ABSTRACT
The B7-H1/PD-1 immune co-inhibitory pathway is functionally bi-directional. We showed previously that B7-H1 could be widely induced on various types of cells and, in addition to be a ligand for PD-1 on T-cells, also serve as an anti-apoptotic receptor upon interacting with PD-1. We explored the role of B7-H1 as a receptor in protecting allogeneic T-cell mediated host cell destruction and systemic inflammation using mouse models of graft-versus-host disease (GVHD). Administer of by PD-1Ig or a B7-H1 monoclonal antibody (mAb) led to accelerated progression and rapid death in mice transferred with wild type allogeneic T-cells, supporting a dominant role of this pathway in the suppression of allogeneic T-cell response. In sharp contrast, PD-1Ig or B7-H1 mAb could behave as the B7-H1 agonists and drastically ameliorate the progression of GVHD and induced long-term tolerance in the context of transferring PD-1 deficient allogeneic T-cells. We further demonstrated that B7-H1 agonists decreased susceptibility of normal hematopoietic cells to allogenic T-cell lysis in vitro and in vivo. More importantly, mice that developed tolerance could still mount graft-versus-leukemia response. Our findings indicate a role for intrinsic B7-H1 in protecting host cells during systemic inflammation and have implications for treating human diseases including GVHD.
Introduction
B7 Homolog One (B7-H1, PD-L1, CD274) is a member of the B7-CD28 immune regulatory gene family and its mRNA is found at low levels in various hematopoietic and stromal cells. Importantly, expression of cell surface B7-H1 can be upregulated by inflammatory cytokines, mainly by IFN-γ in virtually all cell types tested thus farCitation1-Citation4. Upon binding Programmed Death One (PD-1, CD279), B7-H1 suppresses T cell immunity by different mechanisms, including induction of apoptosis, anergy, and exhaustion, as well as suppression of innate immunity involving dendritic cells and macrophagesCitation4,Citation5. Inducible expression of B7-H1 by IFN-γ secreted from effector T cells constitutes an important mechanism of adaptive resistance for tumor immunity. Hence, specific mAb that block the B7-H1/PD-1 interaction can enhance anti-tumor immunityCitation2,Citation6 and induce tumor regression which can increase survival benefits of a fraction of patients with advanced cancersCitation6,Citation7. While B7-H1 acts as a ligand for PD-1, B7-H1 could also act as a receptor that prevents deathCitation8 and regulates the glucose metabolism of tumor cellsCitation9. To what extent this function of B7-H1 acts in normal physiology versus disease states has yet to be addressed.
With the broadly inducible expression pattern of B7-H1 on hematopoietic cells and somatic cells, enhancing B7-H1 signaling may protect inflammatory tissues from the damage caused by host immune responses. Allogeneic hematopoietic stem-cell transplantation is a therapy for a variety of disorders but the occurrence of graft versus host disease (GVHD) is a major limitation for its applicationCitation10,Citation11. Alloreactive donor T cell-mediated cytotoxic responses against tissues of the recipient result in organ damage. Blockade of the B7-H1/PD-1 pathway was shown to aggravate GVHD in mouse models using blocking antibodiesCitation12-Citation15. We reasoned that B7-H1 will be broadly upregulated on host cells, including those in vital organs, by IFN-γ from alloreactive T cells upon recognition of allogeneic antigens and engagement of B7-H1 helps prevent the death of host cells and ameliorate GVHD. To test this possibility, we employed a mouse model in which PD-1 deficient B6 (C57BL/6) T cells were transferred into CB6F1 (C57BL/6× BALB/c, H-2b×d) mice to induce allogeneic CTL-mediated GVHD. We demonstrate that engagement of B7-H1 could ameliorate GVHD in this model by protecting host cell death.
