?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Tumor-infiltrating lymphocytes (TILs) are correlated to prognosis of several kinds of cancer. Most studies focused on T cells, while the role of tumor-associated B cells (TABs) has only recently gained more attention. TABs contain subpopulations with distinct functions, potentially promoting or inhibiting immune responses. This study provides a detailed analysis of TABs in gastro-esophageal adenocarcinoma (EAC). Flow cytometric analyses of single cell suspensions of tumor samples, mucosa, lymph nodes and peripheral blood mononuclear cells (PBMC) of EAC patients and healthy controls revealed a distinct B cell compartment in cancer patients. B cells were increased in tumor samples and subset-analyses of TILs showed increased proportions of differentiated and activated B cells and an enrichment for follicular T helper cells. Confocal microscopy demonstrated that TABs were mainly organized in tertiary lymphoid structures (TLS), which resemble lymphoid follicles in secondary lymphoid organs. A panel of 34 tumor-associated antigens (TAAs) expressed in EAC was identified based on public databases and TCGA data to analyze tumor-specific B cell responses using a LUMINEXTM bead assay and flow cytometry. Structural analyses of TLS and the detection of tumor-specific antibodies against one or more TAAs in 48.1% of analyzed serum samples underline presence of anti-tumor B cell responses in EAC. Interestingly, B cells were decreased in tumors with expression of Programmed Death Ligand 1 or impaired HLA-I expression. These data demonstrate that anti-tumor B cell responses are an additional and underestimated aspect of EAC. Our results are of immediate translational relevance to emerging immunotherapies.
Introduction
Adenocarcinomas of the stomach and the esophago-gastric junction are among the most frequent causes of cancer-related deaths worldwide.Citation1 Modern multimodal treatments combining surgery with neoadjuvant radiochemotherapy or perioperative chemotherapy significantly improved survival of patients with locally advanced disease.Citation2,Citation3 Nevertheless, the overall prognosis remains poor and more than 50% of patients will experience disease recurrence after neoadjuvant radiochemotherapy and curatively intended resection.Citation2 As therapeutic options in metastatic or recurrent disease are highly limited, there is a high medical need in this complex disease.Citation4
Cancer immunotherapy has extended therapeutic options across different tumor entities. Especially immune checkpoint inhibition represents a major breakthrough for cancer therapy,Citation5,Citation6 including treatment of gastric cancer: The programmed death 1 (PD-1) inhibitor pembrolizumab has recently been approved for the treatment of programmed death ligand 1 (PD-L1) positive gastric and esophageal adenocarcinoma. In contrast to molecular targeted therapies, response to immune checkpoint inhibition can only partially be predicted by expression of the targeted molecule on cancer cells and susceptibility to immunotherapy seems to depend on a large panel of immune related factors (e.g. endogenous immune response, neoantigen burden, composition of the lymphocytic immune infiltrate or site-specific features of the tumor microenvironment).Citation7
The lymphocytic composition of the tumor microenvironment is a possible correlate of tumor immunogenicity. A standardized quantification of tumor infiltrating lymphocytes has been proposed based on the observation that tumor infiltrating effector memory T cells are associated with superior survival in colorectal cancer.Citation8,Citation9 In line with these results, Erdag et al. described a positive prognostic impact of a high infiltration by CD8+ T cells in melanoma. Interestingly, a high B cell infiltration was also associated with superior prognosis in this cohort of melanoma patients.Citation10 The impact of tumor associated B cells on the prognosis of cancer has only been addressed by few studies. In pancreatic adenocarcinoma or hepatocellular carcinoma, a high B cell infiltrate is associated with superior prognosis and a recent publication identified T and B cells as major lymphocytic subsets of prognostic relevance also for gastro-esophageal adenocarcinoma.Citation11–Citation13 In contrast, patients with B cells or plasma cells in the tumor microenvironment of melanoma or lung adenocarcinoma showed an inferior prognosis.Citation14,Citation15 These opposing results can be explained by different B cell functions. Similar to T cells, specific phenotypes define functionally distinct B cell subsets, which can promote or inhibit immune responses. The distribution of functionally different B cell subsets within the whole B cell infiltrate in cancer has only been addressed by very few studies and is still widely unknown in gastro-esophageal adenocarcinoma.Citation12 The relevance of B cells for immune responses to cancer is further highlighted by recent publications focusing on the spatial distribution of tumor associated B cells in cancer. B cells often form clusters in the tumor microenvironment, which are similar to lymphoid follicles in secondary lymphoid organs and have therefore been termed tertiary or ectopic lymphoid structures. Tertiary lymphoid structures (TLS) seem to contribute to anti-tumor immune responses in several kinds of cancer and a formation of these peritumoral B cell clusters in the microenvironment is often associated with superior prognosis.Citation13,Citation16 Functionally, these anti-tumor effects could be mediated by antigen presentation or antibody production. First evidence described a prognostic relevance of B cells in the tumor microenvironment (based on retrospective immunohistochemical analyses) and we decided to further investigate the composition of tumor associated B cells in gastro-esophageal adenocarcinoma.Citation11 This study provides a comprehensive prospective analysis of tumor associated B cell subsets in gastro-esophageal adenocarcinoma including tumor-specific B cell response. Furthermore, we examined the impact of factors enhancing or inhibiting anti-tumor immune responses on tumor associated B cells.
Results
Tumor infiltrating T and B cells are increased in primary tumor samples and mainly show an activated and differentiated phenotype
Tumor infiltrating lymphocytes (TILs) could be isolated from tumor tissue of 44 patients. Primary tumor samples contained significantly more CD45+ lymphocytes (8.5% ± 2.5) than samples obtained from patients following neoadjuvant radiochemotherapy or chemotherapy (3.0% ± 0.96, p < 0.05) or mucosa samples (0.29% ± 0.1, p < 0.01; ). Overall lymphocyte counts were low in most pretreated tumor samples and normal gastric mucosa (only 5/19 single cell suspensions of pretreated tumor samples and 4/23 analyzed single cell suspensions of mucosa samples contained relevant B cell infiltrates). As these proportions are too low to allow quantification of B cell subsets, we only present data showing B cell infiltrates in PBMCs, TDLNs and TILs from patients that did not receive any preoperative treatment. As additional analyses, the 19 patients treated with neoadjuvant Radiochemotherapy according to the CROSS protocol were stratified in minor (> 10% vital tumor cells, n = 9) and major responders (< 10% vital tumor cells, n = 10). Comparison of B cell subsets in PBMC of these patients did not show significant differences (data not shown).
