ABSTRACT
Hepatocellular carcinoma (HCC) is one type of cancers whose carcinogenesis and progression are closely related to chronic inflammation. Identifying the molecular mechanisms for inflammation-related HCC progression will contribute to improve the efficacy of current therapeutics for HCC patients. Many kinds of epigenetic factors, including long non-coding RNAs (lncRNAs), have been discovered to be important in HCC growth and metastasis. However, how the lncRNAs promote HCC progression and what’s the application of lncRNA silencing in vivo in suppressing HCC remain to be further investigated. Here, we found that lncRNA metastasis associated lung adenocarcinoma transcript1 (MALAT1) was upregulated in HCC tumor tissues, and knockdown of MALAT1 suppressed proliferation, cell cycle and invasion of HCC cells in response to lipopolysaccharide (LPS) stimulation. Knockdown of MALAT1 significantly inhibited LPS-induced pro-inflammatory mediators IL-6 and CXCL8 expression in HCC cells, which could be restored by overexpressing MALAT1. Mechanistically, MALAT1 recruited Brahma-related gene 1 (BRG1), a catalytic subunit of chromatin remodeling complex switching/sucrose non-fermentable (SWI/SNF), to the promoter region of IL-6 and CXCL8, and thus facilitated NF-κB to induce the expression of these inflammatory factors. Importantly, in vivo silencing of MALAT1 in HCC tissues inhibited growth of HCC xenografts, and also suppressed the expression of pro-inflammatory factors in HCC tissues accordingly. Our results demonstrate that MALAT1 promotes HCC progression by binding BRG1 to epigenetically enhance inflammatory response in HCC tissues, and silencing of MALAT1 may be a potential approach to the treatment of HCC.
Introduction
Hepatocellular carcinoma (HCC) is frequently linked to chronic inflammation.Citation1,Citation2 Inappropriate activation of the innate signaling can lead to prolonged inflammation and contribute to inflammation-related tumorigenesis.Citation3,Citation4 Toll-like receptor 4 (TLR4), the receptor for LPS, induces the host inflammatory response to infection but also promotes the development and progression of various human cancers including HCC.Citation5–Citation8 Inflammatory cytokines like tumor necrosis factor alpha (TNF-α), lymphotoxin-β and interleukin-6 (IL-6) play important roles in HCC progression.Citation9–Citation11 Among them, IL-6 is one of the most critical pro-tumor factors during HCC development. Mice deficient in IL-6 develop much less HCC in response to diethylnitrosamine (DEN), and the gender-biased production of IL-6 accounts for much higher HCC load in males.Citation12 High levels of circulating IL-6 are associated with HCC risk factors, including hepatosteatosis, obesity, and liver cirrhosis, and are the important predictors of rapid progression from viral hepatitis to HCC in humans.Citation13 In addition, chemokine C-X-C motif ligand 8 (CXCL8) expression is significantly higher in numerous types of cancer as compared to healthy tissues.Citation14 In line with this, high CXCL8 concentration has been detected in the serum of HCC patients, and CXCL8 levels correlate with cumulative tumor size, stage, and prognosis.Citation15,Citation16 In fact, patients with higher serum CXCL8 levels have worse overall survival.Citation17 Now, identifying the molecular mechanism and pathological significance of tumor-derived inflammatory factors attracts much attention because it would contribute to the better understanding of tumor progression and provide the potential targets to treat cancer growth and metastasis. However, molecular mechanisms for inflammation-induced HCC progression are still elusive.