Results
Blockade of the B7-H1/PD-1 pathway aggravated GVHD in wild type mice
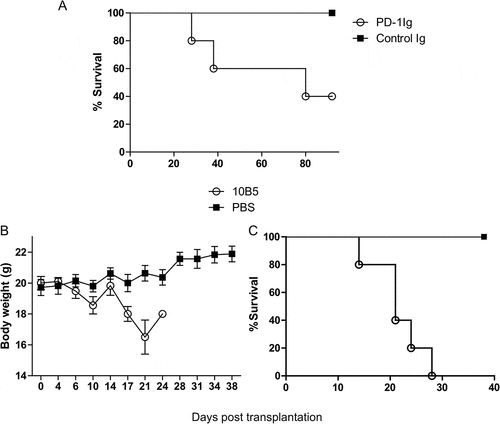
Blockade of the B7-H1/PD-1 pathway by 10B5, an anti-B7-H1 mAb, was shown to aggravate GVHD in a mouse model in which the transfer of irradiated CB6F1 mice with allogeneic spleen cells from B6 mice () and GVHD was monitored by accelerated death, loss of body weight and GVHD score (see Methods), a result similar to those reported previouslyCitation10,Citation12,Citation13,Citation15. 10B5 could block the B7-H1/PD-1 interaction and is the first mAb showing to promote T-cell mediated immune responses in mouse tumor modelsCitation2. In addition, we infused a plasmid encoding a recombinant fusion protein of extracellular murine PD-1 and IgG2a Fc (PD-1Ig) with high pressure to enforce the expression in the recipient CB6F1 mice. This hydrodynamic injection of PD-1Ig plasmid led to rapid elevation of PD-1Ig in sera in a few hours, and serum PD-1Ig levels were maintained up to 8 days with a half-life of approximately 15 days based on sandwich enzyme-linked immunosorbent assay (ELISA) (Fig. S1). Mice treated with PD-1Ig had significantly decreased survival than the control (). Our results thus confirm previous finding that blockade of the B7-H1/PD-1 interaction could promote GVHD.
Figure 1. Blockade of the B7-H1/PD-1 pathway aggravated GVHD in mice. (A) PD-1Ig or control Ig plasmids were injected in high pressure into the tail veins of CB6F1 mice that received 5Gy total body irradiation two days prior to receiving 6 × 10Citation7 splenocytes from B6 donor. Survival rates were shown between the mice receiving PD-1Ig (n = 5) and control Ig plasmid (n = 5). P < 0.05. B6D2F1 mice were also received 9 × 10Citation7 splenocytes from B6 mice and were administrated with 10B5 (anti-B7-H1 mAb, n = 5) or control antibody (PBS, n = 5) at day 0, 3, 7, 10, 14, 17. The body weight (B) and GVHD clinical score (C) were shown as mean ± SEM. The differences between two groups of mice were statistically significant, P < 0.01. The data are representative of at least two independent experiments and were shown as mean ± SEM
Engagement by PD-1Ig of B7-H1 receptor ameliorated GVHD in the absence of intrinsic PD-1
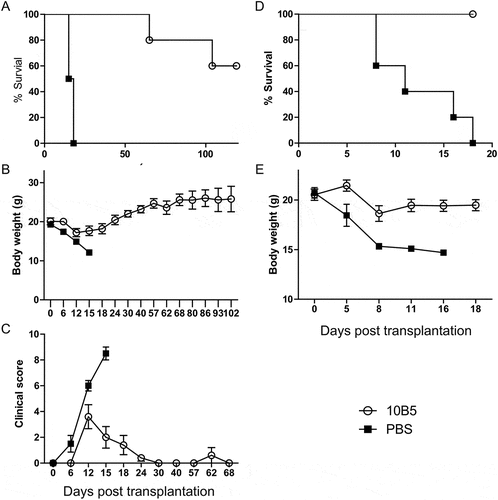
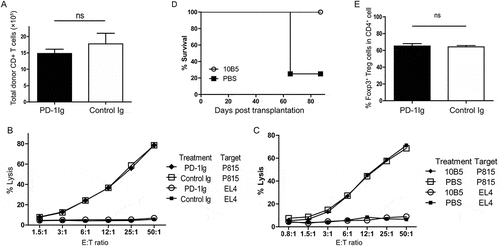
We next tested the hypothesis that if B7-H1 agonists protect mice from GVHD. To avoid the dominant effect of PD-1, we modified the GVHD model by the transfer of PD-1 deficient (PD-1KO) allogeneic T-cells. As expected, transfer of PD-1KO T cells into CB6F1 mice led to accelerated death of mice than that of wild type T cells (). In sharp contrast, the treatment with PD-1Ig showed significantly higher survival rates (), increased body weights (), and lower GVHD clinical scores () than the control. These findings indicate that, in the absence of PD-1 signaling to T cells, engagement of B7-H1 by PD-1Ig could suppress the progress of GVHD.