Figure 1. Comprehensive flow cytometric analyses of lymphocytic subsets in gastro esophageal adenocarcinoma. Single cell suspensions of primary tumor samples (n = 28), tumors of patients following neoadjuvant treatment (n = 19) or normal mucosa (n = 23) were analyzed for the percentage of CD45+ lymphocytes by flow cytometry (A). Lymphocytic subsets in PBMC of healthy controls (PBMC HC, n = 20), PBMC of untreated tumor patients (PBMC AC, n = 46), tumor samples (n = 28) and tumor-draining lymph nodes (TDLN, n = 23) were analyzed for the percentages of total B cells (B), CD86+ activated B cells (C), IgD−CD27+ memory B cells (D), CD3+PD-1+ activated T cells (E) and CCR7−CD45RA− effector memory T cells (F). Additional plots compare B cell subsets in low (UICC I and II) and high (UICC III and IV) tumor stages (B-F). Graphs show mean values ± SEM, p = ANOVA, *p < 0.05.
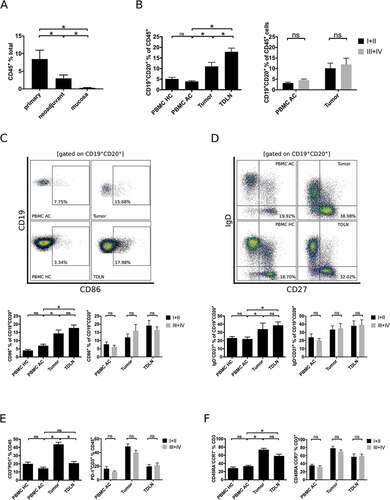
B cells (CD19+CD20+ in % of CD45+ cells) were enriched in tumor samples (11.0% ± 3.5) compared to PBMC of tumor patients (3.9% ± 2.4, p < 0.01; PBMC AC) or PBMC of healthy controls (5.1% ± 3.5, p < 0.01; PBMC HC; ). Single cells suspensions of TDLN contained the highest fraction of B cells (17.9% ± 1.8, p < 0.01; ). Analyses of tumor associated B cell subsets revealed a higher percentage of activated (CD86+ in % of CD19+CD20+ lymphocytes, ) and effector memory B cell subsets (IgD−CD27+ in % of CD19+CD20+ lymphocytes, ) in the tumor microenvironment (14.4% ± 2.1 and 34.0% ± 3.6, respectively) compared to PBMC AC (6.9% and 22.0%, respectively, p < 0.01). In line with increased differentiation of B cells, T cells in tumor samples were also mainly of an activated (CD3+PD-1+ in % of CD3+ cells, ) and effector-memory phenotype (CCR7−CD45RA− in % of CD3+ cells, ). The total percentage of B or T cells and the relative proportions of the aforementioned T and B cell subsets did not differ between PBMC of tumor patients and PBMC of healthy controls (-F). The distribution of B cell subsets did not show significant differences, if samples were stratified according to tumor grading (G1/G2 vs. G3) and localization of the tumor (esophago-gastric junction vs. stomach).
B cells in the tumor microenvironment are organized in tertiary lymphoid structures
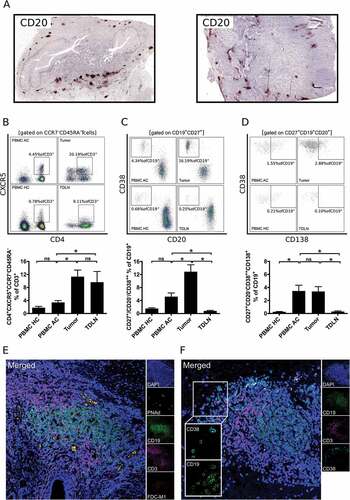
As we observed partial similarities of B cell subsets in tumor samples and TDLN, we hypothesized structural similarities. The spatial distribution of tumor associated B cells (TABs) was analyzed by immunohistochemical staining of CD20 in 72 available paraffin embedded tumor samples. The decreased lymphocytic infiltrate of pretreated samples obtained by flow cytometric analyses was confirmed by immunohistochemical analyses of CD20 in tumor specimens. Whereas 51.6% (15/31) of pretreated tumor samples did not contain B cells in the tumor microenvironment, 92.7% (38/41) of primary tumors showed a relevant B cell infiltrate (p < 0.01). TABs in these primary samples were mainly organized in B cell clusters along the invasive margin (). In line with a formation of tertiary lymphoid structures, follicular T helper cells (TFH; CD3+CD4+CCR7−CD45RA−CXCR5+ in % of CD3+ lymphocytes) were elevated in tumor samples (11.3% ± 2.0) and TDLN (9.7% ± 3.3) compared to PBMC AC (3.4% ± 0.6; , E; p < 0.05). Plasmablasts (CD20−CD27+CD38++ in % of CD19+ lymphocytes) were also enriched in tumor samples (12.9% ± 2.1) compared to PBMC AC (5.2% ± 1.1) and TDLN (0.8% ± 0.2; p < 0.05; ). Whereas the percentage of plasmablasts was not increased in PBMC AC compared to PBMC HC, our analyses revealed a systemic plasmacytosis (CD20−CD27+CD38++CD138+ in % of CD19+ lymphocytes) in PBMC of tumor patients (3.5% ± 0.9 vs. 0.3% ± 0.1; p < 0.01). Plasmacells were also detectable in tumor samples (3.4% ± 0.7), but not in TDLN (0.3% ± 0.1; p < 0.01; ). Presence of follicular dendritic cells and lymphatic vessels as additional important structural components of TLS could be visualized by multicolor confocal immunofluorescence microscopy (). CD19+CD38+ plasmablasts were mainly located in the surrounding of TLS along the invasive margin of tumors ().
