ABSTRACT
Hepatocellular carcinoma (HCC) is a typical inflammation-associated cancer. IL-21 regulates both innate and adaptive immune responses and has key roles in antitumor and antiviral responses. However, the role of IL-21 in HCC development is poorly defined. In the current study, we explored the role of IL-21R signaling in HCC growth by using IL-21R knockout mice and HCC mouse models. We discovered that IL-21R signaling deficiency promoted HCC growth in tumor-bearing mice. We showed that IL-21R deletion reduced T cells infiltration and activation as well as their function but increased the accumulation of myeloid-derived suppressor cells in tumor tissues to enhance HCC growth. Furthermore, loss of IL-21R signaling in tumor-bearing mice resulted in an imbalance of the systemic immune system characterized by decreased antitumor immune cells and increased immunosuppressive cells in the spleen and lymph nodes. In addition, we revealed that IL-21R signaling is critical for the expansion of antitumor immune cells in the memory immune response to tumor rechallenge. Finally, we showed that the transcriptional levels of IL-21 in the peritumoral region and IL-21R within the tumor are associated with survival and recurrence of HCC patients. In conclusion, our study demonstrates that IL-21R signaling is essential for controlling the development of HCC and immunological memory response to tumor challenge.
Introduction
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related death and is a typical inflammation-associated cancer.Citation1 The interaction between HCC antigenicity and the immunological microenvironment and the immunosuppressive microenvironment in HCC jointly drives the initiation and development of HCC.Citation2 The immunological microenvironment, which is composed of antitumor cells and molecules, such as CD8+ T cells, CD4+ T cells, NK cell and NKT cells, exerts protective functions to control HCC growth. Previous studies have demonstrated that T lymphocyte infiltration in tumors is closely associated with prognosis of cancer patients, such as HCC and colorectal cancer patients.Citation3–Citation5 HCC development can lead to the formation of an immunosuppressive microenvironment composed of protumor cells and molecules, such as Treg cells, myeloid-derived suppressor cells (MDSCs) and M2 macrophages. Many clinical studies have shown that there is a negative association between MDSCs and HCC patient outcome.Citation6–Citation8 The recruitment of these cells in HCC is induced by chemokines from cancer cells,Citation9,Citation10 which show immunosuppressive and tumor-promoting effects in HCC patients.Citation11 Therefore, the balance between immunological response factors and immunosuppressive response factors is critical for HCC growth, progression and metastasis in patients. However, little is known about the mechanism that controls the balance of immune responses and immunosuppressive responses in HCC growth.
IL-21 is a member of the common γc cytokine family composed of IL-2, IL-4, IL-7, IL-9, and IL-15 and is synthesized by activated CD4+ T cells, activated NKT cells, and Tfh cells.Citation12,Citation13 IL-21 has a pleiotropic role in modulating multiple lymphocyte subsets and plays a crucial role in many pathological responses, such as viral infection, allergy, autoimmunity and tumors.Citation13 Many studies have confirmed the antitumor role of IL-21 in animal tumor models, such as models of melanoma,Citation14 pancreatic carcinomas,Citation15 mammary adenocarcinoma and bladder cancer.Citation16,Citation17 In contrast, other studies have shown that IL-21 can promote a protumorigenic inflammatory circuit to induce colitis-associated colon cancer (CAC) development.Citation18,Citation19 These results demonstrate that IL-21R signaling may play a different role in the development of tumor depending on different tumor type. However, the role of IL-21R signaling in HCC development is unclear. Although previous study has shown that IL-21R signaling is important for memory anti-viral CD8+ T cell response,Citation20 the action of IL-21R signaling in memory anti-tumor immune response is not well explored.
Herein, we investigated the role of IL-21R signaling in HCC growth and memory immune response to tumor challenge by using IL-21R deletion mice and HCC mouse models. We demonstrated that IL-21R signaling inhibited HCC growth by enhancing the immune responses to tumor cells and decreasing the accumulation of immunosuppressive cells and is critical for expansion of antitumor immune cells in the memory immune response to tumor rechallenge.
Results
IL-21R signaling suppresses tumor growth in HCC murine models
To determine the role of IL-21R in HCC development, we compared tumor growth in WT and IL-21R KO mice in HCC murine models. First, we used a subcutaneous HCC model to observe tumor growth in mice. After implantation, the body weight markedly decreased and the tumor volume significantly increased over time in IL-21R KO mice compared with WT mice ( and B). Histological analysis showed that lymphocytes infiltration in IL-21R KO mice significantly decreased in the peritumoral zone (Fig. S1A). We then used an orthotopic HCC model to examine the effects of IL-21R signaling on HCC growth. On day 22 post-inoculation, the IL-21R KO mice exhibited an increase in tumor nodule size compared with WT mice (), and the hepatosomatic indices and spleen index were higher in IL-21R KO mice than WT mice ( and Fig. S1B). Moreover, survival analysis showed that the survival time was significantly shorter in IL-21R KO mice than WT mice (median survival 33.5 days versus 48.5 days, P < 0.0001) (). Together, these results demonstrate that IL-21R signaling deficiency promotes tumor growth in murine HCC models.
Figure 1. IL-21R deletion promoted tumor growth in hepatocellular carcinoma mouse models.
A–B, Hepa1–6 cells (1 × 107) were subcutaneously inoculated into WT mice and IL-21R KO mice for 25 days. The changes in body weight (A) and tumor volume and gross morphology of the tumors (B) are shown in WT mice and IL-21R KO mice over time. The data are representative of four independent experiments, each using 4 to 6 mice per group. C–D, Hepa1–6 cells (2 × 106) were intrahepatically inoculated into WT mice and IL-21R KO mice for 22 days. Data shown are the mean tumor size and representative tumor morphology (IL-21R KO, n = 12, WT, n = 10) (C) and hepatosomatic indices (IL-21R KO, n = 21, WT, n = 20) (D). E, Survival time was determined in WT mice (n = 28) and IL-21R KO mice (n = 36) with orthotopic HCC inoculation. Data represent the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
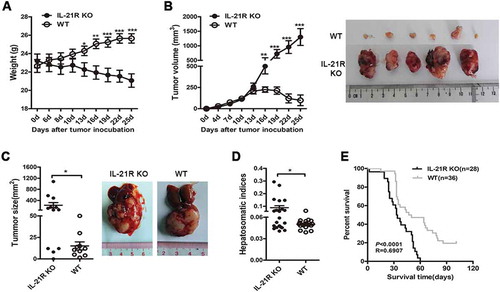
Loss of IL-21R signaling impairs antitumor immunity
To determine how IL-21R signaling mediates tumor protection, we first investigated whether IL-21 could directly inhibit tumor growth in vitro. MTT tests showed that IL-21 had no direct effect on Hepa1-6 cell proliferation (Fig. S1C). IL-21R is primarily expressed in lymphoid tissues,Citation21 and HE staining showed decreased lymphocytes infiltration in subcutaneous tumors of IL-21R KO mice (). Therefore, we hypothesized that IL-21R signaling inhibited HCC growth by regulating the antitumor immune system. As expected, immunohistochemical (IHC) staining of subcutaneous tumors showed that peritumoral tissues in IL-21R KO mice had decreased infiltration of CD4+ T cells, CD8+ T cells, CD20+ B cells, CD68+ macrophage and IL21+ cells (). We then examined intrahepatic infiltrating leukocytes (IILs) in the liver from orthotopic HCC mice. We found that CD8+ and CD4+ T cells in IL-21R KO mice were significantly reduced compared to those in WT mice on day 15 and 22 post-inoculation (). The CD44+ and CD62L+ cell populations in CD8+ T cells, but not CD4+ T cells, substantially decreased in the livers of IL-21R KO mice ( and D). Given that CD44 is an adhesion molecule and a marker of activation and maturation of T cells,Citation22,Citation23 and CD62L is a lymphocyte homing receptor and mediates T cell migration,Citation24,Citation25 these results indicate that IL-21R deletion likely impaired the activation and migration of CD8+ T cells. We also discovered that effector memory CD8+ and CD4+ T cells were significantly decreased in IL-21R KO mice on day 22 but not day 15 (-G), which imply that IL-21R signaling may have an influence on differentiation of memory T cell. These results demonstrate that IL-21R deletion contributes to decreased antitumor lymphocyte infiltration and impaired T cell differentiation in tumors. We next determined the cytokine profile of the infiltrating lymphocytes of the livers from mice on day 22 post-inoculation. IFN-γ and IL-21 production in CD4+ T cells was decreased in IL-21R KO mice compared with WT mice, but IL-17A production was considerably increased in IL-21R KO mice, and IL-10 production was not different between the two groups (). Furthermore, CD8+ T cells from IL-21R KO mice had a decreased capacity to produce IFN-γ, but not CD107A, compared to cells from WT mice (), indicating that IL-21R defects decrease the ability of CD8+ T to protect against tumor growth. Collectively, these data demonstrate that IL-21R signaling deficiency promotes HCC growth by suppressing antitumor immune responses.