Figure 2. PD-1Ig ameliorated GVHD in a mouse model. PD-1Ig or control Ig plasmids were injected into CB6F1 mice two days prior to receiving 3.5 × 10Citation7 splenocytes from B6 PD-1KO donor. (A) Survival rates were shown between cohorts receiving PD-1Ig (n = 4) and control Ig plasmid (n = 5). P < 0.01. (B) The body weight of each mouse was measured regularly after the transplantation and data were shown as mean ± SEM. (C) GVHD clinical score of cohorts after giving splenocytes and data shown as mean ± SEM clinical score of cohorts at each time. All data shown are representative of at least two independent experiments
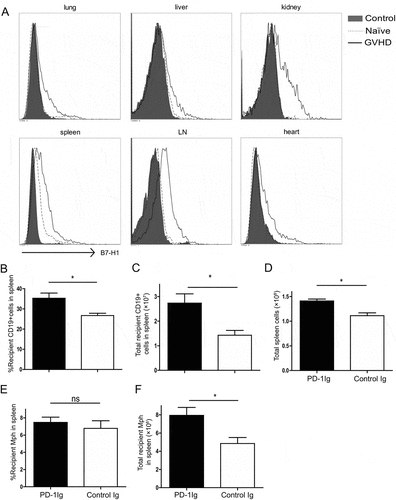
Since B7-H1 could be induced on various tissues and cells during inflammation via IFN-γ, we tested the hypothesis that broad induction of B7-H1 expression on peripheral tissues during disseminated inflammation helps host cells resist CTL destruction. Donor PD-1 deficient B6 T cells respond to allogenic H-2d antigens and mounts rapid CTL responses accompanied by upregulation of B7-H1 expression on cells isolated from lung, liver, kidney, spleen, lymph nodes, and heart (). This is most likely due to the release of IFN-γ from alloreactive T cells as suggested previouslyCitation2,Citation4. This effect is also dependent on the recognition of allogeneic antigen because the transfer of B6 naïve or anti-CD3 activated PD-1KO T cells into syngeneic B6 mice, however, did not upregulate B7-H1 expression on these tissues (Fig. S2). Eight days after allogeneic T cell transfer, the recipient mice were sacrificed; spleen and LN removed and cells were analyzed by flow cytometry. In the spleen, the mice that received PD-1Ig plasmid had significantly more CD19 + B cells () and total spleen cells () compared with the control. While the difference in percent of recipient macrophages (Mph) was insignificant (), the absolute number of recipient Mph in the spleen was significantly higher in the PD-1Ig group (). Similar results were obtained in the LN cells (data not shown). The loss of B cells due to allogeneic CTL lysis was an early sign of allogeneic GVHD and was followed by the loss of other hematopoietic cellsCitation16. Our finding supports a protective effect for B7-H1 on host cells upon PD-1Ig engagement in this GVHD setting.
Figure 3. Agonist effect of PD-1Ig on protecting host cells in a murine GVHD model. (A) Upregulation of B7-H1 after allogeneic T cell transplantation. Tissues of lung, liver, kidney, heart, spleen, and lymph node (LN) taken from naïve CB6F1 mice or mice 9 days after PD-1 KO B6 splenocytes transfer (control and GVHD), were digested with collagenase D for 40 minutes. Single cell suspensions were stained with anti-mouse B7-H1 mAb (clone 10B5) (GVHD) or control mAb (control) and tested by flow cytometry. (B-F) CB6F1 mice were injected with PD-1Ig or control Ig plasmids. Two days later, splenocytes from B6 PD-1KO mice were transferred to non-irradiated CB6F1 mice at 3.5 × 10Citation7 per mouse. Day 8 after the transfer, splenocytes were stained with anti-H-2d and H-2b mAb; donor (H-2b) and recipient cells (H-2bxd) were identified by flow cytometry. The % of CD19 + B cells in total recipient splenocytes (B), absolute numbers of recipient CD19 + B cells (C), total spleen cells (D), the % of recipient macrophages in recipient splenocytes (E) and absolute numbers of recipient macrophages (F) were shown. Data were analyzed by Student’s t test (*P < 0.05, ns P > 0.05) and shown as mean ± SEM
B7-H1 mAb ameliorated GVHD in both MHC semi- and fully mismatched models
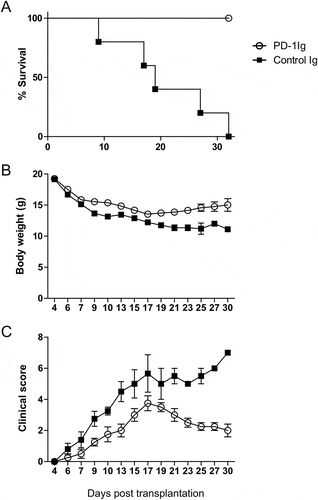
In addition to B7-H1, PD-1Ig could also engage B7-DC (PD-L2) and its role as a receptor remains unclearCitation4. To exclude this possibility, mice were treated with anti-murine B7-H1 mAb instead of PD-1Ig, and showed significantly improved GVHD symptoms and increased survival (), increased body weight and lower GVHD clinical score () compared to controls. Similar results were also found when the same treatments were used in a MHC fully mismatched GVHD model (). In our GVHD model, however, 10B5 showed an agonistic effect, likely due to the lack of PD-1 on donor T cells. Our results support that engagement of B7-H1 on host cells prevents GVHD by allogeneic T cells.