Continuing advances in transcriptomics revealed that long non-coding RNAs (lncRNAs) are one of the most important mediators in regulating differentiation and developmental processes and pathological responses. Abnormal expressions of lncRNAs like MALAT1, PCAT7, PVT1, and HOTAIR have been demonstrated to play important roles in tumorigenesis and tumor progression.Citation18,Citation19 LncRNAs can regulate gene expression at different levels, such as chromatin modification, transcription and post-transcriptional processing. Xist (X inactive specific transcript), the first identified lncRNA, recruits the polycomb complex to the X chromosome and then trimethylates lysine 27 residue of histone H3 to induce heterochromatin formation, finally rendering the silence of X chromosome.Citation20 LncRNA NRON can bind to the transcription factor NFAT (nuclear factor of activated T cells) and impede nuclear accumulation of NFAT to inhibit downstream reactions.Citation21
MALAT1, a conserved nuclear-retained lncRNA, was originally recommended to control lung cancer metastasis and cancer cell survival.Citation22 Recent studies showed that MALAT1 was overexpressed in various human carcinomas, including gastric adenocarcinoma,Citation23 renal carcinoma,Citation24 breast cancerCitation25 and glioblastoma,Citation26 and was considered as a potential prognostic biomarker for cancers diagnosis.Citation27,Citation28 Inhibition of MALAT1 could effectively reduce cell proliferation, viability and metastasis, while increasing the susceptible to apoptosis.Citation27,Citation29 However, the mechanism for MALAT1’s promoting role in HCC remains unclear, which needs to be investigated.
In the present study, we analyzed the expression pattern of MALAT1 in HCC clinical samples and investigated its role in tumor progression in vivo and in vitro. We showed that the up-regulated MALAT1 recruited chromatin remodeling component BRG1 to transcription factor nuclear factor kappa-B/P65 (NF-κB/P65) binding loci within the promoter region of IL-6 and CXCL8, promoting the secretion of IL-6 and CXCL8, and further facilitated HCC cell proliferation and invasion. Our results revealed a role for MALAT1 in inflammation-promoted HCC progression, which deepened our understanding of how lncRNA function in tumor progression.
Results
Increased expression of MALAT1 in human HCC cells and tissues
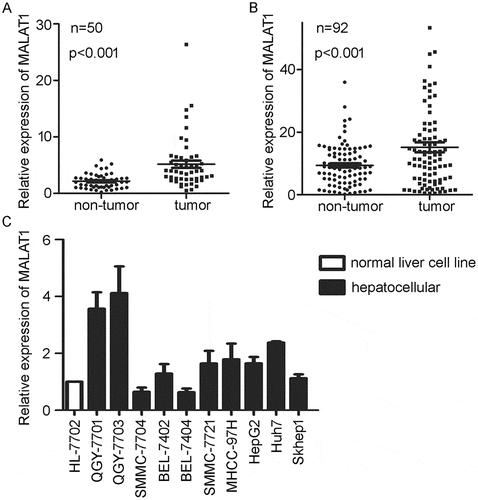
MALAT1 was up-regulated in multiple types of human solid tumors. Analysis of the clinical data from The Cancer Genome Atlas (TCGA) database (http://cancergenome.nih.gov/) confirmed the higher expression of MALAT1 in tumors than that in paired non-tumorous tissues of HCC (). Next, we examined 92 pairs of HCC tissues and matched paracancerous tissues. As expected, up-regulation of MALAT1 was detected in 60 (65.2%) cases, and higher expression of MALAT1 was obviously observed in HCC tumors than that in adjacent non-cancerous liver tissues (). In addition, we examined the expression of MALAT1 in eleven hepatoma cell lines. QGY-7703 and QGY-7701, among the HCC cell lines detected, expressed the highest level of MALAT1 than the normal liver cell line HL-7702 () and were selected for further investigation.
Figure 1. Higher expression of MALAT1 in HCC clinical samples and cell lines. A) Relative expression of MALAT1 in HCC tumor and paired non-tumorous tissues from TCGA. B) Relative expression of MALAT1 was detected in tumors and paired non-tumorous tissues of 92 HCC patients. C) Relative expression of MALAT1 in normal liver cell line and HCC cell lines. Results are normalized by human GAPDH and presented relative to normal liver cell line HL-7702. Data are shown as mean± s.e.m. (n = 3), *P < 0.05, **P < 0.01, ***P < 0.001.