Figure 2. IL-21R deficiency decreased antitumor lymphocyte infiltration and function in tumors in the HCC mouse model.
A, Representative images of HE and IHC staining for CD4, CD8, CD20, CD68, and IL-21 in subcutaneous tumors in WT and IL-21R KO mice on day 25. Original magnification, × 200 for HE, × 400 for IHC; scale bar, 50 μm. B-G, IILs from orthotopic HCC mice were collected on day 15 and 22 post-inoculation and analyzed by flow cytometry. The percentages of CD4+ T cells and CD8+ T cells in intrahepatic lymphocytes from tumor-bearing mice were examined on day 15 and 22 post-inoculation (B). The percentages of CD8+CD44+, CD4+CD44+, CD8+CD62L+ and CD4+CD62L+ T cells in intrahepatic lymphocytes from tumor-bearing mice were examined on day 15 and 22 post-inoculation (C, D). The gating strategy for memory CD4+ and CD8+ T cell in the liver of orthotopic HCC mice (E). The frequencies of memory CD4+ or CD8+ T cells in figure E are shown (F,G). H, After stimulation with anti-CD3/28 mAbs, the cytokine production of IFNγ, IL-21, IL-17A and IL-10 from WT or IL-21R KO CD4+ T cells from the livers of orthotopic HCC mice was examined on day 22 post-inoculation. I, Representative flow cytometric plots and frequencies of WT or IL-21R KO CD8+ T cells from the livers of orthotopic HCC mice producing IFNγ and CD107A after stimulation with PMA/ionomycin were shown on day 22 post-inoculation. Data represent the means of four to six mice per group ± SEM and are representative of two experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
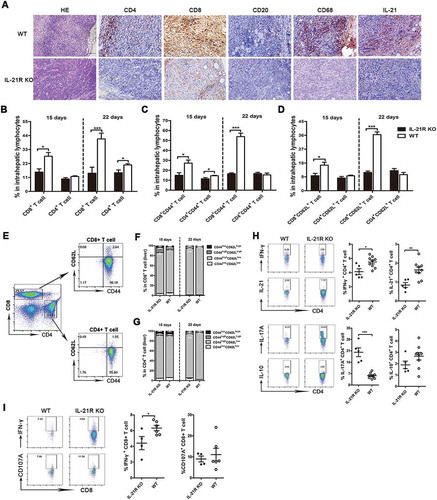
Increased accumulation of immunosuppressive cells in IL-21R KO tumor-bearing mice
MDSCs mainly consist of granulocytic-MDSCs (G-MDSCs) and monocytic-MDSCs (M-MDSCs), which have immunosuppressive activity on T cell function.Citation26,Citation27 To explore whether IL-21R deficiency affected accumulation of immunosuppressive cells, we isolated IILs from orthotopic HCC mice and subjected them to flow cytometric analysis. Interestingly, CD11b+ cell, MDSC, G-MDSC and M-MDSC populations substantially increased in IL-21R KO mice compared with WT mice on day 15, and the accumulation of these cells was further aggravated on day 22. The F4/80+CD11b+ tumor-associated macrophage population marginally increased in IL-21R KO mice on day 15 (). Based on the fact that MDSCs exert their immunosuppressive function primarily by producing arginase-1 and inducible nitric oxide synthase (known as NOS2), their expression levels of M-MDSCs and G-MDSCs in liver tumors were examined. However, there were no differences in Arg-1 and NOS2 levels in M-MDSCs and G-MDSCs between the two groups ( and D). To determine whether increased MDSCs accumulation was due to the enhancement in MDSC induction or migration, we then examined the gene expression of a variety of soluble factors involving the generation, migration and activities of MDSCs in tumor tissues. We found that the expression of chemokines that are important for tumor recruitment of MDSC, such as CCL2, CCL7 and CXCL5, were mostly enhanced in IL-21R KO mice (). However, IL-21R deficiency had no effect on the expression of cytokines that are important for MDSC generation (Fig. S1D). Additionally, the gene expression of MDSC expansion (S100A8/9) and proangiogenic factors (Angpt2, MMP9 and MMP12) were significantly increased in liver tumor in IL-21R KO mice (). Collectively, these findings suggest that IL-21R defects could increase accumulation of MDSCs through promoting chemokines production, leading to increased angiogenisis in orthotopic HCC mice.
Figure 3. IL-21R deficiency increased immunosuppressive cells in liver tumors from orthotopic HCC mice.