Figure 2. B cells in gastro-esophageal adenocarcinoma are mainly localized in tertiary lymphoid structures (TLS). B cells in tumor sections of gastro-esophageal adenocarcinoma were stained by immunohistochemistry for CD20 (A). Lymphocytic subsets in PBMC of healthy controls (PBMC HC, n = 20), PBMC of untreated tumor patients (PBMC AC, n = 46), tumor samples (n = 28) and tumor-draining lymph nodes (TDLN, n = 23) were analyzed by flow cytometry for the percentages of CD4+CXCR5+CCR7−CD45RA− follicular T helper cells (B), CD20−CD27+CD38++ plasmablasts (C) and CD20−CD27+CD38++CD138+ plasmacells (D). Presence of lymphatic vessels (PNAd) and follicular dendritic cells (FDC-M1) as additional components of TLS is demonstrated by confocal microscopy (E). CD38+ B cells were visualized by confocal microscopy of CD19, CD38 and CD3 (F). Graphs show mean values ± SEM, p = ANOVA, *p < 0.05.
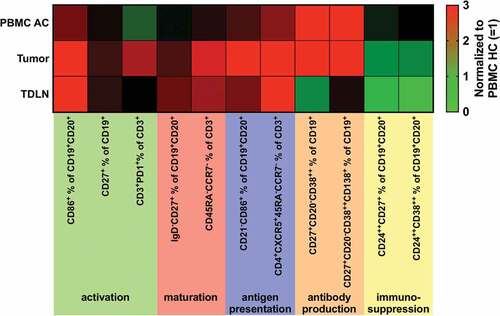
summarizes results revealed for B and T cell subsets in PBMC AC, tumor and TDLN normalized for results obtained in PBMC HC. The pattern of differential expression was similar to TDLN for B and T cell subsets involved in antigen presentation (antigen-presenting B cells (BAPC, CD21−CD86+ in % of CD19+CD20+) and T follicular helper cells (TFH, CD3+CD4+CCR7−CD45RA−CXCR5+ in % of CD3+), whereas other subpopulations (e.g. CD3+PD-1+) were only increased in tumor samples or tumor samples and PBMC (e.g. plasmablasts (CD20−CD27+CD38++ in % of CD19+, ).
Tumor specific B cell response
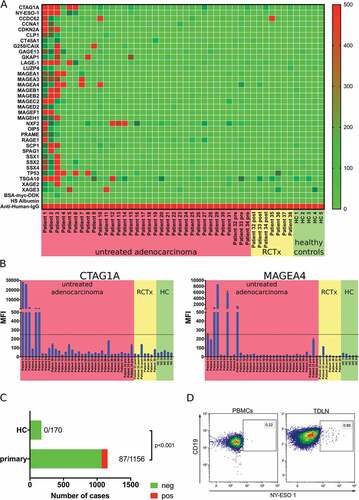
We searched databases (CTDatabase, Proteinatlas) to identify cancer testis antigens and other tumor associated antigens frequently expressed in esophageal adenocarcinoma. Expression of identified antigens was analyzed using a dataset combining data from The Cancer Genome Atlas (TCGA) with data from the Genotype Tissue Expression program (GTEx) and ArrayExpress (n = 185 EAC samples, 319 normal esophageal samples and 207 normal testicle samples). All analyzed antigens were highly expressed in normal human testicle and several antigens showed higher expression in tumor samples compared to normal tissue (Supplementary Figure 2 and ).
Table 1. Mean fluorescence intensities for 34 tumor associated antigens analyzed in serum samples of gastroesophageal adenocarcinoma patients (positive = MFI> 250, negative = MFI< 250) and healthy controls. Expression levels of analyzed antigens in external cohorts of esophageal adenocarcinoma samples (TCGA n = 185) and normal esophageal mucosa samples (GTEx n = 300, ArrayExpress n = 6, TCGA n = 13).
Based on these results, we designed a custom LUMINEXTM bead array and could detect antibody responses against 31/34 tested tumor associated antigens (TAAs) in serum samples of tumor patients. Antibodies against at least one of the analyzed TAAs could be detected in 16 out of 33 analyzed serum samples of untreated patients ( and ). We did not detect antibody responses against CT45A and LUZP4. In contrast, antibodies targeting CTAG1A and MAGEA4 could be detected in 5/33 analyzed serum samples of previously untreated patients ( left and 4B right, respectively). MFI of positive results showed a large variety. Using MFI > 250 as cutoff, our analyses did not reveal any positive results in serum samples of healthy controls, whereas 87 out of 1156 tests in samples of tumor patients revealed an MFI > 250 (p < 0.001; ). In addition, we analyzed antibody responses in serum samples of patients which received neoadjuvant radiochemotherapy. CTA-specific antibodies were detected in 2/7 samples. Comparison of antibodies against TAA prior and post neoadjuvant treatment (approximately 2–3 months between the two samples) revealed similar MFIs for most analyzed TAAs (Patient 32–34, ). Indirect staining of the B cell receptor using biotinylated protein and PE-conjugated Streptavidin tetramers can be used as an alternative method to identify antigen-specific B cell response. Using this approach, we identified NY-ESO specific B cells in tumor draining lymph nodes of 1/3 patients with esophageal adenocarcinoma ().
Figure 4. Analysis of the tumor antigen-specific B cell response in gastro-esophageal adenocarcinoma. LUMINEXTM analyses of 34 TAAs were performed in serum samples of untreated gastro-esophageal adenocarcinoma patients (n = 34), serum samples following neoadjuvant chemoradiotherapy (n = 7) and healthy controls (n = 5) was analyzed by LUMINEXTM (A). Individual MFIs for CTAG1A and MAGEA4 (B). Summary of antibody responses detected in serum samples of tumor patients and healthy controls (C) Exemplary flow cytometry analyses of b cells specific for NY-ESO-1 (CTAG1A) in PBMC and TDLN of a gastro-esophageal adenocarcinoma patient using biotinylated NY-ESO-1 and a streptavidin tetramer (D). Heatmap and bar graphs of LUMINEXTM data show mean MFIs of duplicates, p = ChiCitation2 test.