MALAT1 promotes HCC cell growth and invasion in vitro
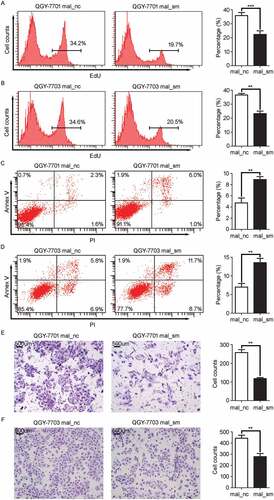
We next evaluated the impact of MALAT1 on inflammation-induced HCC progression. Loss-of-function experiments were performed in HCC cell lines following with LPS stimulation. The 5-Ethynyl-2ʹ-deoxyuridine (EdU) incorporation experiment was performed in QGY-7701/7703 cells after MALAT1 knockdown (Fig. S1A and 1B). The flow cytometer showed knockdown of MALAT1 significantly suppressed HCC cell proliferation ( and ). Cell cycle analysis showed that knockdown of MALAT1 retained more QGY-7701/7703 cells in G1 phase (Fig. S1C and 1D). Apoptosis assays showed that QGY-7701/7703 cells with MALAT1 knockdown were less resistant to apoptosis induction by Doxorubicin, a chemotherapeutic drug ( and ). In matrigel invasion assays, knockdown of MALAT1 impaired migration of QGY-7701/7703 cells (109 versus 284, p < 0.01 and 223 versus 396, p < 0.01, respectively) ( and ). Together, these results reveal that MALAT1 promotes HCC cell growth and invasion in vitro.
Figure 2. MALAT1 suppresses cell apoptosis while promotes proliferation, invasion of HCC cells. HCC cells, QGY-7701 and QGY-7703, were transfected with negative control (mal_nc) or MALAT1 smart silence (mal_sm) at 50nM for 48h. A-B) QGY-7701 A) and QGY-7703 B) cell growth were measured by EdU incorporation. C-D) Apoptotic QGY-7701 C) and QGY-7703 D) cells were analyzed by FACS. The AnnexinV positive cells were regarded as apoptosis cells. E-F) The invasive ability of QGY-7701 E) and QGY-7703 F) cells were evaluated by in vitro invasion assays. Scale bar = 500 μm. Data are shown one representative experiment. Similar results were obtained in at least three independent experiments.
MALAT1 promotes LPS-induced IL-6 and CXCL8 expression in HCC cells
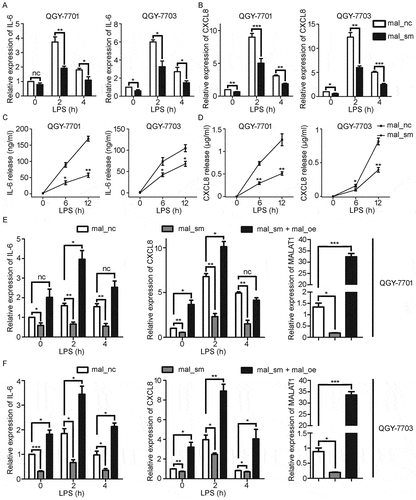
Chronic inflammation is a critical factor in promoting tumor progression, especially in HCC.Citation30 Thus we wondered whether the upregulated expression of MALAT1 could enhance inflammatory response in HCC cells. LPS-TLR4 signaling was able to activate the NF-κB pathway in HCC.Citation5,Citation31 As shown in and , knockdown of MALAT1 significantly decreased the mRNA expressions of IL-6 and CXCL8 in QGY-7701/7703 cells following LPS stimulation while mRNA expression of TNF-α, IL-1β and MALAT1 remained unchanged in LPS-stimulated QGY-7701/7703 cells (Fig. S2A, 2B and 2C). Meanwhile, the protein levels of IL-6 and CXCL8 in supernatants of QGY-7701/7703 cells decreased once knockdown of MALAT1 ( and ). Moreover, the suppression of LPS-induced IL-6 and CXCL8 expression was restored after re-expression of MALAT1 in QGY-7701/7703 cells 24 h after MALAT1 knockdown ( and ). These data indicate that MALAT1 could promote LPS-induced production of inflammatory factors IL-6 and CXCL8 in HCC cells, indicating MALAT1 might involve the inflammation-related HCC progression.