Figure 4. B7-H1 mAb ameliorated GVHD in both MHC semi- and fully mismatched models. CB6F1 mice received 3.5 × 10Citation7 splenocytes from B6 PD-1KO mice. Mice were administered with 10B5 (anti-B7-H1 mAb, n = 5) or control (PBS, n = 4) at day 0, 9, 12, 15. (A) Survival rates, P < 0.01, (B) the body weight and data were shown as mean ± SEM, and (C) GVHD clinical score were shown as mean ± SEM. In a fully MHC-mismatched GVHD model, Balb/c mice received 6Gy total body irradiation and injected i.v. with 5 × 10Citation6 T cell-depleted BM from PD-1 KO B6 background mice. Twelve days later, 5 × 10Citation6 PD-1 KO splenocytes were injected into these recipient mice. Mice were administrated with 10B5 (anti-B7-H1 mAb, n = 5) or control (PBS, n = 5) at day 0, 6, 10, 15. (D) Survival rates, P < 0.01, (E) the body weight and data were shown as mean ± SEM. All data shown are representative of at least two independent experiments
PD-1Ig or mAb treatment in host mice did not affect allogenic CTL activity
To explore the mechanism of B7-H1-mediated protection of GVHD, we first examined if donor T cells expand upon treatment with PD-1Ig after transfer of PD-1KO B6 T cells to CB6F1. As shown in , the number of H-2b donor CD8 + T cells, a major subset of T cells that mediate GVHDCitation10, did not show significant expansion versus the control. Allogeneic CTL activity was also assessed using the cytolytic activity of spleen cells from CFSE-labeled P815 cells (H-2d) in vitro on day 8 after donor T cell transfer. Allogeneic CTLs could lyse P815 cells but not syngeneic EL4 (H-2b) cells. Interestingly, treatment with PD-1Ig or 10B5 did not change allogeneic CTL activity as compared with controls in all tested effector/target ratios (). Mice that survived upon 10B5 treatment could resist to the challenge of A20 lymphoma while 75 percent of the control mice (CB6F1 mice) were died of rapid tumor growth (). We also excluded possible role of T regulatory cells (Treg) because there was no significant increase of Treg numbers in the spleens of mice which were tolerated to GVHD after the PD-1Ig treatment (). These findings indicate that the protective effect of PD-1Ig on normal tissues is neither due to expansion nor general suppression of allogeneic CTL.
Figure 5. PD-1Ig or mAb treatment in host mice did not affect allogenic CTL activity. (A) PD-1Ig or control Ig plasmids were injected into CB6F1 mice two days prior to receiving 3.5 × 10Citation7 splenocytes from B6 PD-1KO donor. Splenocytes were stained with the mAb to H-2d, H-2b and CD8 mAb, and analyzed by flow cytometry on day 8. (B, C) Spleen cells from CB6F1 mice harvested 8 days post donor cell transfer were incubated with CFSE-labeled target cells at the indicated effector/target (E/T) ratios for 4 hours at 37◦C, 0.1μg DAPI were added and analyzed by flow cytometry immediately. Cytolytic activity of allogenic CTL was calculated as described in Methods. (D) CB6F1 mice received 3.5 × 10Citation7 splenocytes from B6 PD-1KO mice, were administrated with 10B5 (anti-B7-H1 mAb, n = 4) at day 0, 9, 12, 15. Thirty-two days later (indicated as the day 0), 1 × 10Citation6 A20 cells were injected intravenously into the treated CB6F1. Naïve CB6F1 (n = 4) were also challenged with identifical number of A20 as the controls. Survival rates, P < 0.05. (E) PD-1Ig or control Ig plasmids were injected into CB6F1 mice two days prior to receiving 3.5 × 10Citation7 splenocytes from B6 PD-1KO donor. Splenocytes were stained with the mAb to CD4 and foxp3, and analyzed by flow cytometry on day 8. The data are representative of at least two experiments and were shown as mean ± SEM. The results were analyzed by Student’s t test; *P < 0.05, **P < 0.01, ***P < 0.001
