ABSTRACT
Combination of radiotherapy with immunotherapy has become an attractive concept for the treatment of cancer. The objective of this study was to assess the effect of curative, normofractionated radiotherapy on peripheral immune lymphocytes in prostate cancer patients, in order to propose a rationale for scheduling of normofractionated radiotherapy with T-cell based immunotherapy.
In a prospective study (clinicaltrials.gov: NCT01376674), eighteen patients with localized prostate cancer were treated with radiotherapy with or without hormonal therapy. Irradiation volumes encompassed prostate and, in select cases, elective pelvic nodal regions. Blood samples were collected from all patients before, during, and after radiotherapy, as well as from 6 healthy individuals as control.
Normofractionated radiotherapy of prostate cancer over eight weeks had a significant influence on the systemic immune status of patients compared to healthy controls. Absolute leukocyte and lymphocyte counts decreased during treatment as did peripheral blood immune subsets (T cells, CD8+ and naïve CD4+ T cells, B cells). Regulatory T cells and NK cells increased. Proliferation of all immune cells except regulatory T cells increased during RT. Most of these changes were transient. Importantly, the functionality of T lymphocytes and the frequency of antigen-specific CD8+ T cells were not affected during therapy.
Our data indicate that combination of normofractionated radiotherapy with immunotherapy might be feasible for patients with prostate cancer. Conceptually, beginning with immunotherapy early during the course of radiotherapy could be beneficial, as the percentage of T cells is highest, the percentage of regulatory T cells is lowest, and as the effects of radiotherapy did not completely subside 3 months after end of radiotherapy.
Introduction
Immunotherapy has recently evolved as a fourth pillar in oncology besides systemic therapy (chemotherapy and targeted therapy), radiotherapy (RT), and surgery.Citation1 Therapy with immune-checkpoint inhibitors in particular has shown remarkable long-term remissions in a substantial subset of metastasized melanoma patients and is meanwhile established as standard therapy in several malignancies.Citation2–Citation4 RT is being used for inducing immunosuppression before stem cell transplantationCitation5 and for the treatment of arthrodegenerative and inflammatory diseases,Citation6 and has long been considered as being essentially immunosuppressive. This view has changed in recent years, as it was found that radiation can support anti-tumor immunity via induction of immunogenic cell death and increased antigen crosspresentation.Citation7–Citation10 However, these effects are weak and rare with RT alone, and combination with immunotherapies might induce stronger adaptive immune responses.Citation11–Citation13 Hence, the combination of RT with different immunotherapeutic strategies is being explored, including for prostate cancer.Citation8,Citation10 Preclinical models as well as clinical observations reported better local control as well as abscopal effects compared to monotherapies.Citation14–Citation16
Immunotherapy for prostate cancer has mostly been tested for metastatic castration-resistant disease so far. Sipuleucel-T was the first FDA approved therapeutic anti-cancer vaccine, but has shown limited clinical benefit.Citation17,Citation18 Further approaches for activating anti-tumor T-cell responses are currently in clinical phases I to III, including checkpoint inhibitors, recombinant viruses and anti-cancer vaccines.Citation19–Citation22 The society of immunotherapy of cancer has recently published consensus guidelines on the use of immunotherapy in prostate cancer patients.Citation23 Checkpoint inhibitors have shown limited efficacy so far, which might be related to the low mutational load in this tumor.Citation24,Citation25 A randomized phase III trial was performed in patients with metastatic, castration resistant prostate cancer for testing RT only (8 Gy in one fraction to 1–5 bone metastases) versus RT followed by Ipilimumab treatment. Although the trial did not meet the primary endpoint of significantly improved overall survival, disease-free survival was prolonged, and an overall survival benefit was observed in the subgroup of patients with favourable prognostic factors.Citation26 Cancer vaccines have also shown encouraging results either in metastatic, castration-resistant patients or in patients with biochemical relapse.Citation19,Citation21,Citation27,Citation28 Hence, combination with RT might enhance clinical benefit. However, the optimal dose and fractionation of the RT regimen and its scheduling with immunotherapy have not been systematically explored.
Whereas RT in metastatic cancers is often hypofractionated or given in a single fraction encompassing only the tumor region,Citation29 curative RT of localized disease is mostly given in normofractionated regimens over several weeks, especially if elective nodal regions are included.Citation30 Curative RT for localized prostate cancer is tailored to the D’Amico risk stratificationCitation31 and is combined with androgen deprivation therapy and/or radiation of pelvic lymph node regions.Citation32–Citation34 Curative RT, especially elective nodal irradiation, includes relevant volumes of normal tissue with a risk of microscopic tumor spread. Irradiation of normal tissue with curative RT dose is only possible with fractionated therapy (small doses of RT once daily over several weeks) to avoid risking severe side effects, such as gastrointestinal and genitourinary dysfunctions or tissue fibrosis in case of pelvic irradiation.
Impact of curative, normofractionated RT on patients’ immune cells has been poorly studied so far. Here, we assess its effects on peripheral lymphocyte subsets during and shortly after treatment (in most patients RT was combined with hormonal treatment that had started at least 6 weeks earlier). The aim of this prospective study was to propose an optimal time window for combination of RT with T-cell based immunotherapy.
Results
Changes in peripheral immune cell subsets during RT
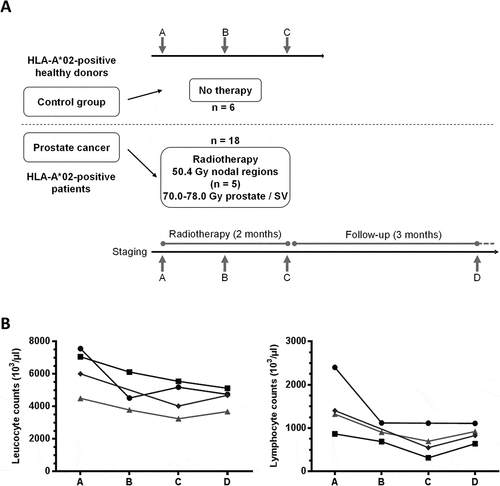
Peripheral blood mononuclear cells (PBMCs) from 18 patients were collected at four timepoints during the study (). Blood cell differential counts of four patients treated with localized RT were available at all successive time points. Leukocyte counts, but even more lymphocyte counts, decreased during the course of therapy (). However, changes were overall not significant and cell counts appeared to recover three months after end of radiation (timepoints D vs B or C).