IILs from orthotopic HCC mice were collected on day 22 post-inoculation and analyzed by flow cytometry. A, The gating strategy for MDSCs, G-MDSCs, M-MDSCs and macrophages in livers of orthotopic HCC mice. B, The frequencies of myeloid populations in liver mononuclear cell orthotopic HCC mice were examined on day 15 and 22 post-inoculation. C-D, The frequencies of WT or IL-21R KO G-MDSCs (C) and M-MDSCs (D) from livers of orthotopic HCC mice producing Arg-1 and NOS2 after stimulation with PMA/ionomycin were determined on day 22 post-inoculation. E-F, Quantitative reverse transcriptase (qRT)-PCR analysis of the expression of chemokine genes (E), MDSCs expansion and proangiogenic genes (F) in liver tumor tissues from WT or IL-21R KO mice. Data represent the means of four to six mice per group ± SEM and are representative of two experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
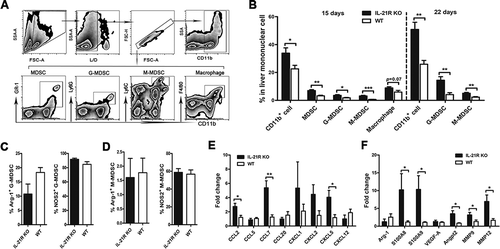
IL-21R deficiency leads to disequilibrium of the systemic immune system in tumor-bearing mice
Prompted by the above findings that increased protumor immune cells and decreased antitumor immune cells were present in IL-21R KO mouse tumors, we investigated whether IL-21R deficiency induces an imbalance of the systemic immune system in tumor-bearing mice. We therefore examined the influence of IL-21R signaling on systemic immune cells in the spleen and mesenteric lymph node (MLN) of mice. As expected, IL-21R KO mice had significantly decreased CD8+, CD4+, CD8+CD62L+, CD4+CD62L+, CD8+CD44+ and CD4+CD44+ T cell expansion in the spleen on both day 15 and 22 (-C), and similar results were observed in the MLN on day 22 ( and H). Consistent with the findings in the orthotopic HCC model, similar results were observed in the spleen of subcutaneous HCC mice on day 20 (Fig. S1E and S1F). These results indicate the IL-21R deficiency suppresses the expansion of antitumor immune cells in lymphatic organs. As expected, the MDSC, G-MDSC and M-MDSC populations substantially increased in the spleens from IL-21R KO mice on day 22 but not day 15, but the macrophage population significantly decreased in IL-21R KO mice (). Similarly, levels of these cells in the MLN were higher in IL-21R KO mice than WT mice (). These results suggest that IL-21R deletion increased the accumulation of MDSCs in immunological organs. In addition, while no obvious differences in antigen-presenting cells (APCs), such as B cells, CD11c+ DCs, conventional DCs (cDCs) and plasmatoid DCs (pDCs), were found in the MLN between the two groups (), these cells markedly decreased in the spleen, and the expression of the presentation marker MHCⅡ in B cells and DCs was significantly reduced in IL-21R KO mice ( and F). These findings suggested that IL-21R deletion inhibits the presentation ability of APCs. Collectively, these data indicate that IL-21R signaling deficiency leads to disorder of immune cells in immune organs of tumor-bearing mice.
Figure 4. Loss of IL-21R led to decreased antitumor lymphocytes and increased accumulation of myeloid-derived suppressor cells in the spleen and LN in orthotopic HCC mice.
Splenocytes and MLN cells from orthotopic HCC mice were collected on day 15 and 22 post-inoculation and analyzed by flow cytometry. A, The frequencies of CD4+ T cells and CD8+ T cells in the spleen from orthotopic HCC mice were examined on day 15 and 22 post-inoculation. B-C, The percentages of CD8+CD44+, CD4+CD44+, CD8+CD62L+ and CD4+CD62L+ T cells in the spleen from orthotopic HCC mice were examined on day 15 and 22 post-inoculation. D, The frequencies of myeloid populations in the spleen of orthotopic HCC mice was examined on day 15 and 22 post-inoculation. The frequencies of B cells, CD11c+ DCs, cDCs, and pDCs of splenocytes (E) and MLN (J) from orthotopic HCC mice were determined on day 22 post-inoculation. F, Expression levels of MHCII in B cells and CD11c+ DCs in the spleen were determined on day 22 post-inoculation. G, The frequencies of CD4+ T cells and CD8+ T cells in the MLN from orthotopic HCC mice were examined on day 22 post-inoculation. H, The frequencies of CD8+CD44+, CD8+CD62L+, CD4+CD44+, and CD4+CD62L+ T cells of MLN from orthotopic HCC mice were examined on day 22 post-inoculation. I, The frequencies of myeloid populations in MLN from orthotopic HCC mice were determined on day 22 post-inoculation. Data represent the means of four to six mice per group ± SEM and are representative of two experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
IL-21R signaling is essential for tumor memory immunity
To explore the role of IL-21R signaling in the memory response to tumor inoculation, we inoculated Hepa1-6 cells in the left groin of IL-21R KO and WT mice and reinoculated Hepa1-6 cells in the right groin of these mice on day 25 after the first inoculation. After day 19, the first subcutaneous tumor grew faster and was larger in IL-21R KO mice than WT mice; however, tumor growth decreased gradually and even disappeared in WT mice after day 19 (). We next comparatively analyzed two-time subcutaneous tumor growth within 10 days from the time of inoculation. Compared with the first subcutaneous tumor in WT mice, the second subcutaneous tumor showed slower growth, and tumor volume decreased, reached a peak on day 29 and then almost disappeared on day 35 (), suggesting that WT mice generated tumor-specific memory responses to tumor cells. However, no significant differences were found in the growth speed and tumor volume between the first and second subcutaneous tumors in IL-21R KO mice (), indicating that IL-21R KO mice failed to generate tumor-specific memory responses to tumor rechallenge. Thus, this experiment showed that IL-21R signaling is important for the generation of tumor-specific memory responses to tumor rechallenge.
Figure 5. Defects in secondary immune response in IL-21R KO tumor-bearing mice.
WT and IL-21R KO mice were subcutaneously inoculated with Hepa1-6 cells in the left groin and then reinoculated with Hepa1-6 cells in the right groin on day 25, and kinetics and profiles of primary and secondary immune cells in PB were determined over time. A, Tumor volume of the tumors is shown in WT mice and IL-21R KO mice subcutaneously inoculated with Hepa1-6 cells in the left groin for 35 days. B, Tumor volume of two inoculations is shown in WT mice and IL-21R KO mice subcutaneously inoculated with Hepa1-6 cells for 10 days. C, The frequency of CD3+ T cells in the PB of individual mice was examined. D, The frequencies of CD4+ T cells, CD4+CD44+ cells, and CD4+CD62L+ T cells in the PB of individual mice were examined. E, The frequencies of CD8+ T cells, CD8+CD44+ cells, and CD8+CD62L+ T cells in the PB of individual mice were examined. F, The frequencies of CD11b+ T cells, MDSCs, G-MDSCs, M-MDSCs, and macrophages in the PB of individual mice were measured. Data represent the means of four to six mice per group ± SEM and are representative of two experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
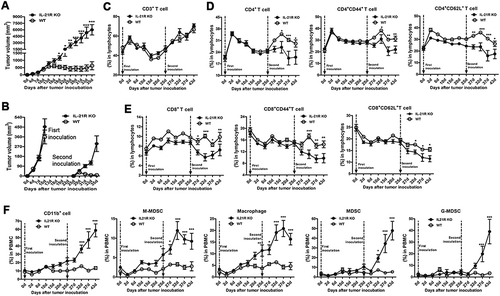
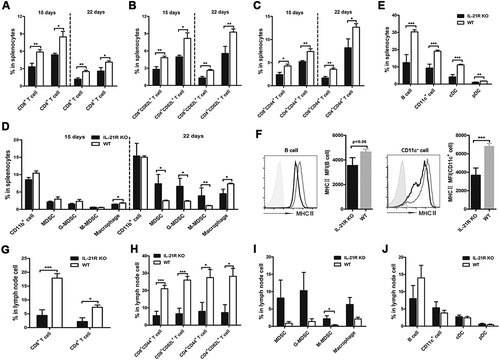
To determine the impact of IL-21R signaling defects on the systemic immune response in mice following two-time inoculation, we monitored the kinetics and profiles of immune cells in the peripheral blood (PB) in mice. We found that there were no differences in immune cells in PB between the two groups before tumor inoculation (day 0). Surprisingly, the CD3+ cell response was indistinguishable throughout the whole process in the two groups (). We then examined the kinetics of CD4+ and CD8+ T cells and their subpopulations ( and E). During the period of the first inoculation (day 1–25), the kinetics of CD4+, CD4+CD44+, CD4+CD62L+, CD8+, CD8+CD44+ and CD8+CD62L+ T cell responses appeared the same, but the magnitude of CD4+CD62L+, CD8+, CD8+CD44+ and CD8+CD62L+ T cell response observed in WT mice was slightly higher than that in IL-21R KO mice, indicating that the T cell response to tumor challenge was weakened in IL-21R KO mice. During the period of the second inoculation (day 25–43), however, these cell populations significantly decreased in IL-21R KO mice compared with WT mice. These data demonstrate that IL-21R deficiency weakened T cell responses to tumor challenge in the primary response and impaired T cell response in the secondary response in PB.