B cell infiltrates are decreased in tumors containing factors conferring low immunogenicity (HLA-loss and PD-L1 expression)
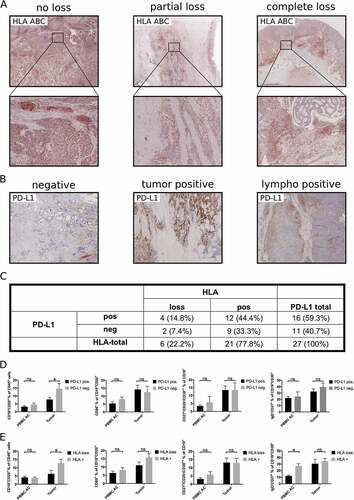
According to the “cancer immunoediting” hypothesis, reciprocal shaping of tumors and their immune microenvironment frequently induces immune escape.Citation17 To assess the impact of immune escape on tumor associated B cell subsets, we analyzed loss of HLA-I (HLA-ABC) and expression of PD-L1 as two mechanisms of immune evasion described in gastroesophageal adenocarcinoma.Citation18,Citation19 Altered HLA-expression with either partial or complete loss was detected in 6/27 (22.2%) of primary tumor samples (, C). Immunohistochemistry showed positive staining for PD-L1 on tumor cells or tumor infiltrating lymphocytes in 16/27 (59.3%) of analyzed samples (, C). Expression of PD-L1 and HLA-loss were not correlated (p = 0.68, ). B cells (CD19+CD20+ in % of CD45+ lymphocytes) were reduced in the tumor microenvironment of patients with expression of PD-L1 (7.8% ± 1.4) or loss of HLA-ABC (6.5% ± 2.1) compared to patients without expression of PD-L1 (14.6% ± 3.2, p < 0.01; ) or unaltered HLA-ABC expression (14.2% ± 2.0; p < 0.01; ), respectively. Comparison of B cell subsets revealed similar percentages of activated B cells, plasmablasts and memory B cells in patients with and without HLA-loss or PD-L1 expression in the tumor microenvironment ( D, E). Microsatellite instability can increase anti-tumor immune response and is correlated to improved response to immune checkpoint inhibition.Citation20,Citation21 As only 3/28 primary tumor samples were identified as microsatellite instable, our cohort was too small to allow conclusions regarding the impact of MSI on TABs.
Figure 5. Influence of immune escape mechanisms on tumor associated B cells. HLA-loss (A, C) and expression of PD-L1 (B, C) was assessed by immunohistochemistry in 27 primary tumor samples. PBMC of these patients (PBMC AC) or single cell suspensions of tumor samples were analyzed by flow cytometry for the presence of different B cell subsets according to their expression of PD-L1 (D) or HLA-loss (E). Bar graphs show mean percentages of flow cytometric data, p = ANOVA, p < 0.05.
Immunosuppressive B cell subsets, which inhibit immune responses by production of Interleukin-10 (IL-10), Granzyme B or expression of PD-L1 have been described.Citation22–Citation25 However, in our study regulatory B cell subsets (CD24++CD38++ or CD24highCD27+ in % of CD 19+CD20+) were not increased in tumor samples (1.6% ± 0.3 and 10.1% ± 2.0) compared to TDLN (0.5% ± 0.1 and 5.3% ± 1.1, p = 0.4 and 0.1), PBMC of EAC patients (3.1% ± 0.5 and 18.5% ± 2.1, p < 0.05) and PBMC of healthy controls (3.1% ± 0.4 and 20.0% ± 2.2, p < 0.05 and 0.2; Supplementary Figure 3A, B). In addition, PD-L1+ B cells were not enriched in the tumor microenvironment (1.9% ± 0.4) compared to PBMC AC (1.8% ± 0.3, p = 1.0) and PBMC HC (1.0% ± 0.1, respectively, p = 0.9, Supplemetary Figure 3C). In contrast, regulatory T cells (CD3+CD4+CD25+CD127low in % of CD3+) were elevated in tumor samples (8.2% ± 0.8) compared to PBMC AC (4.4% ± 0.3) and PBMC HC (2.0% ± 0.4, p < 0.01; Supplementary Figure 3D).
Discussion
Type, density and localization of tumor infiltrating T cells is correlated to prognosis in several kinds of cancer.Citation20,Citation26–Citation28 Only a small subset of these studies included quantification of TABs.Citation28 A high density of TABs (analyzed based on detection of CD19 or CD20 by immunohistochemistry) is associated with superior survival in the majority of studies in gastro-esophageal adenocarcinoma.Citation11,Citation29–Citation31 Svensson et al. analyzed tumor associated B and T cells in a cohort of esophageal adenocarcinoma patients. Patients with a combination of a high B and T cell infiltrate had the best prognosis, suggesting that tumor associated B and T cells synergistically inhibit tumor growth.Citation29
Distinct B cell subsets have been identified, which potentially promote or inhibit anti-tumor immune response.Citation32–Citation34 The data described above highlight the prognostic relevance of B cells in solid cancer, but only very few studies analyzed the composition of B cell subsets in the tumor microenvironment.Citation35–Citation37 Our current study demonstrates, that B cells are regularly detectable in the microenvironment of gastro- esophageal adenocarcinoma and predominantly consist of activated and differentiated subsets. Plasmablasts were particularly elevated in PBMC and tumor samples of gastro-esophageal adenocarcinoma patients. A similar systemic plasmacytosis and elevation of plasmablasts in tumor samples has been described in melanoma and other solid tumors, most likely reflecting anti-tumor B cell responses in these patients.Citation38–Citation41
Garnelo et al. found a similar increase of plasmablasts using multicolor immunofluorescence microscopy to identify B cell subpopulations in hepatocellular carcinoma. They demonstrated a positive prognostic impact of B cells and CD38+ plasmacells in this disease. As costimulatory molecules and inflammatory cytokines were elevated in tumor samples with a high B cell infiltrate, they concluded that B and T cells act synergistically in the tumor microenvironment.Citation12 Furthermore, TABs in hepatocellular carcinoma were localized in tertiary lymphoid structures (TLS), whose presence is correlated to superior prognosis in several other types of solid cancer.Citation13,Citation16,Citation34,Citation42,Citation43 In line with an increase of differentiated B cell subsets in our flow cytometric data, TLS were detectable in the vast majority of our primary tumor samples (40/43). In addition to a clustering of T and B cells we could confirm the presence of high endothelial vessels and follicular dendritic cells as additional important structural components of TLS (). CD19+CD38+ plasmablasts were mainly localized along the invasive margin and outside of TLS (), which has been described similarly for other tumor entities.Citation12,Citation34,Citation41 One recent publication suggested that immunosuppressive effects of circulating regulatory B cell subsets promote tumor growth in esophageal adenocarcinoma.Citation44 Our results do not confirm these data, since the two immunosuppressive B cell populations (CD24highCD38high and CD24highCD27+ B cells) were not enriched in the tumor microenvironment.