Figure 1. Flow chart of the study and white blood cells counts. (A) HLA-A*02+ patients undergoing primary RT for prostate cancer were included (n = 18). RT regimens are shown (SV = seminal vesicles). Blood samples were obtained before start of treatment (timepoint A), twice during therapy at 1 month intervals (timepoints B, C) and three months after the end of treatment at a follow-up visit (timepoint D). As controls, three consecutive blood samples (A, B, C) were obtained at one month intervals from HLA-A*02+ healthy donors (n = 6). (B) Absolute numbers of leucocytes (left) and lymphocytes (right) are shown before (A), during (B, C) and post-therapy (D) for four patients (four timepoints each, except for one patient for whom only 3 samples were available)
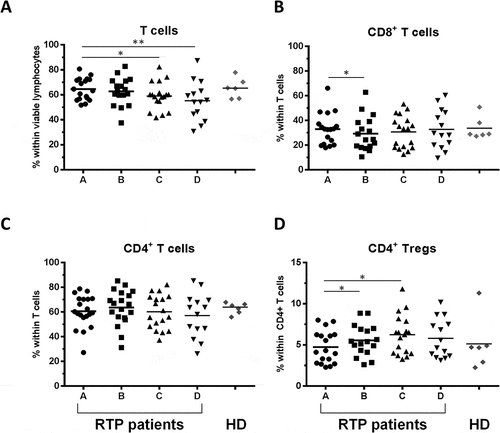
To gain a more precise view on which cell subsets might be affected by RT, we assessed the fractions of T-, B- and natural killer (NK) cells within patient PBMC lymphocytes and compared the results to that obtained from healthy donor PBMCs. The percentages of CD3+ T cells within viable lymphocytes decreased significantly over the course of RT (, median 63.9% vs 59.3% for timepoints A and C, respectively). Within CD3+ lymphocytes, the proportion of CD8+ cells transiently decreased after the first four weeks of RT (, median 32.9% vs 24.6% for timepoints A and B, respectively). Whereas total CD4+ T cell levels did not change over the course of treatment, regulatory T cells (Tregs, defined as CD4+CD25+FoxP3+) increased significantly (C-D; Tregs within CD4+ T cells: median 4.2% at timepoint A vs 6.4% at timepoint C). Normal levels of both CD8+ (median 32.9% vs 28.9% for timepoints A and D, respectively) and Tregs (median 4.2% vs 5.1% for timepoints A and D, respectively) were restored after therapy.
Figure 2. Impact of RT on T lymphocyte subsets. (A) Percentage of total T cells among viable lymphocytes defined as CD3+ positive cells, (B, C) CD8+ and CD4+ T cells and (D) CD4+CD25+Foxp3+ (Treg) cells within CD4+ cells are shown for the 4 timepoints A-D; n = 18 RT patients are included. Healthy donors are shown in comparison (n = 6, mean of three timepoints per donor). Bars indicate means. Significant differences: * p < 0.05; ** p < 0.01
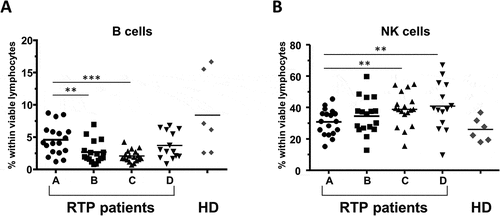
The most significant impact of RT was a decrease in the percentage of B cells (CD3−CD19+), which was however normalized after end of treatment (, median 4.1%, 1.8% and 2.8% at timepoints A, C and D, respectively). Inversely, the proportion of NK cells (CD3−CD19−) was continuously enhanced over the time of therapy (, 31.1% pre-RT and 38.8% at timepoint C).
Figure 3. Impact of RT on B cells and NK cells. Percentage of (A) B lymphocytes, and (B) NK cells within viable lymphocytes for the 4 timepoints A-D; n = 18 RT patients are included. Healthy donors are shown in comparison (n = 6, mean of three timepoints per donor). Bars indicate means. Significant differences: ** p < 0.01; *** p < 0.001
Three months after the end of the RT, only the proportion of NK and T cells remained significantly affected (median 31.1% vs 39.2% and 63.9% vs 54.9% in pre- and post-treatment samples, for NK- cells and T- cells, respectively), while other transient changes had returned to normal levels. Altogether, no significant difference was observed in the proportion of T-, B- and NK-cell subsets between patients before treatment (timepoint A) and healthy donors. Thus, a significant effect of six weeks of hormonal treatment preceding RT is unlikely.
Radiotherapy increases lymphocyte proliferation
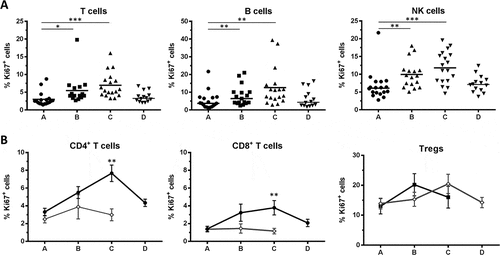
Intracellular Ki67 staining indicating proliferating cells was assessed for all lymphocyte subsets. T cells, B cells and NK cells showed similar dynamics with a significant increase in proliferation during treatment, which receded after the end of RT (). For all three subsets, the median percentage of Ki67+ cells was approximately doubled at the end of RT (T cells: 2.5% vs 5.6%; B cells: 3.7% vs 9.6%; NK cells: 6.1% vs 11.8% for timepoints A and C, respectively). Compared to healthy donors, the proliferation rate of patient CD4+ and CD8+ T cells did not differ before therapy, but was increased at the end of RT (approximately 2.5 fold for both subsets, ). These changes resumed three months after treatment. In contrast, the proliferation of patient Tregs, which was highest within T cells before RT, was not affected over the course of RT () and did not differ from that in healthy donors (). These results suggest that the proliferation of effector T cells (CD4+ and CD8+), rather than Tregs, is increased upon RT.