Figure 3. Knockdown of MALAT1 decreases LPS-induced IL-6 and CXCL8 expression in HCC cells. HCC cells, QGY-7701 and QGY-7703, were treated with mal_nc or mal_sm at 50nM for 48h. A, B) mRNA levels of IL-6 A) and CXCL8 B) in QGY-7701 and QGY-7703 cells were examined by RT-qPCR after exposure to LPS (1μg/ml) for 0 h, 2 h and 4 h respectively. C-D) Protein production of IL-6 C) and CXCL8 D) from QGY-7701 and QGY-7703 cells were determined using ELISA in response to LPS (1μg/ml) stimulation for 0 h, 6 h and 12 h. E-F) Transfection of 1 μg MALAT1 overexpression plasmid for 48 h after mal_nc/mal_sm transfected, relative expression of IL-6 and CXCL8 in QGY-7701 E) and QGY-7703 F) cells were examined by RT-qPCR after LPS (1 μg/ml) treatment for 0 h, 2 h and 4 h. GAPDH was used as an internal reference for RT-qPCR. Data are shown as mean± s.e.m. (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
MALAT1 associates with chromatin remodeling subunit BRG1 to promote inflammatory factors production in HCC cells
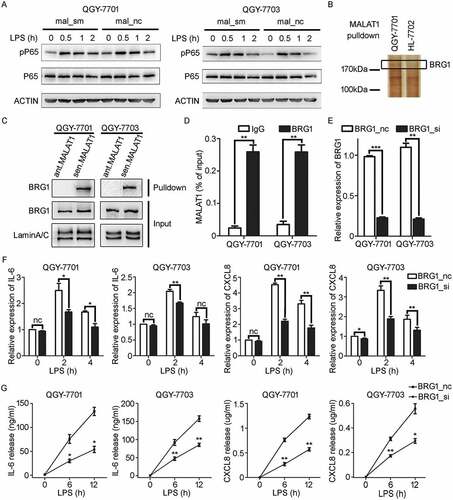
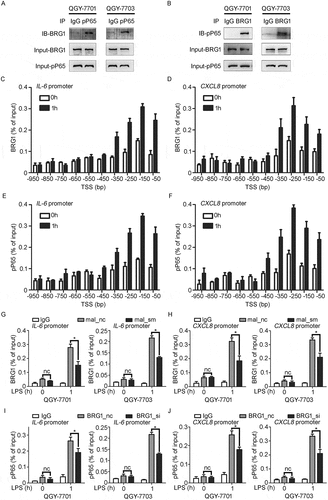
For investigating how MALAT1 regulated the expression of IL-6 and CXCL8 in HCC cells, phosphorylated NF-κB P65 (pP65) was firstly detected and found unaffected in QGY-7701/7703 cells with or without knockdown of MALAT1 (). LncRNAs are widely accepted as sinks for microRNA-RNA reciprocityCitation32 or as scaffolds for protein-DNA interactions.Citation33 We speculated that MALAT1 might associate with specific proteins in the nuclear in this process. The nuclear proteins interacting with biotinylated MALAT1 were separated, and further identified by mass spectrometry (MS). BRG1, a catalytic subunit of chromatin remodeling complex SWI/SNF, was indicated to be associated with MALAT1 in QGY-7701 cells because analysis of the difference of MALAT1-associated proteins suggested that there was no indication of MALAT1- BRG1 association in normal liver cells HL-7702 (). RNA pull-down and BRG1-RIP experiments further verified the specific interaction of MALAT1- BRG1 in HCC cells QGY-7701/7703 ( and ). Furthermore, silencing of BRG1 () was found to down-regulate LPS-induced expression of IL-6 and CXCL8 ( and ), which was consistent with that of MALAT1 knockdown. These data suggest that MALAT1 could interact with BRG1 to regulate expression of IL-6 and CXCL8 in HCC cells in response to inflammatory signals.