On the contrary, CD11b+ cell, M-MDSC and macrophage populations gradually increased during the whole process (), which is consistent with the trend of the subcutaneous tumor growth (). The results suggested there is a strong association between these cells and tumor growth, and these cells significantly increased in IL-21R KO mice after the second inoculation. MDSC and G-MDSC populations in the two groups were similar on day 1–25, but they significantly increased in IL-21R KO mice after day 32 (). These data suggest that IL-21R signaling deletion led to increased accumulation of MDSCs in PB in tumor-bearing mice.
IL-21R deletion causes immune cell expansion deficiency in the secondary immune response in tumor-bearing mice
As mentioned above, IL-21R defects cause T cell expansion deficiency in PB. To further determine the effects of IL-21R signaling on immune cells of the systemic immune system in the memory response, we analyzed the changes in these cells in central immune organs. Splenocytes on days 35 and 43 were collected and analyzed in two groups of mice following two-time inoculation. Interestingly, T cells and their subpopulations ( and B), and APCs () in the spleen gradually increased in tumor-reinoculated WT mice from day 35 to day 43, and these cells were significantly higher in tumor-reinoculated WT mice than IL-21R KO mice on days 35 and 43. Moreover, these cell populations recovered to the levels in normal spleen compared to those of naive WT mice on day 43 (-C). Surprisingly, B cell populations in tumor-reinoculated WT mice surpassed the levels of these cells in naive WT mice on day 43 (). Similarly, these cells in the bone marrow (BM) were significantly higher in tumor-reinoculated WT mice than tumor-reinoculated IL-21R KO mice and naive WT mice on day 43 (Fig. S2A-C). Conversely, these cells in spleen and BM from tumor-reinoculated IL-21R KO mice did not expand like those in tumor-reinoculated WT mice (-C and Fig. S2A-C), suggesting that IL-21R deletion impaired the expansion of these cells in spleen and BM following tumor rechallenge.
Figure 6. Defective expansion of antitumor immune cells in the spleen in IL-21R KO mice after secondary inoculation.
WT and IL-21R KO mice were subcutaneously inoculated with Hepa1-6 cells in the left groin and then reinoculated with Hepa1-6 cells in the right groin on day 25 after the first inoculation. After days 35 and 43, splenocytes were collected and measured by flow cytometry. A, The frequencies of CD4+ T cell, CD4+CD44+ and CD4+CD62L+ T cells in splenocytes from tumor-bearing WT and IL21R KO mice and naive mice are shown. B, The frequencies of CD8+ T cell, CD8+CD44+ and CD8+CD62L+ T cells in splenocytes from tumor-bearing WT and IL21R KO mice and naive mice were measured. C, The frequencies of B cells, DCs, cDCs, and pDCs in splenocytes from tumor-bearing WT and IL21R KO mice and naive mice were examined. D, The frequencies of MDSCs, G-MDSCs, M-MDSCs, and macrophages from splenocytes from tumor-bearing WT and IL21R KO mice and naive mice are shown. Data represent the means of five to six mice per group ± SEM and are representative of two experiments. s.c., subcutaneous injection. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
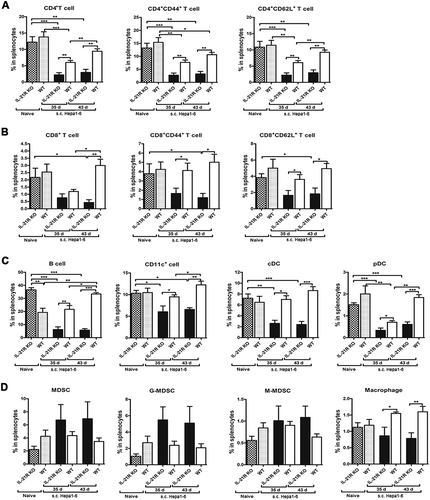
No obvious differences in the proportions of MDSCs, G-MDSCs, M-MDSCs and macrophages in the spleen and BM were found between tumor-reinoculated and naive WT mice on days 35 and 43 ( and Fig. S2E). However, MDSCs showed a marginal increase in the spleens of tumor-reinoculated IL-21R KO mice compared to those of naive IL-21R KO mice and tumor-reinoculated WT mice on days 35 and 43, but macrophages were significantly decreased in tumor-reinoculated IL-21R KO mice on days 35 and 43 (). Similarly, CD11b+ cell, MDSC and M-MDSC populations in the BM markedly increased in tumor-reinoculated IL-21R KO mice compared with naive IL-21R KO mice on day 43, but the G-MDSC population was substantially reduced (Fig. S2E). Compared with cells from tumor-reinoculated WT mice, M-MDSC and macrophage populations in the BM showed a significant increase, but the G-MDSC population substantially decreased in tumor-reinoculated IL-21R KO mice on day 43 (Fig. S2E). These results suggest that IL-21R deficiency contributed to increased accumulation of MDSCs in the spleen and BM in tumor-reinoculated mice. Additionally, compared with naive WT mice, B cells in the spleen were substantially increased in naive IL-21R KO mice (), and B cells, CD11c+ DCs and macrophages in the BM as well (Fig. S2C and D), however, CD11b+ cells and MDSCs in the BM were significantly decreased in naive IL-21R KO mice (Fig. S2E). These findings suggest that IL-21R signaling may have an important role in the development of different immune cells. Altogether, these results demonstrate that IL-21R signaling is indispensable for the expansion of antitumor immune cells in the memory immune response to tumor rechallenge.
IL-21/IL-21R expression correlates with prognosis of human HCC
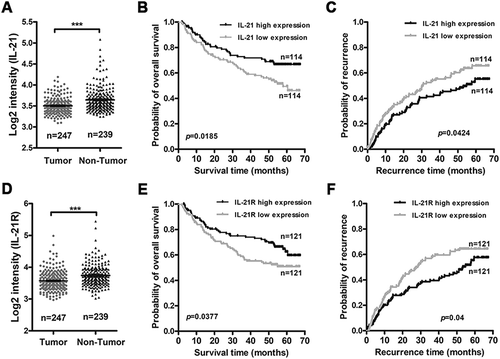
To study the clinical correlation of IL-21/IL-21R signals, we analyzed an HCC GEO RNA-seq dataset (GSE14520). After analysis, we found that IL-21 and IL-21R transcriptional levels in non-tumorous tissues were significantly higher than those in tumorous tissues ( and D), which is histopathologically consistent with the increased lymphocytes accumulation around the tumor and decreased lymphocytes infiltration within the tumor in HCC patients based on IL-21/IL-21R expression mainly on lymphocytes. Moreover, we found a positive correlation between IL-21 expression in non-tumorous tissues and overall survival and recurrence in HCC patients ( and C). Furthermore, high IL-21R expression in tumor tissues was associated with increased overall survival and recurrence in HCC patients ( and F). These findings hint that high IL-21R expression in tumor tissues may reflect increased lymphocytes infiltration and immune activation in the tumors of these patients because IL-21R is primarily expressed on immune cells and may be beneficial for control of HCC progression. However, no correlation between IL-21R expression in non-tumorous tissues or IL-21 expression in tumorous tissues and overall survival and recurrence in HCC patients was found (Fig. S3A-D). These results demonstrate that IL-21/IL-21R signaling may be a protective factor in controlling HCC development in HCC patients.