Figure 4. Proliferation of lymphocyte subsets. (A) Proliferation was assessed by intracellular Ki67 staining in T cells, B cells and NK cells. Bars indicate means. (B) Mean ± SEM of Ki67 expressing cells in total CD4+, total CD8+ and Treg subsets for n = 18 RT patients and n = 6 HD. Significant differences are shown as * p < 0.05; ** p < 0.01; *** p < 0.001
Antigen-specific T cells are not affected during RT
Next, we analysed in more detail several aspects of T cell differentiation and function within the CD4+ and CD8+ cell subsets. The proportion of naïve CD4+ T cells (CD45RA+CD28+) decreased over the course of RT and stayed low three months thereafter (, left panel: median 18.1% before RT, 10.4% at the end of therapy, i.e. timepoint C, and 9.0% three months after, i.e. timepoint D). Inversely, the levels of antigen-experienced CD4+, defined as CD45RA+CD28− and CD45RA−CD28±, decreased but without reaching significance (data not shown). The proportion of naïve (CD45RA+CD28+) or effector (CD45RA+CD28−) CD8+ cells did not vary (, middle and right panels).
Figure 5. Differentiation status and function of T cells. (A) Naïve cells (CD45RA+CD28+) and effectors (CD45RA+CD28−) are shown. n = 16 RT patients. Bars indicate means and significant differences are shown as * p < 0.05; *** p < 0.001. (B) Viral-specific CD8+ T cells stained with four different HLA-A*02 multimers over time. Intermediate to high frequencies (> 0.1% of the CD8+ subset) are shown on the left panel and low frequencies (< 0.1% of the CD8+ subset) on the right panel. Altogether 20 specificities were detected in n = 9 RT patients. (C) Example of EBV-BMLF1 multimer stainings in one patient. Cells are gated on living CD4− lymphocytes; timepoints and % CD8+multimer+ cells are indicated. (D) Intracellular cytokine production of CD4+ (left, TNF and IL-2) and CD8+ (right, TNF and IFNγ) T cells after activation with SEB. Timepoints A and C are shown for n = 7 RT patients, mean and SD are indicated. The lower panels show the pairwise percentage of TNF+ and IL-2+ and TNF+ and IFNγ+ T cells in the subset of CD4+ and CD8+ T cells, respectively
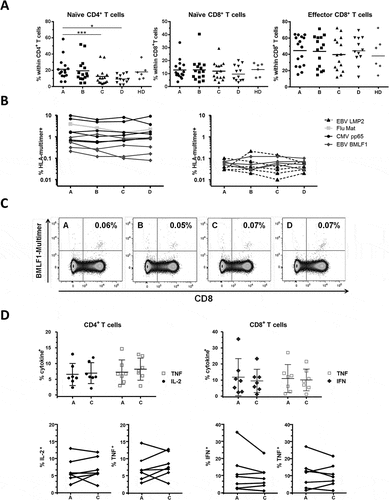
To assess antigen-specific T cells, we then performed HLA-peptide multimer stainings. Four specificities were tested, CMV-pp65, EBV-LMP2 and -BMLF1, and Flu-Matrix, which represent known immunodominant epitopes, all presented by HLA-A*02. As shown in and , the percentages of virus-specific CD8+ T cells were stable overtime in all patients and for all specificities tested, even for cells present at low frequencies (range: 0.03% to 9.68% of the CD8+ subset). Finally, we tested T cell functionality by stimulating patients´ PBMCs overnight with the superantigen Staphylococcus Enterotoxin B (SEB), followed by intracellular staining for the cytokines TNF and IL-2 or TNF and IFNγ, for CD4+ cells and CD8+ cells, respectively. We found that cytokine production in the two subsets was not significantly impaired at the end of RT compared to pre-treatment status (timepoints C vs A, ). Altogether, we concluded that the frequencies and functionality of antigen-specific CD8+ T cells appear to remain stable during RT; the proportion of naïve precursors was however decreased within the CD4+ subset, but overall functionality was not affected.
Radiation volume impacts immune cells
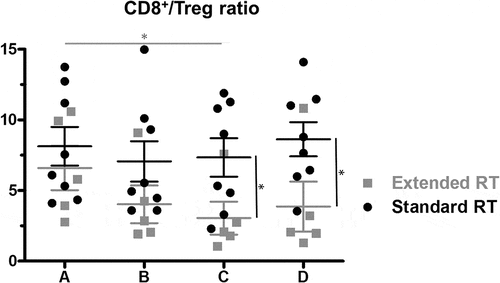
To determine whether immune changes are dependent of the extent of RT (radiation volume), we evaluated two patient groups separately, i.e. patients treated with RT of prostate and seminal vesicles only and patients receiving additional irradiation of pelvic nodal regions (see suppl. Table 1, suppl. ). When considering all available datapoints, we found that the fraction of T lymphocytes was significantly lower in patients receiving extended RT vs. standard RT after end of the radiotherapy treatment (timepoint D), whereas NK cells were higher at the same timepoint. There was no statistically significant difference between the two treatment groups for B-, CD4+ and CD8+ cells, however the CD4+ Treg fraction was significantly higher in the extended vs standard RT groups at timepoint C only (data not shown). As a correlate of potential anti-tumoral T cell activity that could be harnessed by immunotherapy approaches, we next assessed the ratio between CD8+ cytotoxic effectors and CD4+CD25+FoxP3+ regulatory cells (CD8+/Tregs) within blood T cells. The CD8+/Treg ratio was similar between the two patient groups before treatment (n = 8 and n = 5 patients receiving standard vs. extended RT with all timepoints tested), and showed only minimal changes over the course of treatment in patients treated with small radiation volumes. In contrast, we observed a progressive decrease of the CD8+/Treg ratio in the group of patients with large pelvic treatment volumes (extended RT; p = 0.03 between timepoints A and C). Altogether, we found that the CD8+/Treg ratio was significantly lowered by extended RT, and did not recover three months after end of treatment (timepoints C and D, both p = 0.03, ) compared to therapy with limited treatment volumes. Although obtained in a limited number of patients, these data suggest that extended RT might exert a stronger suppressive effect on peripheral effector T cells compared to small treatment volumes.