Figure 4. MALAT1 associates with BRG1 to promote IL-6 and CXCL8 expression in HCC cells in response to LPS stimulation. A) Western blotting (WB) was performed to analyze the levels of pP65 in mal_nc/mal_sm transfected HCC cells QGY-7701 and QGY-7703 in response to LPS (1 μg/ml) stimulation at indicate time. B) Biotinylated MALAT1 RNA pull down experiment combined with mass spectrometry is performed to identify its interacted protein. C) Immunoblot detection of BRG1 pulled down by sense (sen.) or antisense (ant.) MALAT1 transcripts from nuclear extract of HCC QGY-7701/QGY-7703 cells. D) RT-qPCR detection of MALAT1 in the immunoprecipitation complex by IgG or BRG1 antibody from HCC QGY-7701/QGY-7703 cell lysate. E) Relative expression of BRG1 in HCC QGY-7701/QGY-7703 cells after BRG1 siRNA/NC treating for 48 h at 20 nM was determined by RT-qPCR. F, G) mRNA and protein levels of IL-6 and CXCL8 were analyzed by RT-qPCR F) and ELISA G) respectively in HCC QGY-7701/QGY-7703 cells in response to LPS (1 μg/ml) stimulation for the indicated time after BRG1 siRNA/NC treating for 48 h. Data are shown as mean± s.e.m. (n = 3) (D, E, F, G). Data are shown one representative experiment (A, B, C). Similar results were obtained in three independent experiments. * P < 0.05, ** P < 0.01.
MALAT1 recruits BRG1 to the promoter region of IL-6 and CXCL8 following LPS stimulation
Next, we explored the underlying molecular mechanism by which MALAT1 enhanced inflammatory responses in HCC cells. BRG1 has been reported to regulate pro-inflammatory factors like IL-6, IL-1 and monocyte chemotactic protein 1 (MCP-1), and be associated with pP65.Citation34 We speculated that MALAT1 might recruit BRG1 to the promoters of IL-6 and CXCL8 in response to LPS stimulation, and then promoted the transcription of IL-6 and CXCL8 initiated by pP65. To confirm this, co-immunoprecipitation (co-IP) was performed using anti-BRG1 or anti-pP65, followed by Western blotting to assess relationship between BRG1 and pP65. A robust interaction between BRG1 and pP65 was observed ( and ). Then, primers about ~ 1kb upstream of the transcription start site of IL-6 and CXCL8 were designed for CHIP-qPCR detection (Table. S1 and S2). The results showed a significant increase in BRG1 recruitment at the IL-6 −250 ~ −50bp of TSS (encompassed by primer set 3) and CXCL8 −350 ~ −150bp of TSS (encompassed by primer set 3) in response to LPS stimulation, respectively ( and ). Intriguingly, it appears that BRG1 and pP65 occupy the same region of the IL-6 and CXCL8 promoter, both encompassed by primer set 3 ( and ). Furthermore, knockdown of MALAT1 attenuated the recruitment of BRG1 to IL-6 and CXCL8 promoter induced by LPS ( and ). Knockdown of BRG1 impaired the recruitment of pP65 to IL-6 and CXCL8 promoter in response to LPS stimulation ( and ). Taken together, these results suggest that lncRNA MALAT1 connects BRG1 with pP65 as a scaffold in response to LPS stimulation, and thus enhances their binding to IL-6/CXCL8 gene promoters to facilitate gene expression of the pro-inflammatory cytokines.
Figure 5. BRG1, recruited by MALAT1, binds to the promoter regions of IL-6 and CXCL8 loci in HCC cells in response to LPS stimulation. A, B) WB detection of the indicated proteins in input samples and in pP65- A) or BRG1- B) specific antibody immunoprecipitated samples in QGY-7701 or QGY-7703 cells with LPS (1 μg/ml) stimulation for 1 h. C, D) ChIP assay analysis for the recruitment of BRG1 to the promoter region of IL-6 C) and CXCL8 D) genes in QGY-7701 cells with LPS (1 μg/ml) stimulation for 1 h. E, F) ChIP assay analysis for the recruitment of pP65 to the promoter region of IL-6 E) and CXCL8 F) genes in QGY-7701 cells with LPS (1 μg/ml) stimulation for 1h. G, H) ChIP assay was performed to analyze the recruitment of BRG1 to promotors of IL-6 G) and CXCL8 H) in HCC cells once MALAT1 knockdown and followed by exposure to LPS (1 μg/ml) stimulation for 1 h. I, J) BRG1 si/nc treated cells further exposed to LPS (1 μg/ml) for 1 h, ChIP assay was performed to analyze the recruitment of pP65 to promotors of IL-6 I) and CXCL8 J) in HCC cells. Data are shown as mean± s.e.m. (n = 3) (C, D, E, F, G, H, I, J). Data are shown one representative experiment (A, B). Similar results were obtained in three independent experiments. * P < 0.05, ** P < 0.01.