PD-1Ig treated B7-H1+ cells resistance to alloreactive CTLs lysis
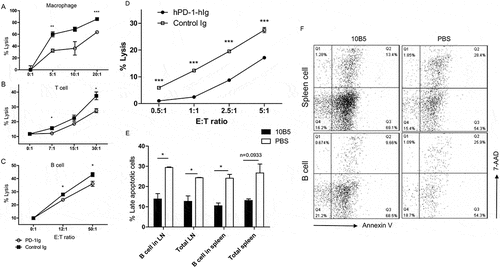
To test the possibility that B7-H1 on target cells confers resistance to allogeneic CTL lysis upon PD-1Ig engagement, macrophages (Mph), B cells, and T cells from CB6F1 mice were treated with IFN-γ to upregulate B7-H1 as detected by flow cytometry. Naïve Mph, B and T cells expressed negligible cell surface B7-H1, treatment with IFN-γ significantly upregulated B7-H1 (Fig. S3). Cells were subsequently treated with plate-coated PD-1Ig and were co-cultured with allogeneic CTL to test their susceptibility to lysis. PD-1Ig treated Mph, T, and B cells were significantly less sensitive to allogeneic CTL lysis (). In addition, PD-1Ig-treated human PBMCs were also significantly less sensitive to the lysis by allogeneic human CTLs (). To determine if the resistance to allogenic CTL lysis is due to deceased apoptosis, we isolated B cells from the spleens of mice that were treated with or without 10B5 and evaluated apoptosis. With 10B5 treatment, B cells had significantly less apoptosis as shown less 7-AAD and annexin V double positive cells (). These data indicate that B7-H1 agonists may decrease susceptibility of host cells by suppressing cell apoptosis.
Figure 6. Cells from mice and human resistance to lysis after treated with PD-1Ig. Freshly isolated macrophages (A), T cells (B), and B cells (C) from CB6F1 mice were lysis by CTLs. (D) The lysis of human PBMCs by allo-PBMC CTLs in vitro. (E and F) Splenocytes from mice received 3.5 × 10Citation7 splenocytes from B6 PD-1KO donor and treated with 10B5 or PBS were stained with anti-CD19 mAb, annexin V, and 7-AAD, and analyzed by flow cytometry on day 8. The data are representative of at least two experiments and were shown as mean ± SEM. The results were analyzed by Student’s t test; *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
Although a major role of B7-H1 is a ligand for PD-1 to suppress T cell response, several studies including ours showed that B7-H1 could act as a receptor to transmit an anti-apoptotic signal. In this study, we showed that engagement of B7-H1 on host cells could protect them from the destruction by allogeneic T cells and ameliorate GVHD. These results extend our previously findings and support a potential application of this strategy for the suppression of systemic inflammation.
Due to the lack of PD-1 on donor allogeneic T cells, protection of recipient hematopoietic cells upon PD-1Ig treatment could not be interpreted as blockade of the PD-1/B7-H1 interaction. Therefore, the effect of PD-1Ig supports a major role for B7-H1 as a receptor of PD-1Ig to deliver a protective signal to the host cells. 10B5 has been shown to block B7-H1/CD80 interactions which suppresses T cell responses. In our GVHD model, 10B5 showed an agonistic effect, likely due to the lack of PD-1 on donor T cells. However, our data suggest that this interaction only plays a minor role in the presence of PD-1 because 10B5 treatment led to accelerated GVHD upon the transfer of PD-1 ± T cells. Currently the mechanism of action for this agonistic effect of PD-1Ig/B7-H1 mAb is yet to be clarified. It is possible that PD-1 on T cell surface may have different role as compared to free mAb to the cell membrane receptor. We showed previously that expression of PD-1 without intracellular domain on T cells is sufficient to transmit an anti-apoptotic signal to B7-H1 on tumor cellsCitation8. Taken together with the finding in PD-1Ig, our results support that engagement of B7-H1 on host cells prevents GVHD by allogeneic T cells.