Figure 6. The ratio of CD8+ T cells to Tregs within CD4+ cells at the four timepoints for patients receiving standard (n = 8) vs extended (n = 5) RT. Mean and SEM are shown. Significant differences were detectable in the group treated with extended RT volumes at timepoint C compared to initial CD8+/Treg ratio, as well as between the treatment groups at timepoints C and D. * p < 0.05
Discussion
RT of the prostate and seminal vesicles with or without androgen deprivation therapy and with or without irradiation of elective pelvic nodal regions is one of the curative treatment options for patients with localized prostate cancer which achieves satisfying results for most patients. However, according to a large metaanalysisCitation35 patients with high risk cancers still face a risk of appr. 30% to develop a biochemical relapse and thus might profit from combinatorial approaches with immunotherapy. Here we observed that the standard treatment of RT to 70–78 Gy, in most cases (15/18 patients) combined with anti-hormonal therapy, has significant effects on the cellular immune status of the patients. Whereas T cells and B lymphocytes decreased during RT, NK cells and Tregs were proportionally increased. Notably, all subpopulations except Tregs showed an increased proliferation rate during RT, which resolved to normal levels three months after the end of treatment. Importantly, antigen-specific T cell frequencies and T cell function against a model superantigen were not hampered by local irradiation. Based on our previous results Citation19,Citation21 and unpublished observations, we performed multimer staining for tumor-antigen reacting T cells. We could not detect any CD8+ cells specific for any of the prostate-associated epitopes tested (derived from PSA, PSMA, TRPP8 and Prostein, n = 6 RTP patients; data not shown). This is in line with published data that tumor-antigen specific T cells can be found in the blood of prostate cancer patients at very low frequencies only.Citation36–Citation39 Altogether, and since our patient cohort was limited (especially only 5 patients with extended RT regimen were included), results could be strengthened by comparison of larger groups of patients receiving standard vs extended RT. Various immunological effects of irradiation have been described in preclinical tumor models. In vitro, irradiation induces co-stimulatory and decreases co-inhibitory molecules on the surface of prostate cancer cells;Citation40,Citation41 it was also shown to induce the release of HMGB1 and the expression of calreticulin on the cell surface of tumor cell lines, and to increase tumor cell killing by T cells.Citation42 However, mouse models also indicate immunosuppressive effects; for example, RT alone induces an increase in the proportion and the expansion of Tregs in the spleen but also within the tumor microenvironment. Indeed, regulatory T cells have been described to be more radioresistant than effector T cells, both in mice and in patients.Citation43–Citation45 Our data are well in line with these observations and strongly suggest that especially extended radiation volumes applied to patients with a high-risk of recurrence might have a negative effect on anti-tumor immune effector cells over the course of radiotherapy. Schaue et al. also described an increase of Tregs during RT of rectal cancer (including pelvic regions), not of prostate cancer (standard RT limited to the prostate region).Citation46
In patients, the effects of radiation on peripheral immune cell subsets in urological malignancies were already described in 1976 with a decrease in T cell numbers and function.Citation47 RT induces proinflammatory markers such as IL-18 and Hsp21.Citation48,Citation49 Lymphocyte subset changes have also been described for different radiation modalities like hypofractionated RT or carbon ion therapy.Citation39,Citation50 Comparisons of peripheral immune cells after definitive versus salvage RT after radical prostatectomy showed results comparable to our data with a transient decrease of B cells within lymphocytes, and inversely an increase of the NK cell fraction. A notable difference to our study was that RT was restricted to the prostate region without pelvic nodal target volume, the dose was 60–74 Gy and that only 10 of 33 patients received hormonal therapy.Citation51 Both androgen deprivation therapy and RT for prostate cancer have intrinsic immunological properties like promoting pro-inflammatory cytokine microenvironment, Th1 differentiation of T cells, and increased tumor immune cell infiltration. Several clinical trials with combination regimens are ongoing.Citation52 In contrast to these and our data, radio(chemo)therapy for cervical cancer induced a profound and long lasting (up to 9 weeks after treatment completion) decrease in CD4+ and CD8+ T cell subsets, but not in Tregs, hence does not seem promising for combination with immunotherapy.Citation53 Thus, it is important to test the effects of RT on the immune system in each clinical setting considered for combination of radio(chemo)therapy and immunotherapy. Notably, while peripheral changes are easy to monitor with routine blood sampling, they might not fully reflect the intratumoral situation, in which several immunological parameters have been described to predict outcome and therapy response. Hence, it would be interesting to also assess intra-tumoral immune infiltrates after local radiotherapyCitation54.
Whereas several new systemic treatment options are available for metastatic prostate cancer,Citation55 the treatment of localized disease still consists of local therapy, in advanced stages combined with androgen deprivation.Citation56 The role of immunotherapeutic strategies in the curative setting has to be established in the context of possible benefits of new systemic therapies in addition to local treatment in this patient population. The limited success of checkpoint inhibitors in prostate cancerCitation26 (most possibly due to low mutational burdenCitation25) might point to a role of different immunotherapy strategies such as vaccinationCitation19 in this tumor entity. A number of non-mutated, but prostate or prostate carcinoma specific- and associated-antigens are known that could be used for immunizing patients.
Limited data is available on the combination of curative RT with immunotherapy for prostate cancer. In a spontaneous tumor model (TRAMP mice) treated with a combination of radiation, adoptive transfer and anti-tumor vaccination, T-cell expansion was strictly dependent on timing and sequencing of the treatment modalities.Citation57 Gulley et al. conducted a clinical trial comparing standard RT with a combination of RT and vaccination starting three months prior to RT. Vaccine-specific T cell responses were induced in most patients, along with evidences of antigen spreading; hence, although the study did not include patients receiving the vaccine only, it can be concluded that RT did not (drastically) impair anti-vaccine T cells.Citation58 Two further studies reported preliminary data for intraprostatic vaccination and RT.Citation54,Citation59 Finally, three clinical trials for metastatic prostate cancer and one trial with curative RT in combination with vaccination are ongoing.Citation60
In summary, our data suggest that a favourable time window would be to start T-cell based immunotherapy prior to or early during the course of curative fractionated RT. We found that four weeks after start of RT (timepoint B), T cell levels were only slightly affected, the proportion of naïve cells was intact, the ratio CD8+/Tregs was unchanged, and T cell proliferation was increased. The combination of curative treatment such as RT with immunotherapy should be most effective in high-risk patients with a significant risk of relapse and distant metastases. This population is offered RT to the pelvic nodal regions, thus hypofractionated irradiation as mostly described for RT in combination with immunotherapyCitation7,Citation61 is not an option for this patient population. Combination of T-cell immunotherapy with this irradiation regimen might be a promising strategy to decrease the risk of local recurrence and metastatic spread in high-risk prostate cancer patients.