In vivo silencing of MALAT1 in HCC tissues inhibits growth of HCC xenografts and induction of pro-inflammatory factors
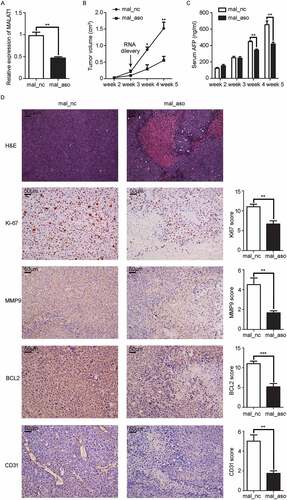
We further investigated whether in vivo silencing of MALAT1 could inhibit HCC growth. SMMC-LTNM, a human HCC-bearing nude mouse model, was used for in vivo study because this model is more close to clinical progression of HCC as high level of α-fetoprotein (AFP) could be detected in sera.Citation35,Citation36 After intratumoral injection of cholesterol-conjugated MALAT1 antisense oligonucleotide (ASO) for 3 weeks, we confirmed the reduced expression of MALAT1 in HCC xenografts () and found tumor growth was significantly inhibited in mice treated with MALAT1 ASO, almost one third of that treated with MALAT1 NC (). Similarly, serum AFP collected from mice treated with MALAT1 ASO reduced obviously till the last week (). Histologic analysis showed that extensive necrosis accompanied with MALAT1 ASO delivery. Immunohistochemical analysis revealed expressions of proliferation indicator Ki-67, invasion indicator matrix metalloproteinase 9 (MMP9), apoptosis indicator B-cell lymphoma 2 (BCL2), and angiogenesis indicator CD31 were reduced greatly in HCC tumors bearing MALAT1 knockdown (). These results indicated that MALAT1 promotes HCC xenografts growth in vivo.
Figure 6. In vivo knockdown of MALAT1 in HCC tissues suppresses HCC growth. A) RT-qPCR analysis of MALAT1 expression in SMMC-LTNM tumor tissue two weeks after intratumoral injection of cholesterol-conjugated MALAT1 silence (mal_aso) or negative control (mal_nc). B, C) Two weeks after subcutaneous inoculation of SMMC-LTNM tumor cells, HCC-bearing nude mice were treated by intratumoral injection of mal_aso or mal_nc, respectively. Tumor volume B) and serum AFP levels C) were shown. D) H&E staining and detection of Ki-67, MMP9, BCL2 and CD31 by IHC in HCC tissues was performed two weeks after intratumoral injection of cholesterol-conjugated mal_aso or mal_nc. IHC staining score were analyzed statistically (right panel). Scale bars = 50μm or 500μm. Data are shown as mean± s.e.m. (n = 3) (A, B, C and D). Data are shown one representative experiment (D) Similar results were obtained in three independent experiments. * P < 0.05, ** P < 0.01.