In early GVHD, donor T cells were activated and secreted inflammatory factors, including IFN-γ, which could upregulate B7-H1 expression on host tissues. B7-H1+ tissues resistance the lysis of alloreactive cytotoxic T cells, resulted in higher survival. Treated with PD-1Ig decreased the loss of B cells, B cells lost due to allogeneic CTL lysis was an early sign of allogeneic GVHD and was followed by the loss of other hematopoietic cellsCitation16. Our finding supports a protective effect for B7-H1 on host cells upon PD-1Ig engagement in this GVHD setting. However, the activity of donor alloreactive T cell and the host Treg cells is not affected by treatment. These findings indicate that the protective effect of PD-1Ig on normal tissues is not due to suppression of allogeneic CTL. It was further confirmed by the result that less 7-AAD and annexin V double positive host B cells were found in the mice spleen treated with anti-B7-H1 mAb that stimulated B7-H1 delivers anti-apoptosis signals to B7-H1+ cells. It is interesting that, upon engagement of B7-H1, a durable resistance to GVHD was induced and it appears that a long-term tolerance of T cells was developed. This tolerance is specific because specific T cell responses to other antigens were still present, as indicated by the resistance of the tolerated mice to A20 lymphoma challenge. Therefore, PD-1Ig or B7-H1 mAb did not dampen T cell responses in general but were highly selective because GVHD-tolerated mice were still resistant to the challenge of A20 lymphoma.
Our findings suggest an intrinsic mechanism of host cells to protect against allogeneic CTL mediated destruction, namely by IFN-γ mediated upregulation of B7-H1 to decrease apoptosis. It has been shown that upregulation of B7-H1 on cancer or host cells in tumor microenvironment correlates with poor survival in several retrospective studiesCitation17-Citation19. However, B7-H1 is largely induced in vivo by IFN-γ, a T cell effector molecule which could also enhance immune responses by upregulation of MHC class I, antigen processing and T cell differentiationCitation20. In our results also found B7-H1+ cells upregulated by IFN-γ resistance to CTL lysis when pre-treated with PD-1Ig. Therefore, it is not surprise that the expression of B7-H1 in some types of cancer was also reversely associated with patient survival1Citation9,Citation21. B7-H1-mediated protection of tumor cells has been described previouslyCitation8, however, its role in vivo has not been demonstrated. To our knowledge this is the first in vivo evidence that B7-H1 protects cells from CTL destruction. Therefore, it is possible that increased B7-H1 expression on target organs of patients with GVHD may predict better prognosis whereas low B7-H1 expression may be less favorable.
Our findings have several implications in the current efforts to manipulate the B7-H1/PD-1 pathway for therapeutic applications. Our results indicate that the 10B5 mAb specific for B7-H1 could be agonistic and help ameliorate diseases like GVHD and possibly viral infections to prevent excessive damage of normal organs. Current forms of B7-H1 agonists, however, could not be used directly for therapy because the dominant effect of PD-1 as a receptor on T cells. Nevertheless, our findings provide a mechanistic insight and possible approach to enhance B7-H1 receptor function for protecting host cells from death. With this understanding, further engineering of B7-H1 mAb or agonists without antagonistic effect will need to be developed in the future.
Materials and methods
Mice and cells
Female and male C57BL/6 (B6) and BALB/c (B/c) mice at 5–8 weeks of age were purchased from the Experimental Animal Center of Sun Yat-sen University. The CB6F1 mice were generated by breeding female B/c with male B6. All mice were maintained in specific pathogen-free facilities and all mouse experiments were carried out in accordance with the guidelines of Sun Yat-sen University Animal Care and Use Committee and ethical guidelines for investigation of experimental pain in conscious animals. PD-1KO mice in B6 background, EL4 thymoma and P815 mastocytoma cell lines were described previously. Macrophages (Mph) were harvested from peritoneal cavities of mice inoculated with 3% Brewer thioglycollate medium for 5 days. All cells were cultured in DMEM or RPMI 1640 media supplemented with 10% FBS, 10 mM HEPES, 1 mM sodium pyruvate, 100 U/ml penicillin G and 100 µg/ml streptomycin sulfate. T and B cells were purified from CB6F1 mice using MACS negative selection (Miltenyi Biotec).
Antibodies
Armenian hamster mAb to mouse B7-H1 (clone 10B5) and PD-1 (clone G4), recombinant mouse PD-1/murine IgFc and Flag/murine IgFc fusion protein (control) were generated and purified as described previouslyCitation1,Citation22. Cells were stained with mAb and fluorescence detected by flow cytometry (BD FACSVerse) and analyzed with Cell Quest software (BD Biosciences).