Figure 7. High IL-21 expression in non-tumor tissues and high IL-21R expression in tumor tissues are associated with good prognosis in HCC patients.
The correlations of IL-21 expression level in non-tumor tissues and IL-21R expression level in tumors and the prognosis of HCC patients were analyzed by the Kaplan-Meier method (data from GSE14520). The expression levels of IL-21 (A) and IL-21R (D) in tumor and non-tumor tissues from HCC patients. B-C, The association of IL-21 expression level in non-tumor tissues and the overall survival (B) and the recurrence (C) of patients with HCC. E-F, The association of IL-21R expression level in tumors and the overall survival (E) and the recurrence (F) of patients with HCC. Data represent the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Discussion
In this study, we demonstrated that IL-21R signaling has a protective role in controlling HCC growth. We found that rapid HCC growth in IL-21R KO mice characterized by suppressed antitumor immune responses and increased accumulation of protumor immunosuppressive cells in the tumor microenvironment. Similar to immunological disorder in the local tumor microenvironment, IL-21R deficiency causes an imbalance of the systemic immune system in tumor-bearing mice characterized by decreased activation and expansion of antitumor lymphocytes, increased accumulation of MDSCs and a decreased presentation ability of APCs in immune organs. We also demonstrated that IL-21R signaling plays a critical role in primary and secondary tumor-specific immune responses.
A previous study showed that IL-21 increased CD62L expression in T cells upon secondary antigen stimulation in vitro.Citation28 Similarly, our results showed that IL-21R deletion significantly reduced CD4+CD62L+ and CD8+CD62L+ T cell populations in the spleen, PB, MLN and liver tumors after tumor inoculation, especially after secondary inoculation. Several studies have demonstrated that adoptively transferred T cells utilize CD62L to target professional APCs and induce their in vivo activation, proliferation, and subsequent antitumor activity.Citation29,Citation30 Based on these findings, IL-21R deficiency may decrease CD62L expression in T cells after tumor inoculation, which likely caused decreased migration of T cells, failure of the interaction between T cells and professional APCs and finally inhibition of tumor-specific T cell generation. These findings suggested that IL-21R deletion impaired the memory response to tumor rechallenge in our study due to lack of tumor-specific T cells. In contrast to previous reports showing that IL-21 inhibited CD44 expression in CD8+ T cells with antigen stimulationCitation28 and blocked IL-15-dependent expansion of CD44highCD8+ TCR-independent T cells in vitro,Citation31 the current data showed a reduction of CD4+CD44+ and CD8+CD44+ T cells in IL-21R KO tumor-bearing mice, indicating that there are probably other signaling pathways that influence CD44 expression in T cells from IL-21R KO mice after tumor inoculation. Notably, Fröhlich A et al have showed that IL-21R signaling was appeared as superfluous for Th1 and Th17 responses by using IL-21R KO mice with Leishmania major infection and experimental autoimmune myocarditis, respectively.Citation32 But IL-21–mediated signaling also supports the generation and stabilization of pathogenic Th17 cells and development of spontaneous autoimmunity.Citation33 However, Andrew L. Rankin et al found a decrease of Th1 cell and an increase of Th17 cell in splenocytes in MRLlpr.IL-21R−/- mice.Citation34 Similarly, the data in our HCC mouse model also showed that IL-21R KO mice decreased production of IFN-γ and IL-21 and increased secretion of IL-17A in CD4+ T cells in liver tumor. These results suggested that IL-21R signaling have divergent effect on Th1 and Th17 response according to the different disease models. Thus, we speculated that the effects of IL-21R signaling on Th1 and Th17 response probably depend on the immune status of local microenvironment. Additionally, IL-21R deletion in our study inhibited IFN-γ production in CD8+ T cells to accelerate HCC growth.Citation16 Therefore, our results demonstrate that IL-21R signaling is important for maintaining the migration, activation, expansion and function of T cells after tumor challenge.
IL-21 differentially affects B cell fate depending on the signaling context. IL-21 not only induces B cell apoptosis without a T cell signal or with stimulation of a TLR signalCitation35 but also has a proapoptotic effect on many B cell lymphomas.Citation36–Citation40 Our results showed that B cell level in the spleen and BM from naive IL-21R KO mice was significantly higher than that in naive WT mice, presumably due to the suppression of B cell apoptosis in IL-21R KO mice. Furthermore, our results showed that IL-21R KO tumor-bearing mice had substantially reduced levels of B cell and MHCII molecule expression in the spleen, which probably results from the blocked B cell proliferation due to the lack of antigen-specific T cell stimulation in IL-21R KO mice. In contrast to previous studies showing that IL-21 can inhibit the maturation and activation of BM-derived DCs and induce cDC and pDC apoptosis in vitro,Citation41,Citation42 IL-21R deletion in our study had no impact on the development of DCs, pDCs and cDCs in the spleen but slightly increased their populations in BM of naive mice. These findings suggest that IL-21R signaling may not be the major factor in modulating the development and differentiation of DCs, and there may be other factors, such as GM-CSF and IL-15, that maintain DC development and differentiation in vivo.Citation41 However, our study showed that IL-21R deficiency reduced CD11c+ DCs, pDCs and cDCs and inhibited MHCⅡ expression in CD11c+ DCs in the spleen of orthotopic HCC mice, indicating that IL-21R signaling has a distinct effect on DC depending on the context of microenvironment. Thus, further attempts need to be undertaken to elucidate the exact actions of IL-21 on DCs in different situations. Interestingly, Andrew L. Rankin et al demonstrated that MRLlpr mice deficiency for IL-21R exhibited reduced levels of splenomegaly compared to MRLlpr mice with systemic autoimmunity, which was due to the accumulation of DN (CD4− CD8− CD3+) T cells and activated CD4+, CD8+ T, and B cells.Citation34 On the contrary, in our study, IL-21R KO mice with orthotopic HCC had obvious splenomegaly compared to WT mice, and IL-21R KO mice showed a decreased levels of T, and B cell. However, we also found that IL-21R KO mice with HCC inoculation had a hepatomegaly and abdominal swelling compared to WT mice in the process of experiment. So we speculated that the development of splenomegaly in IL-21R KO mice maybe as a consequence of backflow obstruction of hepatic portal vein and compensatory proliferation of spleen due to liver tumor development.
