulator of Ca(2+)-induced Ca2+ release in intestinal longitudinal muscle. J Biol Chem. 1995;270:25488–25494.
- Deaglio S, Zubiaur M, Gregorini A, Bottarel F, Ausiello CM, Dianzani U, et al. Human CD38 and CD16 are functionally dependent and physically associated in natural killer cells. Blood. 2002;99:2490–2498.
- Wang Y, Zhang Y, Hughes T, Zhang J, Caligiuri MA, Benson DM, et al. Fratricide of NK cells in Daratumumab therapy for multiple myeloma overcome by ex vivo expanded autologous NK Cells. Clin Cancer Res. 2018. doi:10.1158/1078-0432.ccr-17-3117.
- Arendt BK, Walters DK, Wu X, Tschumper RC, Jelinek DF. Multiple myeloma dell-derived microvesicles are enriched in CD147 expression and enhance tumor cell proliferation. Oncotarget. 2014;5:5686–5699. doi:10.18632/oncotarget.2159.
- Malavasi F, Chillemi A, Castella B, Schiavoni I, Incarnato D, Oliva S, et al. CD38 and antibody therapy: what can basic science add?Blood. 2016;128:SCI–36.
- Horenstein AL, Chillemi A, Quarona V, Zito A, Roato I, Morandi F, et al. NAD(+)-metabolizing ectoenzymes in remodeling tumor-host interactions: the human myeloma model. Cells. 2015;4:520–537. doi:10.3390/cells4030520.
- Chillemi A, Quarona V, Antonioli L, Ferrari D, Horenstein AL, Malavasi F. Roles and modalities of ectonucleotidases in remodeling the multiple myeloma niche. Front Immunol. 2017;8:305. doi:10.3389/fimmu.2017.00305.
- Costa F, Toscani D, Chillemi A, Quarona V, Bolzoni M, Marchica V, et al. Expression of CD38 in myeloma bone niche: A rational basis for the use of anti-CD38 immunotherapy to inhibit osteoclast formation. Oncotarget. 2017;8:56598–56611. doi:10.18632/oncotarget.17896.
- Herrera AF, Kim HT, Bindra B, Jones KT, Alyea EP, 3rd, Armand P, et al. A phase II study of bortezomib plus prednisone for initial therapy of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20:1737–1743. doi:10.1016/j.bbmt.2014.06.040.
- Koreth J, Kim HT, Lange PB, Bindra B, Reynolds CG, Chammas MJ, et al. A Bortezomib-based regimen offers promising survival and graft-versus-host disease Prophylaxis in Myeloablative HLA-mismatched and unrelated donor transplantation: a phase II Trial. Biol Blood Marrow Transplant. 2015;21:1907–1913. doi:10.1016/j.bbmt.2015.05.027.
- Terrazas C, Oghumu S, Varikuti S, Martinez-Saucedo D, Beverley SM, Satoskar AR. Uncovering Leishmania-macrophage interplay using imaging flow cytometry. J Immunol Methods. 2015;423:93–98. doi:10.1016/j.jim.2015.04.022.
- Stiff A, Caserta E, Sborov DW, Nuovo GJ, Mo X, Schlotter SY, et al. Histone deacetylase inhibitors enhance the therapeutic potential of Reovirus in multiple myeloma. Mol Cancer Ther. 2016;15:830–841. doi:10.1158/1535-7163.MCT-15-0240-T.
- Newton AJ, Kirchhausen T, Murthy VN. Inhibition of dynamin completely blocks compensatory synaptic vesicle endocytosis. Proc Natl Aca Sci U S A. 2006;103:17955–17960. doi:10.1073/pnas.0606212103.