The majority of clinical studies described a positive prognostic impact of tumor associated B cells and our data describing highly differentiated and organized B cell infiltrates further supports this observation.Citation45 However, the exact mode of action of anti-tumor B cell effects is still widely unknown and this aspect needs further investigation. Antibody responses against TAAs could be described in cancers of different origins, mainly focusing on one specific antigen. Frequencies of antibodies against TAAs varied according to analyzed antigens and cancer sites. Some of these publications demonstrated a prognostic relevance of antibody-mediated anti-tumor immune responses.Citation46,Citation47 Two larger studies analyzed antibody responses against a panel of TAAs in gastro-esophageal adenocarcinoma.Citation48,Citation49 Antibodies could be detected in 0–39.1% of patients, depending on the analyzed antigen. Chen et al. described coexpression of more than four out of eight cancer testis antigens in 31% of esophageal adenocarcinoma patients.Citation48 In the present study we identified a larger panel of 34 TAAs and could detect antibody response in at least one patient for 31 of these antigens. Although our expanded panel increased the frequency of detectable antibodies compared to previous studies, we only detected antibodies in 48.5% of serum samples. Using a similarly large panel of antigens, Germain et al. described tumor-specific antibodies in supernatants of isolated and in vitro stimulated tumor infiltrating B cells of lung adenocarcinoma patients in 41% of analyzed samples. In line with our results, some patients showed response to a broad spectrum of antigens, whereas the majority of patients had detectable antibodies against only one or two different TAAs.Citation34
Finally, our study provides first evidence for impaired anti-tumor B cell responses in patients with expression of PD-L1 or HLA-loss as two important mechanisms of immune escape. The total B cell infiltrate was decreased in both conditions, but the composition of infiltrating B cell subsets did not show differences in tumors with and without evidence of immune escape. Decreased lymphocytic infiltrates have been described for patients with evidence of HLA-loss in the tumor microenvironment of other cancers,Citation50,Citation51 whereas increased expression of PD-L1 was mostly associated with increased lymphocytic infiltration.Citation52,Citation53 With few exceptions, these studies did not include B cells into their analyses. Nevertheless, our data provides first evidence for a correlation of the analyzed immune escape mechanisms and B cell infiltrates and the impact of immune escape mechanisms on TAB should be further analyzed. The fraction of MSI-positive tumor samples was too low, as our cohort contained only 6 tumor samples with microsatellite instability of which 2 had received neoadjuvant treatment. In line with previous data showing a link between HLA-loss and MSI, 5 out of 6 MSI+ tumors showed impaired HLA-expression.Citation54
Taken together our results are highly suggestive for tumor-specific B cell responses in gastro-esophageal adenocarcinoma. The present study is of immediate translational relevance since B cells could be an important additional aspect influencing therapeutic efficacy in ongoing clinical trials evaluating the role of immunotherapy in gastro-esophageal adenocarcinoma.
Materials and methods
Patients
89 patients were included into this prospective study. Peripheral blood mononuclear cells (PBMC) were isolated from peripheral blood using density gradient centrifugation (Pancoll, PAN Biotech). Resection specimens were transferred to our Institute of Pathology immediately after surgery (29 gastrectomies, 53 Ivor-Lewis esophagectomies). Samples with very small or undefined tumors had to be excluded to ensure state of the art pathological assessment according to the 8th edition of the Union for International Cancer Control (UICC) staging system. Tumor samples, normal mucosa and tumor draining lymph nodes (TDLN) were provided from 35 primary tumor samples and 20 cases following neoadjuvant chemotherapy or chemoradiotherapy. Processing of 28/35 untreated and 15/20 pretreated tumor samples revealed sufficient cell numbers for further analyses. Patient characteristics are summarized in supplementary Table 1. Written informed consent was obtained from all patients and this study was approved by our institutional ethics committee (No. 11–116).
Production of single cell suspensions and ex vivo 10-color flow cytometry
Tissue specimens were processed to single cell suspensions using mechanical (“GentleMacs” Tissue dissociator, Miltenyi Biotech) and enzymatic digestion (320U/ml Collagenase IV, Worthington, and 100U/ml DNAse I, Applichem). Subsequently, isolated PBMC and single cell suspensions were stained for 10-color flow cytometry (see supplementary table 2 for used clones and providers). Data were acquired on a Gallios flow cytometer (Beckman Coulter) and analyzed using the Kaluza software (Version 1.5a, Beckman Coulter). Dead cells were excluded using aqua dead cell staining (Life Technologies).
Immunohistochemistry (IHC) and immunofluorescence (IF) microscopy
Whole slides of tumor specimens were stained for CD20 and HLA-ABC using standard IHC (AEC EnVision+, Dako). Antigen retrieval for IHC and IF was performed on a LabVision PTmodule (Thermo Fisher) using pH6.0 citrate buffer. Stainings for PD-L1 were performed using the Bond-Max System by Leica. Ready to use anti-MLH-1 and anti-MSH-6 antibodies (Roche/Ventana) were used with DAB on a Ventana Benchmark according to the manufacturer’s instructions. Slides were analyzed by an experienced GI-pathologist (Quaas A.). Expression of HLA-ABC was categorized in overall positivity, partial loss and complete loss. PD-L1 was considered positive, if > 1% of tumor cells or tumor infiltrating lymphocytes showed membranous staining. Patients with loss of MLH-1 or MSH-6 were categorized as microsatellite instable (MSI). Confocal microscopy was performed on a Leica TCS SP8 gSTED superresolution microscope. FIJI ImageJ (Version 2.0.0) was used to adjust for brightness and autofluorescence. See supplementary table 2 for detailed information on used antibodies and dilutions.