Overexpression of IL-21 in mice can increase the myelomonocytic lineage CD11b+ and Gr-1+ cell populations in the spleen.Citation14 Similarly, our results showed that MDSC, G-MDSC and M-MDSC populations in the spleen of naive IL-21R KO mice showed a slight decrease, but CD11b+ cells and MDSCs in BM of naive IL-21R KO mice were significantly decreased compared to those of naive WT mice. These results indicated that IL-21R signaling had a role in the development of myelomonocytic lineage cells, and further studies are needed to clarify their connection. However, our data showed that the accumulation of CD11b+ cells, MDSCs, G-MDSCs and M-MDSCs in the liver tumors, spleen and MLN significantly increased in IL-21R KO tumor-bearing mice, and based on the increased expression of chemokines, such as CCL2Citation43, CCL7 and CXCL5Citation9,Citation26 in liver tumor, we speculated that the accumulation of such cells may result from the chemotaxis. Meanwhile, we found that the MDSCs expansion and proangiogenic genes were upregulated in tumor tissue in IL-21R deficiency mice, which suggest that MDSCs may promote HCC growth by enhancing tumor cell survivalCitation44 and angiogenisis in tumor microenvironment.Citation45
Consistent with viral infection,Citation20 our data demonstrated that IL-21R KO mice cannot generate tumor-specific memory responses to tumor rechallenge, which was confirmed by the impaired expansion of antitumor immune cells and the increased accumulation of MDSCs in PB, spleen and BM in tumor-reinoculated mice. Consistent with our findings, IL-21 can prolong survival time of thymoma-bearing mice, and these long-term survivors showed increased survival times when rechallenged with thymoma, which was dependent on a persistent CD8+ T cell memory population.Citation46 These results demonstrated that IL-21R signaling is essential for the immunological memory response to tumor rechallenge and maintaining the balance of systemic immune responses. Interestingly, our results showed that MDSCs and G-MDSCs in PB quickly increased in tumor-reinoculated IL-21R KO mice after day 32. These findings are probably due to the metastasis of Hepa1-6 cells into the PB, which induces the migration of G-MDSCs from BM into PB, as we found that Hepa1-6 tumor cells had metastasized into the peritoneal cavity when we isolated the tumors on day 43.
Based on these results, we propose a reasonable immunological explanation for the rapid HCC growth in IL-21R KO mice: (1) IL-21R deficiency suppresses APC presentation and prevents DCs from recognizing tumor cells after tumor inoculation; (2) IL-21R deletion inhibits T cell migration and activation and reduces T cell migration to the tumor site to kill the tumors; these immunological deficiencies inhibit the generation of tumor-specific immune responses and increase proliferation of tumor cells in IL-21R KO mice; (3) in turn, tumor cells secrete high levels of chemokines to induce recruitment of immunosuppressive cells at the tumor site, which further suppress the antitumor effects of antitumor immune cells and promote angiogeisis for tumor cell survival. Thus, a vicious cycle to suppress immune responses is generated in IL-21R KO tumor-bearing mice and finally contributes to HCC progression and metastasis. Additionally, clinical data demonstrated that high IL-21 expression in non-tumor tissue or high IL-21R expression in tumor tissue has a positive correlation with overall survival and recurrence in HCC patients, which is supported by our results in IL-21R KO HCC mouse model and suggests IL-21R signaling have a protective role in controlling HCC progression and may act as a biomarker for immune activation in liver tumor.
Overall, our study demonstrated that IL-21R signaling can control the rapid HCC growth through enhancing antitumor immunity and reducing accumulation of MDSCs in tumor microenvironment, and is also critical for maintaining a balance in the systemic immune system and immunological memory response to tumor challenge. Further experiments are needed to explore the molecular mechanism by which IL-21R signaling impacts various immune cells in the tumor microenvironment, which may be useful for the development of novel therapies for HCC.
Methods and materials
Mice
C57BL/6 J mice was purchased from Southern Medical University Animal Center (Guangzhou, PR China) and Guangdong Medical Laboratory Animal Center (FoShan City, PR China). C57BL/6 IL-21R knockout (KO) mice was purchased from the Jackson Laboratory. The homozygous IL-21R KO mice were bred and all mice care and experimental procedures were performed under specific pathogen-free conditions. 8–12 week old age-matched male mice were used in the experiments. All animal protocols were approved by the Institutional Laboratory Animal Care and Use Committee at Southern Medical University (Guangzhou, PR China).
Establishment and assessment of murine hepatocellular carcinoma models
Murine HCC cell line Hepa1-6 cultured in Dulbecco’s Modified Eagle Medium (Gibco) supplemented with 10% FBS, 100 IU/mL penicillin/streptomycin at 37℃, 5% CO2. For subcutaneous HCC model, 1 × 107 Hepa1-6 cells were injected subcutaneously into the left leg groin in 8- to 12-week-old wild-type (WT) or IL-21R KO mice, and tumor growth was monitored every 3 days. Mices were sacrificed at indicated time from tumor inoculation. For second subcutaneous HCC inoculation, 1 × 107 Hepa1-6 cells were injected subcutaneously into the right leg groin on day 25 in WT and IL-21R KO mice after first inoculation, and tumor growth was monitored at indicated time. The subcutaneous tumors were measured with fine digital calipers and tumor volume was calculated by the following formula: tumor volume = 0.5× widthCitation2× length. Orthotopic HCC model was performed as described earlier with minor modification;Citation47 in brief, anesthetized mice were performed with surgical procedures, and then 2 × 106 Hepa1-6 cells in 40 μl PBS were implanted intrahepatically. The mice were sacrificed at indicated time, the tumor size and liver weight and spleen weight were measured.
Flow cytometry
For cell-surface staining, cell samples were stained with fluorescent dye-conjugated mAb against selected markers in PBS containing 0.5% FBS for 30 minutes at 4℃. For intracellular cytokine staining, cells were stimulated for 6 hours with phorbol 12-myristate 13-acetate (PMA; 50 ng/mL) and ionomycin (750 ng/mL) in the presence of brefeldin A (10μg/mL; BD Biosciences) for intacellular cytokine staining of CD8+ T cell and MDSCs; cells were stimulated for 6 hours with Anti-CD3/CD28 (1μg/mL) in the presence of brefeldin A (10μg/mL; BD Biosciences) for intracellular cytokine staining of CD4+ T cell. Cells were harvested, washed, and stained with surface molecule antibodies in PBS containing 0.5% FBS for 30 minutes at 4℃. Following surface staining, intracellular cytokine staining was performed with a Cytofix/Cytoperm Fixation/Permeabilization Kit (BD Biosciences) according to the manufacturer’s instructions. To detect degranulation, cells were stimulated for 6 hours in the presence of PMA (50 ng/ml), brefeldin A and anti-CD107a antibodies (BD Biosciences). The antibodies used for flow cytometry are listed in Supplementary Table 1. Data were collected by using a FACS CantoII and analyzed by FlowJo software (FlowJo).
Histological analysis and IHC
Liver tissue was formalin-fixed, and paraffin-embedded. Liver tissue sections (5μm thick) were stained with hematoxylin and eosin (HE). For IHC, briefly, mouse liver tumor sections were microwaved for 10 min in 0.01 M Na-citrate buffer (pH 6.0) and endogenous peroxidase was blocked with 3% H2O2. Primary antibodies CD4 (1:200, Sino Biological Inc.); CD8 (1:200, Bioss Inc.); CD20 (1:250, Santa Cruz); CD68 (1:200, Abcam); IL-21 (1:250, Bioss Inc.) were applied in blocking buffer (1% BSA, 5% normal goat serum in PBS) to incubate sections overnight at 4℃, followed by the secondary antibody incubation ABC kits and DAB reagents (Gene Tech (shanghai) Company Limited).
In vitro proliferation assay
Cell proliferation rates were determined by Cell Counting MTT assays (Beyotime, Jiangsu, China) according to the manufacturer instructions. Briefly, 1 × 105 Hepa1-6 cells were seeded in 96-well plates and were incubated at different recombined IL-21 (Sino Biological Inc, Beijing, China) concentrations: 0, 10, 20, 50, 100, 200ng/ml. After 48 h inoculation, MTT solution was added to each well and after 4 hours incubation, DMSO was added. The absorbance value was read at 562 nm.