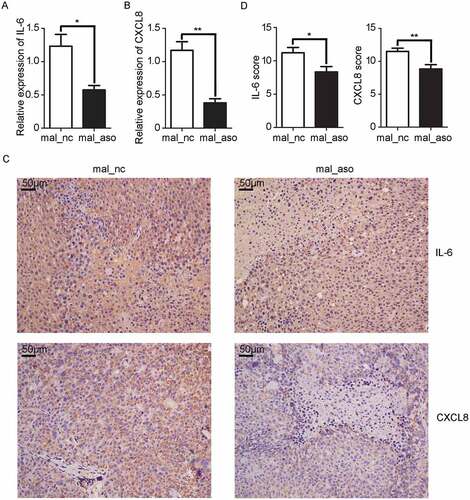
Furthermore, inflammatory cytokines IL-6 and CXCL8 were detected in HCC xenografts. As shown in and , the mRNA levels of IL-6 and CXCL8 in tissues treated with MALAT1 ASO were decreased to one half and one third respectively than that in control mice. Immunohistochemistry results showed that the extracellular distributions of IL-6 and CXCL8 were greatly decreased in HCC tissues with in vivo silencing of MALAT1 ( and ), which further supporting MALAT1 promotes HCC progression by epigenetically inducing pro-inflammation factors, and silencing of MALAT1 could inhibit HCC progression.
Figure 7. In vivo knockdown of MALAT1 inhibits inflammatory response in HCC tissues. A, B) RT-qPCR analysis of IL-6 A) and CXCL8 B) expression in HCC xenografts two weeks after intratumoral injection of cholesterol-conjugated MALAT1 silence (mal-aso) or negative control (mal-nc). C) Detection of IL-6 and CXCL8 by IHC in HCC tissues was performed two weeks after intratumoral injection of cholesterol-conjugated MALAT1 silence mal_aso or mal_nc. Scale bars = 50μm. D) IHC staining score of IL-6 and CXCL8 was made in mal_aso and mal_nc groups. Data are shown as mean± s.e.m. (n = 3) (A, B and D). Data are shown one representative experiment (C). Similar results were obtained in three independent experiments. * P < 0.05, ** P < 0.01.
Discussion
There are a large number of studies proving that lncRNAs serve as regulators in inflammatory responses in immune cells. LncRNA Lethe impeded the NF-κB p65 subunit from binding TNF promoter, negatively regulating TNF expression in mouse macrophages.Citation33 LincRNA-Cox2 assembled with SWI/SNF chromatin remodeling complex recruiting to the promoter region of CCL5 gene and activated transcription in macrophage cell lines after LPS stimulation.Citation37 MALAT1 has also been reported to regulate expression of TNF-α and IL-6 in endothelial cells and other immune cells like macrophages.Citation38,Citation39 We definitely found that MALAT1 promoted IL-6 and CXCL8 production in HCC cells in response to inflammatory signals. It’s well known that lncRNAs usually function through binding to specific protein especially transcription factors. In our study, we observed a close interaction of BRG1 and pP65 mediated by MALAT1 in HCC cells in response to LPS stimulation, which shed new light on relationships among lncRNA, inflammation and cancer.
Mammalian SWI/SNF complexes contain a BRG1 or biological response modifier (BRM) ATPase, and other ~ 10 affiliated subunits to form complexes as gene switch. BRG1, the core catalytic subunit of the SWI/SNF mammalian chromatin remodeling complex, has been previously reported to take part in the transactivation of pro-inflammatory mediators in LPS stimulated macrophages.Citation40 Recent study showed that NF-κB/P65 recruitments of BRG1 and BRM; reciprocally, BRG1 and BRM assistant to the stabilization of P65 binding.Citation34 Data we obtained reinforce the key role of BRG1 in facilitating pro-inflammatory cytokines transcription. The remaining question unsolved was how BRG1 interacted with promoters to promote the binding of pP65. One possibility was that being recruited by MALAT1 after LPS stimulation, BRG1 changed the conformation of chromatin positioning at the IL-6 and CXCL8 promoters, making them more accessible for pP65. Contrary to our result, a recent report described that NF-κB directly binds to the promoter of MALAT1 to activate its transcription. In turn, increased MALAT1 inhibiting its DNA binding activity and consequently decreasing the expression of TNF-α and IL-6 in macrophage.Citation39 Considering different expression level and function of MALAT1 in different cell types, it is reasonable that MALAT1 had different effects in macrophage and HCC cell. As HCC is a disease with multistage process, further studies are necessary to elucidate the transcriptional or post-transcriptional function of MALAT1.