GVHD and GVL models
GVHD and GVL models were described previouslyCitation23,Citation24. In brief, CB6F1 (semi-mismatched model) or Balb/c (fully mismatched model) mice were injected intravenously (i.v.) with 3.5 × 10Citation7 spleen cells from PD-1KO mice on a B6 background. Body weight and clinical scores of the host mice were measured every other day and monitored for survival. The GVHD clinical scores were describedCitation25 incorporating weight loss, posture (hunching), activity, fur texture, and skin integrity. At the time of analysis, mice from coded cages were evaluated and graded from 0 to 2 for each criterion. A clinical index was subsequently generated by summation of the five criteria scores with a maximum index of 10. The mAb was administrated i.v. every 3 days at a dose of 250μg per injection. For in vivo expression of PD-1Ig, 20μg purified murine PD-1Ig plasmid in 2 ml PBS were injected i.v. within 10 seconds and PD-1Ig was detected in mouse sera by sandwich ELISA with B7-H1Ig fusion protein and anti-murine PD-1 mAb (G4 clone). At day 32, mice survived from GVHD were challenged i.v. with 10Citation6 A20 lymphoma (H-2d) and the identical injection of naïve mice as the controls.
Allogeneic CTL activity assay
Spleen cells from CB6F1 mice harvested 8 days after transplantation were incubated with CFSE (Invitrogen, Carlsbad, CA; Cat# V12883) -labeled target cells (P815 or EL4) at the indicated effector/target (E/T) ratios for 4 hours in 37◦C. To analyze target cell lysis, 0.1μg DAPI (Sigma-Aldrich, St. Louis, MO; Cat# D8417) were added and immediately analyzed by flow cytometry. The % lysis = (Number of CFSE+ DAPI+ cells/number of CFSE+ cells) × 100. The spontaneous lysis of target cells in all experiments was less than 10%. For in vitro cytotoxicity assay, Mph were cultured in the wells, which were pre-coated with either control IgG or murine PD-1Ig (PD-1Ig) at 10μg/mL for 18 hours. After extensive washing, CTLs were added and further incubated at the indicated E/T ratios for 12 hours. Upon gentle washing, the remaining cells were fixed with 4% paraformaldehyde for 10 minutes. After washing once with PBS, cells were stained with 0.1% crystal violet for 10 minutes. The optical density (OD) values were read at 570nm. The CTL activity was determined by the relative OD value (the OD value of cells incubated with CTLs/the OD value of cells with no CTLs). The % lysis = (1-OD value of test well/OD value of control well) × 100. For the assay of T and B cells as target cells, T cells, and B cells from CB6F1 mice pre-stimulated with IFN-γ were cultured in the 24 well-plate, which were pre-coated with either control Ig or murine PD-1Ig at 10μg/ml for 18 hours. After extensive washing, T cells and B cells were labeled with CFSE and further incubated with splenocytes harvested from CB6F1 mice 6 ~ 8 days after B6 spleen cell transfer at indicated E/T ratios for 12 hours. CTL activity was determined by the % of DAPI+ cells in CFSE-labeled cells at 37℃. To analyze target cell lysis, 0.1μg DAPI were added and immediately analyzed by flow cytometry. The % lysis = (number of CFSE+ DAPI+ cells/number of CFSE+ cells) × 100. Human PBMCs pre-stimulated with OKT3 were cultured in the 24 well-plate which were pre-coated with either control Ig or human PD-1Ig at 10μg/ml for 40 hours. After extensive washing, cells were labeled with CFSE and further incubated with allogeneic CTL at the indicated effector/target (E/T) ratios for 5 hours at 37°C. DAPI at 0.1μg were added to cells and analyzed by flow cytometry immediately. The data are representative of at least 3 experiments.
Statistical analysis
All presented results were repeated at least three times, the numbers of animals in each model are stated in the figure legends. All data are presented as means ± SEM. Statistical significance were determined using a two-tailed student’s t-test, where *P < 0.05, **P < 0.01, ***P < 0.001.
Abbreviations
| B7-H1 | = | B7 homolog one |
| Mph | = | macrophages |
| mAb | = | monoclonal antibody |
| GVHD | = | graft versus host diseases |
| PD-1 | = | programmed death one |
| PD-L1 | = | Programmed death ligand one |
Disclosure of Potential Conflicts of Interest
L. Chen is scientific advisor/board member of NextCure, Pfizer, Junshi, GenomiCare and Vcanbio. Other authors do not have conflict of interest.