Cell preparation
Spleen cells were prepared by gently crushing the tissues to release the cells. Preparations were filtered to remove debris and washed with sterile PBS before resuspending in 30% Percoll (GE Healthcare). Splenocytes were separated on a 30% to 70% Percoll gradient. The interlayer cells were collected and washed with sterile PBS and suspended with RPMI-1640 complete medium. For interahepatic mononuclear cell, peripheral blood in mice was completely drawed off with orbital blood sampling and liver was isolated and processed into single-cell suspensions. Suspension cells were centrifuged at 50 g for 3 minutes for precipitating liver cells, supernatants were collected and resuspended in 30% Percoll, and interahepatic mononuclear cells were separated on a 30% to 70% Percoll gradient. The interlayer cells were collected and washed with sterile PBS and suspended with RPMI-1640 complete medium. For preparation of mesenteric lymph node (MLN) cell, MLN were isolated from mice, and lymph nodes were gently crushed to release the cells and lymph node cells were filtered to remove debris and washed with PBS and suspended in RPMI-1640 complete medium. For preparation of bone marrow (BM) cell, the femur and shinbone in mice were dissected neatly and BM cells was washed in sterile PBS after the BM was crushed. BM cells were filtered to remove debris and washed with PBS and suspended in RPMI-1640 complete medium. Erythrocytes were lysed by BD FACSTM Lysing Solution (BD Biosciences). Cell counts were calculated for flow cytometery tests.
RNA isolation and quantitative real-time PCR
Total RNA was extracted from liver tumors using Trizol reagent (Invitrogen) and transcribed into cDNA by Transcriptor First Strand cDNA Synthesis Kit (Roche). Quantitative PCR (qPCR) was performed using the TB Green™ Premix Ex Taq™ II (Tli RNaseH Plus) (TaKaRa) with LightCycler 480 System . The relative expression of target gene was calculated using the 2−△△C(t) method. Fold change of target gene expression were calculated by normalization to the expression of the housekeeping gene β-Actin. The primer sequences of all genes for PCR are shown in Supplementary Table S3.
GEO dataset
We analyzed the Gene Expression Omnibus series GSE14520 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE14520), which provides 247 HCC cases (247 tumor tissue samples and 239 non-tumor tissue samples including gene expression data) and 242 HCC patients have follow-up information (242 tumor tissue samples and 228 non-tumor tissue samples including follow-up information). IL-21 (GenBank Accession no. NM_021803) and IL-21R (GenBank Accession no. AF269133) gene expression level in HCC tissues and adjacent non-tumor tissues were analyzed in this study. The overall survival and recurrence of HCC patients were analyzed based on IL-21/IL-21R expression level in tumor tissue and adjacent non-tumor tissues. HCC patients were divided into two groups depend on the expression of IL-21/IL-21R, the expression level greater than median was classified into high expression group, otherwise was classified into low expression group.
Statistical analysis
The comparisons between two groups were analyzed by two-tailed Student’s t-tests and multiple-group comparisons were performed by two-way analysis of variance followed by a Bonferroni correction to compare each group. Survival was determined by Kaplan–Meier method and survival curves between different groups were calculated by log-rank test. Data were expressed as mean ± SEM. Data were analyzed using GraphPad Prism 5 software for Windows (GraphPad Software) and differences were considered statistically significant when P < 0.05. The significance levels are marked as: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed by authors.
Supplemental Material
Download Zip (669.7 KB)Acknowledgments
We would like to thank Dr. Shuqin Gu and Shihong Zhong for their technical assistance and Yong Xie for help with mouse feeding.
Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Makarova-Rusher OV, Medina-Echeverz J, Duffy AG, Greten TF. The yin and yang of evasion and immune activation in HCC. J Hepatol. 2015;62:1420–1429. PMID:25733155. doi:10.1016/j.jhep.2015.02.038.
- Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681–700. PMID:26484443. doi:10.1038/nrgastro.2015.173.
- Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27:407–414. PMID:9462638. doi:10.1002/hep.510270214.
- Unitt E, Marshall A, Gelson W, Rushbrook SM, Davies S, Vowler SL, Morris LS, Coleman N, Alexander GJ. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J Hepatol. 2006;45:246–253. PMID:16580084. doi:10.1016/j.jhep.2005.12.027.
- Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pages F, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–618. PMID:21245428. doi:10.1200/jco.2010.30.5425.
- Wang D, An G, Xie S, Yao Y, Feng G. The clinical and prognostic significance of CD14(+)HLA-DR(-/low) myeloid-derived suppressor cells in hepatocellular carcinoma patients receiving radiotherapy. Tumour Biol. 2016;37:10427–10433. PMID:26846107. doi:10.1007/s13277-016-4916-2.
- Mizukoshi E, Yamashita T, Arai K, Terashima T, Kitahara M, Nakagawa H, Iida N, Fushimi K, Kaneko S. Myeloid-derived suppressor cells correlate with patient outcomes in hepatic arterial infusion chemotherapy for hepatocellular carcinoma. Cancer Immunol Immunother. 2016;65:715–725. PMID:27083166. doi:10.1007/s00262-016-1837-2.
- Gao XH, Tian L, Wu J, Ma XL, Zhang CY, Zhou Y, Sun YF, Hu B, Qiu SJ, Zhou J, et al. Circulating CD14+ HLA-DR-/low myeloid-derived suppressor cells predicted early recurrence of hepatocellular carcinoma after surgery. Hepatol Res. 2016;doi:10.1111/hepr.12831. PMID:27764536
- Lin Y, Yang X, Liu W, Li B, Yin W, Shi Y, He R. Chemerin has a protective role in hepatocellular carcinoma by inhibiting the expression of IL-6 and GM-CSF and MDSC accumulation. Oncogene. 2017;36:3599–3608. PMID:28166197. doi:10.1038/onc.2016.516.
- Kapanadze T, Gamrekelashvili J, Ma C, Chan C, Zhao F, Hewitt S, Zender L, Kapoor V, Felsher DW, Manns MP, et al. Regulation of accumulation and function of myeloid derived suppressor cells in different murine models of hepatocellular carcinoma. J Hepatol. 2013;59:1007–1013. PMID:23796475. doi:10.1016/j.jhep.2013.06.010.
- Kalathil S, Lugade AA, Miller A, Iyer R, Thanavala Y. Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013;73:2435–2444. PMID:23423978. doi:10.1158/0008-5472.can-12-3381.
- Liu SM, King C. IL-21-producing Th cells in immunity and autoimmunity. J Immunol. 2013;191:3501–3506. PMID:24058193. doi:10.4049/jimmunol.1301454.
- Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discov. 2014;13:379–395. PMID:24751819. doi:10.1038/nrd4296.
- Wang G, Tschoi M, Spolski R, Lou Y, Ozaki K, Feng C, Kim G, Leonard WJ, Hwu P. In vivo antitumor activity of interleukin 21 mediated by natural killer cells. Cancer Res. 2003;63:9016–9022. PMID:14695220.
- Ugai S, Shimozato O, Yu L, Wang YQ, Kawamura K, Yamamoto H, Yamaguchi T, Saisho H, Sakiyama S, Tagawa M. Transduction of the IL-21 and IL-23 genes in human pancreatic carcinoma cells produces natural killer cell-dependent and -independent antitumor effects. Cancer Gene Ther. 2003;10:771–778. PMID:14502230. doi:10.1038/sj.cgt.7700630.