Patients and methods
Patients and treatments
From 2011 to 2012, patients undergoing curative primary RT for localized prostate cancer were enrolled in a prospective immunomonitoring study after giving informed consent. Patients with known autoimmune diseases or on immune modulatory drugs like immunosuppressants or corticosteroids were excluded. In addition, six healthy donors were recruited as controls. The study was conducted with the formal approval of the Ethics Committee of Human Experimentation (project 402/2010BO2) and registered at www.clinicaltrials.gov (NCT01376674).
Among the sixty-three patients who underwent HLA screening, twenty-five were HLA-A*02-positive and donated 50 ml of blood (in Lithium-heparin containers) between 1 to 10 days before start of therapy, four and eight weeks after treatment start, and three months after end of treatment (flow chart , timepoints A to D). Eighteen patients were included in the final analysis (Suppl. Fig. 1A).
The mean age of this cohort was 75 years (range 65 to 82 years). Nine patients presented with intermediate risk prostate cancer and nine patients had high-risk tumors (detailed information in Suppl. Table 1). According to our institutional standards, therapy consisted of 70–78 Gy in 35–39 fractions to the prostate and base of seminal vesicles for intermediate-risk disease combined with 6 months of androgen deprivation therapy overlapping the RT time period. This additional LHRH treatment commenced at least 6 weeks before starting RT. For high-risk patients, additional RT to the pelvic nodal regions to 50.4 Gy in 28 fractions and androgen deprivation therapy for 2–3 years were considered. The six healthy donors were in the age range of the screened patient cohort (49 to 63 years vs. 51 to 85 years) and blood was collected at three timepoints four weeks apart.
Oncologic outcome
In our mixed population of 50% intermediate and 50% high-risk prostate cancer patients (n = 18) with a median estimated follow-up of 5.1 ± 0.1 years, two of the eighteen patients experienced a recurrence. Both had high-risk cancers at the time of diagnosis. One patient developed lymph node metastases 1.5 years after radiotherapy and was treated with several systemic therapies and radiotherapy to bone metastases later on. One patient developed a local recurrence 4 years after radiotherapy and received systemic therapy. Estimated biochemical-failure-free survival (BFFS) after 5 years was 89 ± 8%. As expected, high-risk patients had a worse prognosis with a 5-year BFFS of 76.2 ± 14.8% versus 100% for intermediate-risk patients, although without statistical significance (p = 0.13, data not shown).
Cell isolation
Heparinized whole blood was obtained from the included patients and healthy human volunteers through venepuncture after informed consent. Peripheral blood mononuclear cells (PBMCs) were isolated using standard density centrifugation on Biocoll separating solution (Biochrom AG), frozen in 90% FCS and 10% DMSO, and stored in liquid nitrogen until use.
Phenotyping of PBMCs
Frozen cells were thawed in IMDM (Lonza, Verviers, Belgium) supplemented with 2.5% heat-inactivated (h.i.) human AB serum (Life Technologies, Darmstadt, Germany), 1% penicillin/streptomycin (Sigma-Aldrich, Steinheim, Germany), 50 µM beta-mercaptoethanol (Merck, Darmstadt, Germany) and 3µg/ml DNase I (Sigma-Aldrich), according to a standard protocol. In most cases, 1–2 x 106 cells were used per stain, antibody (Ab) panels and staining protocols were established prior to the experiments. Stain I: CD3 PaBlue (Biolegend, Fell, Germany), CD19 BrilliantViolet650 (Biolegend), CD4 APC-Cy7 (BD Biosciences, Heidelberg, Germany), CD8 QDot605 (LifeTechnologies), CD25 APC (BD) and CD56 PE-Cy7 (BD). Stain II: CD4 APC-Cy7 (BD), CD8 PE-Cy7 (Beckman Coulter, Krefeld, Germany), CD45RA PaBlue (LifeTechnologies), CD28 APC (eBioscience), CCR7 PE (BD, not used for the analysis) and four HLA-A*02 multimers: CMV-pp65 (495–503 NLVPMVATV) QDot705 at 5 µg/ml, EBV-BMLF1 (259–267, GLCTLVAML) QDot685 at 10 µg/ml, EBV-LMP2 (426–434, CLGGLLTMV) QDot655 at 5 µg/ml and Influenza-Matrix (58–66, GILGFVFTL) QDot605 at 5 µg/ml. All monomers and multimers were produced in-house and stainings performed as already describedCitation62,Citation63 in PBS containing 0.02% sodium azide, 2 mM EDTA (Roth, Karlsruhe, Germany) and 2% or 50% h.i. foetal calf serum for the Ab or multimers, respectively. After an incubation of 30 min at room temperature (multimers) or 20 min at 4°C (Abs), cells were washed, permeabilized with Fixation/permeabilization solution (eBioscience), followed by an intracellular staining with Foxp3 PE and/or Ki67 AlexaFluor700 for 30 min at 4°C followed by two washes. Isotype controls were used for CD25 APC and Ki67 AlexaFluor700, and each test contained a viability marker (Live/Dead Aqua, LifeTechnologies).
Intracellular cytokine staining
For intracellular cytokine staining (ICS), PBMCs were thawed and 1–2 x 106 cells plated in culture medium IMDM with 10% h.i. human AB serum, 1% penicillin/streptomycin and 50 µM beta-mercaptoethanol at 37°C and 7.5% CO2. After overnight resting, cells were stimulated for five hours with either an HIV-peptide (HIV-RT 476–484, ILKEPVHGV, HLA-A*02 binder) or with SEB (Sigma) at 10 μg/ml in the presence of a CD107a PaBlue antibody (Biolegend, 2 μl/test); GolgiStop (BD) and Brefeldin A (Sigma, 10 μg/ml) were added after one hour. Extracellular stainings contained Abs CD4 APC-Cy 7, CD8 QDot605 and Life/Dead Aqua. After washing and permeabilization with Cytofix/Cytoperm (BD), Th1 cytokines were detected with the following specific Abs: anti IL-2 PE-Cy7 (Biolegend), IFNγ AlexaFluor700 (BD) and TNF BrilliantViolet650 (Biolegend) diluted in PBS containing 0.02% sodium azide, 0.5% BSA and 1% Saponin (both Sigma). Incubations all lasted 20 min at 4°C and washing steps were performed as described.Citation21 After the last incubation, stains were washed twice.