Supplemental Material
Download MS Word (1.1 MB)Acknowledgements
We thank Beth Cadugan for editing the manuscript. This work is partially supported by grants from Guangdong Province Innovative Research Program Project No.2011Y035 and 863 Project grants to Sun Yat-sen University; Fujian Province Department of Science and Technology Research Program 2014Y2001 and 2014Y4008; a Yale University endowed chair from the United Technologies Corporation.
supplementary Material for this article can be accessed here.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi:10.1038/70932
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi:10.1038/nm730
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi:10.1038/nri1349
- Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi:10.1038/nri3405
- Yao S, Zhu Y, Chen L. Advances in targeting cell surface signalling molecules for immune modulation. Nat Rev Drug Discov. 2013;12:130–146. doi:10.1038/nrd3877
- Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125:3384–3391. doi:10.1172/JCI80011
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:324r–328r. doi:10.1126/scitranslmed.aad7118
- Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–3643. doi:10.1182/blood-2007-11-123141
- Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–1241. doi:10.1016/j.cell.2015.08.016
- Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12:443–458. doi:10.1038/nri3212
- Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. doi:10.1038/nri2000
- Fujiwara H, Maeda Y, Kobayashi K, Nishimori H, Matsuoka K, Fujii N, Kondo E, Tanaka T, Chen L, Azuma M, et al. Programmed death-1 pathway in host tissues ameliorates Th17/Th1-mediated experimental chronic graft-versus-host disease. J Immunol. 2014;193:2565–2573. doi:10.4049/jimmunol.1400954
- Schuchmann M, Meyer RG, Distler E, von Stebut E, Kuball J, Schnürer E, Wölfel T, Theobald M, Konur A, Gregor S, et al. The programmed death (PD)-1/PD-ligand 1 pathway regulates graft-versus-host-reactive CD8 T cells after liver transplantation. Am J Transplant. 2008;8:2434–2444. doi:10.1111/j.1600-6143.2008.02401.x
- Blazar BR, Carreno BM, Panoskaltsis-Mortari A, Carter L, Iwai Y, Yagita H, Nishimura H, Taylor PA. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol. 2003;171:1272–1277.
- Li X, Deng R, He W, Liu C, Wang M, Young J, Meng Z, Du C, Huang W, Chen L, et al. Loss of B7-H1 expression by recipient parenchymal cells leads to expansion of infiltrating donor CD8+ T cells and persistence of graft-versus-host disease. J Immunol. 2012;188:724–734. doi:10.4049/jimmunol.1102630
- Flies DB, Higuchi T, Chen L. Mechanistic assessment of pd-1h coinhibitory receptor-induced t cell tolerance to allogeneic antigens. J Immunol. 2015;194:5294–5304. doi:10.4049/jimmunol.1402648
- Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L, et al. Costimulatory B7-H1 in Renal Cell Carcinoma Patients: Indicator of Tumor Aggressiveness and Potential Therapeutic Target. 2004;101(49):17174–17179.
- Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–979. doi:10.1158/1078-0432.CCR-08-1608
- Liu Y, Carlsson R, Ambjorn M, Hasan M, Badn W, Darabi A, Siesjö P, Issazadeh-Navikas S. PD-L1 expression by neurons nearby tumors indicates better prognosis in glioblastoma patients. J Neurosci. 2013;33:14231–14245. doi:10.1523/JNEUROSCI.5812-12.2013
- Farrar MA, Schreiber RD. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi:10.1146/annurev.iy.11.040193.003035
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, et al. Colocalization of inflammatory response with b7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127r–137r. doi:10.1126/scitranslmed.3003689
- Chapoval AI, Zhu G, Chen L. Immunoglobulin fusion proteins as a tool for evaluation of T-cell costimulatory molecules. 2002;21(3):259–264. doi:10.1044/1059-0889(2002/er01)
- Tamada K, Tamura H, Flies D, Fu YX, Celis E, Pease LR, Blazar BR, Chen L. Blockade of LIGHT/LTbeta and CD40 signaling induces allospecific T cell anergy, preventing graft-versus-host disease. J Clin Invest. 2002;109:549–557. doi:10.1172/JCI13604
- Tamada K, Shimozaki K, Chapoval AI, Zhu G, Sica G, Flies D, Boone T, Hsu H, Fu YX, Nagata S, et al. Modulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway. Nat Med. 2000;6:283–289. doi:10.1038/73136
- Cooke KR, Kobzik L, MartinTR, Brewer J, Delmonte J Jr, Crawford JM, Ferrara JL. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The Roles of Minor H Antigens and Endotoxin. Blood. 1996;88:3230–3239.