- Di Carlo E, Comes A, Orengo AM, Rosso O, Meazza R, Musiani P, Colombo MP, Ferrini S. IL-21 induces tumor rejection by specific CTL and IFN-gamma-dependent CXC chemokines in syngeneic mice. J Immunol. 2004;172:1540–1547. PMID:14734732.
- Furukawa J, Hara I, Nagai H, Yao A, Oniki S, Fujisawa M. Interleukin-21 gene transfection into mouse bladder cancer cells results in tumor rejection through the cytotoxic T lymphocyte response. J Urol. 2006;176:1198–1203. PMID:16890725. doi:10.1016/j.juro.2006.04.037.
- Stolfi C, Rizzo A, Franze E, Rotondi A, Fantini MC, Sarra M, Caruso R, Monteleone I, Sileri P, Franceschilli L, et al. Involvement of interleukin-21 in the regulation of colitis-associated colon cancer. J Exp Med. 2011;208:2279–2290. PMID:21987656. doi:10.1084/jem.20111106.
- De Simone V, Ronchetti G, Franze E, Colantoni A, Ortenzi A, Fantini MC, Rizzo A, Sica GS, Sileri P, Rossi P, et al. Interleukin-21 sustains inflammatory signals that contribute to sporadic colon tumorigenesis. Oncotarget. 2015;6:9908–9923. PMID:25839161. doi:10.18632/oncotarget.3532.
- Barker BR, Gladstone MN, Gillard GO, Panas MW, Letvin NL. Critical role for IL-21 in both primary and memory anti-viral CD8+ T-cell responses. Eur J Immunol. 2010;40:3085–3096. PMID:21061439. doi:10.1002/eji.200939939.
- Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. PMID:11081504. doi:10.1038/35040504.
- Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med. 2005;11:1299–1305. PMID:16288282. doi:10.1038/nm1326.
- Hinrichs CS, Gattinoni L, Restifo NP. Programming CD8+ T cells for effective immunotherapy. Curr Opin Immunol. 2006;18:363–370. PMID:16616471. doi:10.1016/j.coi.2006.03.009.
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. PMID:8600538.
- Weninger W, Crowley MA, Manjunath N, Von Andrian UH. Migratory properties of naive, effector, and memory CD8(+) T cells. J Exp Med. 2001;194:953–966. PMID:11581317.
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. PMID:22437938. doi:10.1038/nri3175.
- Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–752. PMID:24060865. doi:10.1038/nrc3581.
- Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. PMID:18276844. doi:10.1182/blood-2007-09-113050.
- Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. PMID:15931392. doi:10.1172/jci24480.
- Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102:9571–9576. PMID:15980149. doi:10.1073/pnas.0503726102.
- Kasaian MT, Whitters MJ, Carter LL, Lowe LD, Jussif JM, Deng B, Johnson KA, Witek JS, Senices M, Konz RF, et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity. 2002;16:559–569. PMID:11970879
- Fröhlich A, Marsland B, Sonderegger I, Kurrer M, Hodge M, Harris N, Kopf M. IL-21 receptor signaling is integral to the development of Th2 effector responses in vivo. Blood. 2007;109:2023–2031. doi:10.1182/blood-2006-05-021600.
- Lee Y, Mitsdoerffer M, Xiao S, Gu G, Sobel RA, Kuchroo VK. IL-21R signaling is critical for induction of spontaneous experimental autoimmune encephalomyelitis. J Clin Invest. 2015;125:4011–4020. PMID:26413871. doi:10.1172/JCI75933.
- Rankin AL, Guay H, Herber D, Bertino SA, Duzanski TA, Carrier Y, Keegan S, Senices M, Stedman N, Ryan M, et al. IL-21 receptor is required for the systemic accumulation of activated B and T lymphocytes in MRL/MpJ-Fas(lpr/lpr)/J mice. J Immunol. 2012;188:1656–1667. PMID:22231702. doi:10.4049/jimmunol.1003871.
- Mehta DS, Wurster AL, Whitters MJ, Young DA, Collins M, Grusby MJ. IL-21 induces the apoptosis of resting and activated primary B cells. J Immunol. 2003;170:4111–4118. PMID:12682241.
- Jahrsdorfer B, Blackwell SE, Wooldridge JE, Huang J, Andreski MW, Jacobus LS, Taylor CM, Weiner GJ. B-chronic lymphocytic leukemia cells and other B cells can produce granzyme B and gain cytotoxic potential after interleukin-21-based activation. Blood. 2006;108:2712–2719. PMID:16809616. doi:10.1182/blood-2006-03-014001.
- Akamatsu N, Yamada Y, Hasegawa H, Makabe K, Asano R, Kumagai I, Murata K, Imaizumi Y, Tsukasaki K, Tsuruda K, et al. High IL-21 receptor expression and apoptosis induction by IL-21 in follicular lymphoma. Cancer Lett. 2007;256:196–206. PMID:17624663. doi:10.1016/j.canlet.2007.06.001.
- Gowda A, Roda J, Hussain SR, Ramanunni A, Joshi T, Schmidt S, Zhang X, Lehman A, Jarjoura D, Carson WE, et al. IL-21 mediates apoptosis through up-regulation of the BH3 family member BIM and enhances both direct and antibody-dependent cellular cytotoxicity in primary chronic lymphocytic leukemia cells in vitro. Blood. 2008;111:4723–4730. PMID:18182577. doi:10.1182/blood-2007-07-099531.
- De Totero D, Capaia M, Fabbi M, Croce M, Meazza R, Cutrona G, Zupo S, Loiacono F, Truini M, Ferrarini M, et al. Heterogeneous expression and function of IL-21R and susceptibility to IL-21-mediated apoptosis in follicular lymphoma cells. Exp Hematol. 2010;38:373–383. PMID:20193734. doi:10.1016/j.exphem.2010.02.008.
- Sarosiek KA, Malumbres R, Nechushtan H, Gentles AJ, Avisar E, Lossos IS. Novel IL-21 signaling pathway up-regulates c-Myc and induces apoptosis of diffuse large B-cell lymphomas. Blood. 2010;115:570–580. PMID:19965678. doi:10.1182/blood-2009-08-239996.
- Brandt K, Bulfone-Paus S, Foster DC, Ruckert R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood. 2003;102:4090–4098. PMID:12893770. doi:10.1182/blood-2003-03-0669.
- Wan CK, Oh J, Li P, West EE, Wong EA, Andraski AB, Spolski R, Yu ZX, He J, Kelsall BL, et al. The cytokines IL-21 and GM-CSF have opposing regulatory roles in the apoptosis of conventional dendritic cells. Immunity. 2013;38:514–527. PMID:23453633. doi:10.1016/j.immuni.2013.02.011.
- Huang B, Lei Z, Zhao J, Gong W, Liu J, Chen Z, Liu Y, Li D, Yuan Y, Zhang GM, et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007;252:86–92. PMID:17257744. doi:10.1016/j.canlet.2006.12.012.
- Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–178. PMID:22770218. doi:10.1016/j.cell.2012.04.042.
- Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. PMID:15488763. doi:10.1016/j.ccr.2004.08.031.
- Moroz A, Eppolito C, Li Q, Tao J, Clegg CH, Shrikant PA. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. J Immunol. 2004;173:900–909. PMID:15240677.
- Wang Q, Luan W, Goz V, Burakoff SJ, Hiotis SP. Non-invasive in vivo imaging for liver tumour progression using an orthotopic hepatocellular carcinoma model in immunocompetent mice. Liver Int. 2011;31:1200–1208. PMID:21745281. doi:10.1111/j.1478-3231.2011.02523.x.