Testing and analysis strategies
All stainings were acquired on an LSR-Fortessa SORP cytometer (BD) operated through Diva version 6.1.2. Analysis was performed using FlowJo version 9.2 for Macintosh. In the case of limited cell numbers for certain time points, phenotypical characterization was prioritized, followed by MHC-multimer staining, then functional analysis.
The following gating strategy was used: time histogram, singlets (FSC-H vs. FSC-A), living cells (Live/Dead Aqua dye vs FSC-A), lymphocytes (FSC-A vs SSC-A). Within lymphocytes, T cells were defined as CD3+CD19−, B cells as CD3−CD19+ and NK cells as CD3−CD19−. CD4+, CD8+ and Tregs (CD4+CD25+Foxp3+) were all assessed within CD3+ lymphocytes. Percentages of multimer+ cells are given within CD4−CD8+ lymphocytes. For the ICS experiments, cytokine production was assessed within the CD4−CD8+ and CD4+CD8− subsets; percentages of SEB-responding cells are given after subtraction of the background cytokine production (measured in the HIV control). Tests were excluded in case of less than 100.000 total events or less than 25% viable lymphocytes and all dot-plots were audited by visual inspection.
Statistics
Patients and timepoints included are shown in detail in Suppl. Table 1. Statistical analysis was performed with Graph prism 5.04 and SPSS statistics V24. First, normal distribution of the values was assessed using a D´Agostino & Pearson test. Differences between timepoints A and B, A and C, or A and D were tested for paired observations (only) with a paired t-test or a Wilcoxon matched-pairs test (same test for all comparisons of the same marker). For HD PBMCs, and since the % of the different immune subsets assessed were very similar, mean of the three timepoints tested were calculated and compared to the single values obtained in RT patients. For comparison of data obtained in patients vs HD (e.g. , timepoint C RT patients vs HD) and between RT groups (), a Mann Whitney test (single group comparison) or a Friedman test (multiple group comparison) were used. If multiple tests were performed in the same experiment, a post-test correction was performed. Estimated patient survival was calculated using the Kaplan Meier method, survival curves were compared by Log-rank test. Significant differences were defined with p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***).
Supplemental Material
Download Zip (609.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data for this article can be access on the publisher’s website.
Additional information
Funding
References
- Perica K, Varela JC, Oelke M, Schneck J. Adoptive T cell immunotherapy for cancer. Rambam Maimonides Med J. 2015;6:e0004. doi:10.5041/RMMJ.20769172.
- Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E, Mennecier B, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. doi:10.1016/S1470-2045(15)70054-9.
- Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD. Pooled analysis of long-term survival data from phase ii and phase iii trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–1894. doi:10.1200/JCO.2014.56.2736.
- Singh P, Black P. Emerging role of checkpoint inhibition in localized bladder cancer. Urologic Oncology. 2016;34:548–555. doi:10.1016/j.urolonc.2016.09.004.
- Adkins DR, DiPersio JF. Total body irradiation before an allogeneic stem cell transplantation: is there a magic dose? Curr Opin Hematol. 2008;15:555–560. doi:10.1097/MOH.0b013e32831188f5.
- Arenas M, Sabater S, Hernandez V, Rovirosa A, Lara PC, Biete A, Panes J. Anti-inflammatory effects of low-dose radiotherapy. Indications, dose, and radiobiological mechanisms involved. Strahlenther Onkol. 2012;188:975–981. doi:10.1007/s00066-012-0170-8.
- Demaria S, Formenti SC. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol. 2012;2:153. doi:10.3389/fonc.2012.00153.
- Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256–265. doi:10.1093/jnci/djs629.
- Golden EB, Apetoh L. Radiotherapy and immunogenic cell death. Semin Radiat Oncol. 2015;25:11–17. doi:10.1016/j.semradonc.2014.07.005.
- Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16:e498–509. doi:10.1016/S1470-2045(15)00007-8.
- Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nat Reviews Clin Oncology. 2017;14:365–379. doi:10.1038/nrclinonc.2016.211.
- Eckert F, Gaipl US, Niedermann G, Hettich M, Schilbach K, Huber SM, Zips D. Beyond checkpoint inhibition - Immunotherapeutical strategies in combination with radiation. Clin Translational Radiat Oncol. 2017;2:29–35. doi:10.1016/j.ctro.2016.12.006.
- Eckert F, Jelas I, Oehme M, Huber SM, Sonntag K, Welker C, Gillies SD, Strittmatter W, Zips D, Handgretinger R, et al. Tumor-targeted IL-12 combined with local irradiation leads to systemic tumor control via abscopal effects in vivo. Oncoimmunology. 2017;6:e1323161. doi:10.1080/2162402X.2017.1323161.
- Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi:10.1056/NEJMoa1112824.
- Schoenhals JE, Seyedin SN, Tang C, Cortez MA, Niknam S, Tsouko E, Chang JY, Hahn SM, Welsh JW. Preclinical rationale and clinical considerations for radiotherapy plus immunotherapy: going beyond local control. Cancer J (Sudbury, Mass). 2016;22:130–137. doi:10.1097/PPO.0000000000000181.
- Shahabi V, Postow MA, Tuck D, Wolchok JD. Immune-priming of the tumor microenvironment by radiotherapy: rationale for combination with immunotherapy to improve anticancer efficacy. Am J Clin Oncol. 2015;38:90–97. doi:10.1097/COC.0b013e3182868ec8.
- Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi:10.1002/cncr.24429.
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi:10.1056/NEJMoa1001294.
- Feyerabend S, Stevanovic S, Gouttefangeas C, Wernet D, Hennenlotter J, Bedke J, Dietz K, Pascolo S, Kuczyk M, Rammensee H-G, et al. Novel multi-peptide vaccination in Hla-A2+ hormone sensitive patients with biochemical relapse of prostate cancer. Prostate. 2009;69:917–927. doi:10.1002/pros.20941.