Gene expression analyses
A set of 34 tumor associated antigens was designed based on available data (Pubmed, Proteinatlas, CTAdatabase). Expression of these antigens in esophageal adenocarcinoma was analyzed in pooled data of 185 esophageal adenocarcinoma patients downloaded from the cancer genome atlas (TCGA),Citation50 and 319 normal controls from GTExCitation55 and ArrayExpress.Citation56 RNA-Seq data were mapped against the human genome version hg19 with TopHat2-2.0.12. R-3.4.1 and Bioconductor 3.6 were used for the RNA-Seq analysis. Reads were counted using the R package GenomicAlignments (mode = ’Union’, inter.feature = FALSE), only primary read alignments were retained. In order to normalize the read counts we used the equation below.
DESeq2 normalization factors were obtained using DESeq2. Log 2 fold change values were obtained by comparing the normalized read counts. P-values were calculated using the Wilcoxon Rank Sum Test and corrected using the Benjamini & Hochberg method. Supplementary Fig. 2 was created using ggplot2_1.0.0 on the log 2 values of the normalized read counts. The dendogram of the heatmap was generated using hierarchical clustering with complete linkage.
Analyses of tumor-specific antibodies
A customized TruePlexTM (Origene) antibody profiling array was used to analyze the predicted 34 tumor associated antigens. Median fluorescence intensities of anti-IgG antibodies were analyzed in 38 serum samples of esophageal adenocarcinoma patients on a LUMINEX 200TM platform according to the manufacturer’s instructions (Origene). Briefly, HEK293T-expressed recombinant human proteins with C-terminal myc-DDK tag or E. coli-overexpressed and purified human proteins with N-terminal His tag were coupled to LUMINEXTM beads and incubated with serum samples. Median fluorescence intensity (MFI) for each of the analyzed antigens was measured using fluorescence-labeled anti-human IgG antibodies. In addition to included negative (human and bovine serum albumin) and positive controls (IgG), serum samples of five healthy controls (living-kidney donors) were analyzed.
Antigen-specific B cell response
NY-ESO-1 specific B cells in TDLN were identified based on our previously established protocol using antigen-biotin-tetramers.Citation57 Briefly, NY-ESO-1 protein (Origene) was biotinylated (EZ-Link NHS-Biotin reagent, Thermo Fisher) and coupled to PE-conjugated streptavidin (Biolegend) in a ratio of 1:3. Cells were incubated with tetramer for 15 min in addition to B cell antibodies and analyzed by flow cytometry.
Statistical analyses
Statistical analyses were performed using Prism 7 (Graphpad). Means of lymphocytic subsets were compared using ANOVA or Kruskal-Wallis, depending on normal distribution as assessed by D’Agostino-Pearson omnibus normality test. Data was corrected for multiple comparisons (either Dunn’s or Tukey’s Test) and p values < 0.05 were considered significant. Results are presented as mean values ± standard error of the mean (SEM).
Conflicts of Interest
S.R.: Honoraria for advisory boards from BMS and MSD. Honoraria for invited talks from BMS. Financial support for research projects from AstraZeneca; M.B.,: Honoraria for advisory boards, for invited talks from BMS and financial support for research projects from Astellas, Roche and MSD. All other authors declare no conflicts of interest. TZ Honoraria for invited talks from MSD, Merck, Roche, Lilly, Novartis, BMS.;
Supplemental Material
Download Zip (1.7 MB)Additional information
Funding
References
- Wang Z, Goodman M, Saba N, El-Rayes BF. Incidence and prognosis of gastroesophageal cancer in rural, urban, and metropolitan areas of the United States. Cancer. 2013;119(22):4020–4027. doi:10.1002/cncr.28313.
- Shapiro J, Jjb VL, Hulshof MCCM, Van Hagen P, Van Berge Henegouwen MI, Wijnhoven BPL, Hwm VL, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–1098. doi:10.1016/S1470-2045(15)00040-6.
- Lorenzen S, Pauligk C, Homann N, Schmalenberg H, Jäger E, Al-Batran S-E. Feasibility of perioperative chemotherapy with infusional 5-FU, leucovorin, and oxaliplatin with (FLOT) or without (FLO) docetaxel in elderly patients with locally advanced esophagogastric cancer. Br. J. Cancer. 2013;108(3):519–526. doi:10.1038/bjc.2012.588.
- Moehler M, Baltin CTH, Ebert M, Fischbach W, Gockel I, Grenacher L, Hölscher AH, Lordick F, Malfertheiner P, Messmann H, et al. International comparison of the German evidence-based S3-guidelines on the diagnosis and multimodal treatment of early and locally advanced gastric cancer, including adenocarcinoma of the lower esophagus. Gastric Cancer. 2014Sep7. doi:10.1007/s10120-014-0403-x.
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015;373(17):1627–1639. doi:10.1056/NEJMoa1507643.
- Johnson LA, June CH. Driving gene-engineered T cell immunotherapy of cancer. Cell Res. 2017;27(1):38–58. doi:10.1038/cr.2016.154.
- McGranahan N, Furness AJS, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–1469. doi:10.1126/science.aaf1490.
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi:10.1126/science.1129139.
- Galon J, Pagès F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G, et al. Cancer classification using the Immunoscore: a worldwide task force. J. Transl. Med. 2012;10:205.doi:10.1186/1479-5876-10-205.
- Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, Patterson JW, Slingluff CL. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72(5):1070–1080. doi:10.1158/0008-5472.CAN-11-3218.
- Hennequin A, Derangère V, Boidot R, Apetoh L, Vincent J, Orry D, Fraisse J, Causeret S, Martin F, Arnould L, et al. Tumor infiltration by Tbet+ effector T cells and CD20+B cells is associated with survival in gastric cancer patients. Oncoimmunology. 2015Jun;3. doi:10.1080/2162402X.2015.1054598.
- Garnelo M, Tan A, Her Z, Yeong J, Lim CJ, Chen J, Lim KH, Weber A, Chow P, Chung A, et al. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut. 2015Dec15. doi:10.1136/gutjnl-2015-310814.
- Hiraoka N, Ino Y, Yamazaki-Itoh R, Kanai Y, Kosuge T, Shimada K. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br. J. Cancer. 2015;112(11):1782–1790. doi:10.1038/bjc.2015.145.
- Ruddell A, Harrell MI, Furuya M, Kirschbaum SB, Iritani BM. B lymphocytes promote lymphogenous metastasis of lymphoma and melanoma. Neoplasia. 2011;13(8):748–757. doi:10.1593/neo.11756.
- Kurebayashi Y, Emoto K, Hayashi Y, Kamiyama I, Ohtsuka T, Asamura H, Sakamoto M. Comprehensive Immune Profiling of Lung Adenocarcinomas Reveals Four Immunosubtypes with Plasma Cell Subtype a Negative Indicator. Cancer Immunol. Res. 2016;4(3):234–247. doi:10.1158/2326-6066.CIR-15-0214.
- Kroeger DR, Milne K, Nelson BH. Tumor-Infiltrating Plasma Cells Are Associated with Tertiary Lymphoid Structures, Cytolytic T-Cell Responses, and Superior Prognosis in Ovarian Cancer. Clin. Cancer Res. 2016;22:12. doi:10.1158/1078-0432.CCR-16-0190.
- Schreiber RD, Old LJ, Smyth MJ. Cancer Immunoediting: integrating Immunity’s Roles in Cancer Suppression and Promotion. Science. (80-.) 2011;331(6024):1565–1570. doi:10.1126/science.1203486.
- Mimura K, Shiraishi K, Mueller A, Izawa S, Kua L-F, So J, Yong W-P, Fujii H, Seliger B, Kiessling R, et al. The MAPK pathway is a predominant regulator of HLA-A expression in esophageal and gastric cancer. J. Immunol. 2013;191(12):6261–6272. doi:10.4049/jimmunol.1301597.
- Schlößer HA, Drebber U, Kloth M, Thelen M, Rothschild SI, Haase S, Garcia-Marquez M, Wennhold K, Berlth F, Urbanski A, et al. Immune checkpoints programmed death 1 ligand 1 and cytotoxic T lymphocyte associated molecule 4 in gastric adenocarcinoma. Oncoimmunology. 2016;5(5):e1100789. doi:10.1080/2162402X.2015.1100789.
- Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, Church SE, Lafontaine L, Fischer M, Fredriksen T, et al. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity. 2016;44(3):698–711. doi:10.1016/j.immuni.2016.02.025.
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. (80-.) 2015;348(6230):124–128. doi:10.1126/science.aaa1348.
- Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res. 2011;71(10):3505–3515. doi:10.1158/0008-5472.CAN-10-4316.
- Iwata Y, Matsushita T, Horikawa M, DiLillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117(2):530–541. doi:10.1182/blood-2010-07-294249.
- Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32(1):129–140. doi:10.1016/j.immuni.2009.11.009.
- Hirose T, Tanaka Y, Tanaka A, Sakai H, Sasaki Y, Shinohara N, Ohdan H. PD-L1/PD-L2-expressing B-1 cells inhibit alloreactive T cells in mice Boussiotis VA, editor. PLoS One. 2017;12(6):e0178765. doi:10.1371/journal.pone.0178765.
- Cr GDA, Sj H, Mb E, Trinh A, Ll L, Beca F, Zi X, Kwak M, Bergholtz H, Su Y, et al. Immune Escape in Breast Cancer During In Situ to Invasive Carcinoma Transition. Cancer Discov. 2017;7(10):CD-17–0222. doi:10.1158/2159-8290.CD-17-0222.
- Turcotte S, Gros A, Tran E, Lee -C-CR, Wunderlich JR, Robbins PF, Rosenberg SA. Tumor-reactive CD8+ T cells in metastatic gastrointestinal cancer refractory to chemotherapy. Clin. Cancer Res. 2014;20(2):331–343. doi:10.1158/1078-0432.CCR-13-1736.
- Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br. J. Cancer. 2013;108(4):914–923. doi:10.1038/bjc.2013.32.
- Svensson MC, Fredrik Warfvinge C, Fristedt R, Hedner C, Borg D, Eberhard J, Micke P, Nodin B, Leandersson K, Jirström K. The integrative clinical impact of tumor-infiltrating T lymphocytes and NK cells in relation to B lymphocyte and plasma cell density in esophageal and gastric adenocarcinoma. Oncotarget. 2017Sep22. doi: 10.18632/oncotarget.19437.
- Dong J, Li J, S-M L, X-Y F, Chen S, Y-B C, X-S Z. CD33+/p-STAT1+ double-positive cell as a prognostic factor for stage IIIa gastric cancer. Med. Oncol. 2013;30(1):442. doi:10.1007/s12032-012-0442-2.
- Nakajima M, Kato H, Miyazaki T. Tumor immune systems in esophageal cancer with special reference to heat-shock protein 70 and humoral immunity. Anticancer. 2009;1606:1595–1606.
- Dilillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann. N. Y. Acad. Sci. 2010;1183: (Table 1)38–57. doi: 10.1111/j.1749-6632.2009.05137.x.
- Woo JR, Liss MA, Mt M, Palazzi K, Strasner A, Ammirante M, Varki N, Shabaik A, Howell S, Cj K, et al. Tumor infiltrating B-cells are increased in prostate cancer tissue. J. Transl. Med. 2014;12:30.doi:10.1186/1479-5876-12-30.
- Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, Lepelley A, Becht E, Katsahian S, Bizouard G, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am. J. Respir. Crit. Care Med. 2014;189(7):832–844. doi:10.1164/rccm.201309-1611OC.
- Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, Mauri C, Coussens LM, Balkwill FR. B regulatory cells and the tumor-promoting actions of TNF-α during squamous carcinogenesis. Proc. Natl. Acad. Sci. U. S. A. 2011;108(26):10662–10667. doi:10.1073/pnas.1100994108.
- Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39(4):782–795. doi:10.1016/j.immuni.2013.10.003.