- Garnett-Benson C, Hodge JW, Gameiro SR. Combination regimens of radiation therapy and therapeutic cancer vaccines: mechanisms and opportunities. Semin Radiat Oncol. 2015;25:46–53. doi:10.1016/j.semradonc.2014.07.002.
- Widenmeyer M, Griesemann H, Stevanovic S, Feyerabend S, Klein R, Attig S, Hennenlotter J, Wernet D, Kuprash DV, Sazykin AY, et al. Promiscuous survivin peptide induces robust CD4+ T-cell responses in the majority of vaccinated cancer patients. Int J Cancer. 2012;131:140–149. doi:10.1002/ijc.26365.
- Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Reviews Immunol. 2010;10:580–593. doi:10.1038/nri2817.
- McNeel DG, Bander NH, Beer TM, Drake CG, Fong L, Harrelson S, Kantoff PW, Madan RA, Oh WK, Peace DJ, et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of prostate carcinoma. J Immunother Cancer. 2016;4:92. doi:10.1186/s40425-016-0198-x.
- Schepisi G, Farolfi A, Conteduca V, Martignano F, De Lisi D, Ravaglia G, Rossi L, Menna C, Bellia SR, Barone D, et al. Immunotherapy for prostate cancer: where we are headed. Int J Mol Sci. 2017;18:2627.
- Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 Inhibition. N Engl J Med. 2017;377:2500–2501. doi:10.1056/NEJMc1713444.
- Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, Van Den Eertwegh AJ, Krainer M, Houede N, Santos R, Mahammedi H, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–712. doi:10.1016/S1470-2045(14)70189-5.
- Baxevanis CN, Papamichail M, Perez SA. Prostate cancer vaccines: the long road to clinical application. Cancer Immunology, Immunotherapy: CII. 2015;64:401–408. doi:10.1007/s00262-015-1667-7.
- Yoshimura K, Minami T, Nozawa M, Kimura T, Egawa S, Fujimoto H, Yamada A, Itoh K, Uemura H. A phase 2 randomized controlled trial of personalized peptide vaccine immunotherapy with low-dose dexamethasone versus dexamethasone alone in chemotherapy-naive castration-resistant prostate cancer. Eur Urol. 2016;70:35–41. doi:10.1016/j.eururo.2015.12.050.
- McDonald R, Chow E, Lam H, Rowbottom L, Soliman H. International patterns of practice in radiotherapy for bone metastases: A review of the literature. J Bone Joint Surg Am. 2014;3:96–102. doi:10.1016/j.jbo.2014.10.003.
- Dirix P, Joniau S, Van Den Bergh L, Isebaert S, Oyen R, Deroose CM, Lerut E, Haustermans K. The role of elective pelvic radiotherapy in clinically node-negative prostate cancer: a systematic review. Radiother Oncol. 2014;110:45–54. doi:10.1016/j.radonc.2013.06.046.
- Hernandez DJ, Nielsen ME, Han M, Partin AW. Contemporary evaluation of the D’amico risk classification of prostate cancer. Urology. 2007;70:931–935. doi:10.1016/j.urology.2007.08.055.
- Jones CU, Hunt D, McGowan DG, Amin MB, Chetner MP, Bruner DW, Leibenhaut MH, Husain SM, Rotman M, Souhami L, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107–118. doi:10.1056/NEJMoa1012348.
- Lawton CA, DeSilvio M, Roach M, 3rd, Uhl V, Kirsch R, Seider M, Rotman M, Jones C, Asbell S, Valicenti R, et al. An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys. 2007;69:646–655. doi:10.1016/j.ijrobp.2007.04.003.
- Zelefsky MJ, Pei X, Chou JF, Schechter M, Kollmeier M, Cox B, Yamada Y, Fidaleo A, Sperling D, Happersett L, et al. Dose escalation for prostate cancer radiotherapy: predictors of long-term biochemical tumor control and distant metastases-free survival outcomes. Eur Urol. 2011;60:1133–1139. doi:10.1016/j.eururo.2011.08.029.
- Grimm P, Billiet I, Bostwick D, Dicker AP, Frank S, Immerzeel J, Keyes M, Kupelian P, Lee WR, Machtens S, et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the prostate cancer results study group. BJU Int. 2012;109(Suppl 1):22–29. doi:10.1111/j.1464-410X.2011.10827.x.
- Forsberg O, Carlsson B, Malmstrom PU, Ullenhag G, Totterman TH, Essand M. High frequency of prostate antigen-directed T cells in cancer patients compared to healthy age-matched individuals. Prostate. 2009;69:70–81. doi:10.1002/pros.20858.
- Kiessling A, Fussel S, Schmitz M, Stevanovic S, Meye A, Weigle B, Klenk U, Wirth MP, Rieber EP. Identification of an HLA-A*0201-restricted T-cell epitope derived from the prostate cancer-associated protein trp-p8. Prostate. 2003;56:270–279. doi:10.1002/pros.10265.
- Komatsu N, Terasaki Y, Moriya F, Suekane S, Noguchi M, Todo S, Itoh K, Shichijo S. A beta-tubulin 5-derived peptide induces cytotoxic T lymphocytes restricted to the HLA-A24 allele in prostate cancer patients. Exp Ther Med. 2010;1:833–839. doi:10.3892/etm.2010.120.
- Tabi Z, Spary LK, Coleman S, Clayton A, Mason MD, Staffurth J. Resistance of CD45RA- T cells to apoptosis and functional impairment, and activation of tumor-antigen specific T cells during radiation therapy of prostate cancer. J Immunol (Baltimore, Md. 1950;2010(185):1330–1339.
- Bernstein MB, Garnett CT, Zhang H, Velcich A, Wattenberg MM, Gameiro SR, Kalnicki S, Hodge JW, Guha C. Radiation-induced modulation of costimulatory and coinhibitory T-cell signaling molecules on human prostate carcinoma cells promotes productive antitumor immune interactions. Cancer Biother Radiopharm. 2014;29:153–161. doi:10.1089/cbr.2013.1578.
- Gameiro SR, Malamas AS, Bernstein MB, Tsang KY, Vassantachart A, Sahoo N, Tailor R, Pidikiti R, Guha CP, Hahn SM, et al. Tumor cells surviving exposure to proton or photon radiation share a common immunogenic modulation signature, rendering them more sensitive to T cell-mediated killing. Int J Radiat Oncol Biol Phys. 2016;95:120–130. doi:10.1016/j.ijrobp.2016.02.022.
- Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5:403–416. doi:10.18632/oncotarget.1719.
- Chaudhary B, Elkord E. Regulatory T cells in the tumor microenvironment and cancer progression: role and therapeutic targeting. Vaccines. 2016;4.
- Komatsu N, Hori S. Full restoration of peripheral Foxp3+ regulatory T cell pool by radioresistant host cells in scurfy bone marrow chimeras. Proc Natl Acad Sci U S A. 2007;104:8959–8964. doi:10.1073/pnas.0702004104.
- Persa E, Balogh A, Safrany G, Lumniczky K. The effect of ionizing radiation on regulatory T cells in health and disease. Cancer Lett. 2015;368:252–261. doi:10.1016/j.canlet.2015.03.003.
- Schaue D, Comin-Anduix B, Ribas A, Zhang L, Goodglick L, Sayre JW, Debucquoy A, Haustermans K, McBride WH. T-cell responses to survivin in cancer patients undergoing radiation therapy. Clin Cancer Res. 2008;14:4883–4890. doi:10.1158/1078-0432.CCR-07-4462.
- Elhilali MM, Paika KD, Brosman S, Fahey JL. Critical evaluation of lymphocyte functions in urological cancer patients. Cancer Res. 1976;36:132–137.
- El-Saghire H, Vandevoorde C, Ost P, Monsieurs P, Michaux A, De Meerleer G, Baatout S, Thierens H. Intensity modulated radiotherapy induces pro-inflammatory and pro-survival responses in prostate cancer patients. Int J Oncol. 2014;44:1073–1083. doi:10.3892/ijo.2014.2260.
- Hurwitz MD, Kaur P, Nagaraja GM, Bausero MA, Manola J, Asea A. Radiation therapy induces circulating serum Hsp72 in patients with prostate cancer. Radiother Oncol. 2010;95:350–358. doi:10.1016/j.radonc.2010.03.024.
- Yang ZR, Zhao N, Meng J, Shi ZL, Li BX, Wu XW, Li P, Zhang Q, Wei XB, Fu S. Peripheral lymphocyte subset variation predicts prostate cancer carbon ion radiotherapy outcomes. Oncotarget. 2016;7:26422–26435. doi:10.18632/oncotarget.8389.
- Sage EK, Schmid TE, Geinitz H, Gehrmann M, Sedelmayr M, Duma MN, Combs SE, Multhoff G. Effects of definitive and salvage radiotherapy on the distribution of lymphocyte subpopulations in prostate cancer patients. Strahlenther Onkol. 2017;193:648–655. doi:10.1007/s00066-017-1144-7.
- Kalina JL, Neilson DS, Comber AP, Rauw JM, Alexander AS, Vergidis J, Lum JJ. Immune modulation by androgen deprivation and radiation therapy: implications for prostate cancer immunotherapy. Cancers. 2017;9.
- Van Meir H, Nout RA, Welters MJ, Loof NM, De Kam ML, Van Ham JJ, Samuels S, Kenter GG, Cohen AF, Melief CJ, et al. Impact of (chemo)radiotherapy on immune cell composition and function in cervical cancer patients. Oncoimmunology. 2016;6:e1267095. doi:10.1080/2162402X.2016.1267095.
- Finkelstein SE, Rodriguez F, Dunn M, Farmello MJ, Smilee R, Janssen W, Kang L, Chuang T, Seigne J, Pow-Sang J, et al. Serial assessment of lymphocytes and apoptosis in the prostate during coordinated intraprostatic dendritic cell injection and radiotherapy. Immunotherapy. 2012;4:373–382. doi:10.2217/imt.12.24.
- Corfield J, Crozier J, Joshua AM, Bolton D, Lawrentschuk N. Understanding the role of new systemic agents in the treatment of prostate cancer. BJU Int. 2016;118(Suppl 3):8–13. doi:10.1111/bju.13633.
- Gillessen S, Attard G, Beer TM, Beltran H, Bossi A, Bristow R, Carver B, Castellano D, Chung BH, Clarke N, et al. Management of patients with advanced prostate cancer: the report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur Urol. 2018;73:178–211. doi:10.1016/j.eururo.2017.06.002.
- Harris TJ, Hipkiss EL, Borzillary S, Wada S, Grosso JF, Yen HR, Getnet D, Bruno TC, Goldberg MV, Pardoll DM, et al. Radiotherapy augments the immune response to prostate cancer in a time-dependent manner. Prostate. 2008;68:1319–1329. doi:10.1002/pros.20794.
- Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, Tsang KY, Yokokawa J, Hodge JW, Menard C, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–3362. doi:10.1158/1078-0432.CCR-04-2062.
- Fujita T, Teh BS, Timme TL, Mai WY, Satoh T, Kusaka N, Fattah EA, Aguilar-Cordova E, Butler EB, et al. Sustained long-term immune responses after in situ gene therapy combined with radiotherapy and hormonal therapy in prostate cancer patients. Int J Radiat Oncol Biol Phys. 2006;65:84–90. doi:10.1016/j.ijrobp.2005.11.009.
- Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer. 2016;4:51. doi:10.1186/s40425-016-0156-7.
- Gandhi SJ, Minn AJ, Vonderheide RH, Wherry EJ, Hahn SM, Maity A. Awakening the immune system with radiation: optimal dose and fractionation. Cancer Lett. 2015;368:185–190. doi:10.1016/j.canlet.2015.03.024.
- Chandran PA, Laske K, Cazaly A, Rusch E, Schmid-Horch B, Rammensee HG, Ottensmeier CH, Gouttefangeas C. Validation of immunomonitoring methods for application in clinical studies: the HLA-peptide multimer staining assay. Cytometry Part B, Clinical Cytometry. 2018;94:342–353.
- Hadrup SR, Maurer D, Laske K, Frosig TM, Andersen SR, Britten CM, van der Burg SH, Walter S, Gouttefangeas C. Cryopreservation of MHC multimers: recommendations for quality assurance in detection of antigen specific T cells. Cytometry A: Journal Int Soc Anal Cytol. 2015;87:37–48. doi:10.1002/cyto.a.22575.