- Linnebacher M, Maletzki C. The ignored players in tumor immunology Tumor-in ltrating. B Cells. 2012;1(7):1186–1188.
- Berntsson J, Nodin B, Eberhard J, Micke P, Jirström K. Prognostic impact of tumour-infiltrating B cells and plasma cells in colorectal cancer. Int. J. Cancer. 2016;139(5):1129–1139. doi:10.1002/ijc.30138.
- Zirakzadeh A, Marits P, Sherif A, Winqvist O. Multiplex B cell characterization in blood, lymph nodes, and tumors from patients with malignancies. J. Immunol. 2013;190(11):5847–5855. doi:10.4049/jimmunol.1203279.
- Carpenter EL, Mick R, Rech AJ, Beatty GL, Colligon T, Mr R, De K, K-M C, Sm D, Kanetsky P, et al. Collapse of the CD27+ B-cell compartment associated with systemic plasmacytosis in patients with advanced melanoma and other cancers. Clin. Cancer Res. 2009;15(13):4277–4287. doi:10.1158/1078-0432.CCR-09-0537.
- Shimabukuro-Vornhagen A, Schlößer HA, Gryschok L, Malcher J, Wennhold K, Garcia-Marquez M, Herbold T, Neuhaus LS, Becker HJ, Fiedler A, et al. Characterization of tumor-associated B-cell subsets in patients with colorectal cancer. Oncotarget. 2014;5(13):4651–4664. doi:10.18632/oncotarget.1701.
- Germain C, Gnjatic S, Dieu-Nosjean M-C. Tertiary Lymphoid Structure-Associated B Cells are Key Players in Anti-Tumor Immunity. Front. Immunol. 2015;6. doi:10.3389/fimmu.2015.00067.
- Vh E, Ab R, Is M, An W, Jd P, Cl S. Immune Cell Infiltration and Tertiary Lymphoid Structures as Determinants of Antitumor Immunity. J. Immunol. 2018;200(2):432–442. doi:10.4049/jimmunol.1701269.
- Qian L, Bian G-R, Zhou Y, Wang Y, Hu J, Liu X, Xu Y. Clinical significance of regulatory B cells in the peripheral blood of patients with oesophageal cancer. Cent. J. Immunol./Polish Soc. Immunol. Elev. Other Cent. Immunol. Soc. 2015;40(2):263–265. doi:10.5114/ceji.2015.52840.
- Wouters MC, Nelson BH. Prognostic significance of tumor-infiltrating B cells and plasma cells in human cancer. Clin. Cancer Res. 2018Jul26. clincanres.1481.2018 doi:10.1158/1078-0432.CCR-18-1481.
- Ohue Y, Wada H, Oka M, Nakayama E. Antibody response to cancer/testis (CT) antigens: A prognostic marker in cancer patients. Oncoimmunology. 2014;3(11):e970032-1-e970032-3. doi:10.4161/21624011.2014.970032.
- Reuschenbach M, Von Knebel Doeberitz M, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol. Immunother. 2009;58(10):1535–1544. doi:10.1007/s00262-009-0733-4.
- Chen Y-T, Panarelli NC, Piotti KC, Yantiss RK. Cancer-testis antigen expression in digestive tract carcinomas: frequent expression in esophageal squamous cell carcinoma and its precursor lesions. Cancer Immunol. Res. 2014;2(5):480–486. doi:10.1158/2326-6066.CIR-13-0124.
- Mashino K, Sadanaga N, Tanaka F, Yamaguchi H, Nagashima H, Inoue H, Sugimachi K, Mori M. Expression of multiple cancer-testis antigen genes in gastrointestinal and breast carcinomas. Br. J. Cancer. 2001;85(5):713–720. doi:10.1054/bjoc.2001.1974.
- Perea F, Sánchez-Palencia A, Gómez-Morales M, Bernal M, Á C, García MM, González-Ramírez AR, Kerick M, Martin J, Garrido F, et al. HLA class I loss and PD-L1 expression in lung cancer: impact on T-cell infiltration and immune escape. Oncotarget. 2018;9(3):4120–4133. doi:10.18632/oncotarget.23469.
- Han LY, Fletcher MS, Urbauer DL, Mueller P, Landen CN, Kamat AA, Lin YG, Merritt WM, Spannuth WA, Deavers MT, et al. HLA class I antigen processing machinery component expression and intratumoral T-Cell infiltrate as independent prognostic markers in ovarian carcinoma. Clin. Cancer Res. 2008;14(11):3372–3379. doi:10.1158/1078-0432.CCR-07-4433.
- Thompson ED, Zahurak M, Murphy A, Cornish T, Cuka N, Abdelfatah E, Yang S, Duncan M, Ahuja N, Taube JM, et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. 2016Jan22;gutjnl-2015–310839. doi:10.1136/gutjnl-2015-310839.
- McLaughlin J, Han G, Schalper KA, Carvajal-Hausdorf D, Pelakanou V, Rehman J, Velcheti V, Herbst R, LoRusso P, Rimm DL. Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non-Small-Cell Lung Cancer. JAMA Oncol. 2015Nov;12:1–9. doi:10.1001/jamaoncol.2015.3638.
- Hirata T, Yamamoto H, Taniguchi H, Horiuchi S, Oki M, Adachi Y, Imai K, Shinomura Y. Characterization of the immune escape phenotype of human gastric cancers with and without high-frequency microsatellite instability. J. Pathol. 2007;211(5):516–523. doi:10.1002/path.2142.
- GTEx Consortium J, Thomas J, Salvatore M, Phillips R, Lo E, Shad S, Hasz R, Walters G, Garcia F, Young N, et al. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013;45(6):580–585. doi:10.1038/ng.2653.
- Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Å S, Kampf C, Sjöstedt E, Asplund A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi:10.1126/science.1260419.
- Wennhold K, Thelen M, Schlößer HA, Haustein N, Reuter S, Garcia-Marquez M, Lechner A, Kobold S, Rataj F, Utermöhlen O, et al. Using antigen-specific B cells to combine antibody and T cell–based cancer immunotherapy. Cancer Immunol. Res.. 2017;5:9.doi:10.1158/2326-6066.CIR-16-0236.