Pro-inflammatory cytokines are associated closely with a series of cell biological processes concerning cancers. IL-6, as a critical mediator of HCC development, not only prevents DNA-damage-induced hepatocyte apoptosis through suppression of p53 and the enhanced beta-catenin activation and tumor proliferation, but also directly induces endothelial cell proliferation to promote tumor angiogenesis.Citation41 Other than these, IL-6 promotes HCC tumor growth and metastasis by activating signal transducers and activators of transcription 3 (STAT3) and its downstream target genes, including Bcl-2, myeloid cell leukemia 1 (Mcl-1), cyclinD1 and matrix metalloproteinase 2 (MMP2).Citation42 CXCL8 is another cytokine mostly implicated in promoting tumorigenesis and angiogenesis.Citation43 Upon CXCL8 stimulation, endothelial cells began angiogenic processes characterized by secretion of MMPs to break-down the extracellular matrix, with proliferation and formation of new vessels.Citation44,Citation45 In addition, CXCL8 secreted by HCC cells makes a tumorigenic inflammatory microenvironment to promote epithelial-mesenchymal transition and HCC invasion by MAPK and NF-κB pathway.Citation46 The CXCL8- chemokine (C-X-C motif) receptor 2 (CXCR2) axis trans-activates epithelial growth factor receptor (EGFR) via receptor phosphorylation to mediate endothelial cell migration and capillary tube formation, and recent study showed the inhibition of this axis could restrain cell proliferation by induction of G0/G1 cell cycle arrest and regarded as a potential therapeutic target against acute myeloid leukemia.Citation47–Citation49 In our study, MALAT1 knockdown significantly impairs LPS-induced IL-6 and CXCL8 secretion, resulting in the decreased HCC cell proliferation by retaining HCC cells in G0 phase, decreased invasion capacity and increased apoptosis by MMP9 and BCL2 downregulation. Considering SMMU-LTNM nude mice with the reduced distribution of IL-6 and CXCL8 after MALAT1 interference in vivo, we speculated that the shrinked HCC xenograft owing to the decrease of IL-6/CXCL8 in the tumor microenvironment.
Considering the specific cancer expression signature, multiple oncogenic lncRNAs hold the potential as clinical therapeutic targets. lncRNA candidates with an oncogenic activity could be targeted by RNA interference (RNAi) or RNAi-like molecules as a potential approach for a reduction of their abundance and thus a diminution of their functional activity. The same effect could be achieved by the binding of short RNA sequences (antagolincs)Citation50 to the lncRNA-protein interfaces, disrupting the interaction. Systematic treatment of tumor-bearing mice with short RNA sequences (antagomirs) targeting miRNA has been proved to be efficient. In vivo treatment with antagomirs targeting miR-10b inhibits tumor metastasis by increasing the expression of its target, a tumor suppressor gene HOXD10.Citation51 Moreover, double-stranded DNA plasmids inducing toxic responses could also been used in the lncRNA targets therapies. BC-819, a double-stranded DNA plasmid carrying the gene for the a subunit of diphtheria toxin under the control of lncRNA H19 regulatory sequences, has been clinically used in vivo to knockdown H19 in different cancers, and has shown promising therapeutic benefit for the cancer patients.Citation52,Citation53 Our in vivo injection of MALAT1 ASO has successfully decreased the expression of MALAT1 and suppressed HCC progression, which suggested targeting MALAT1 in HCC patients is a potential and promising clinical therapy.
In summary, our study suggested that lncRNA MALAT1 connects BRG1 to pP65 as scaffold following LPS stimulation, and promotes expression of pro-inflammatory cytokines IL-6/CXCL8 to accelerate HCC deterioration, which deepened our understanding of the complicated process of HCC initiation and development.
Materials and methods
Patients
Human HCC tissue samples were obtained from HCC patients during operation, and paracancerous tissues were obtained from distal normal liver and all the samples were immediately frozen in liquid nitrogen until analysis. This study was approved by the ethics committee of Second Military Medical University, Shanghai, China and informed consent was obtained from each patient. We confirm that our study’s involvement with human subjects complies with the Declaration of Helsinki.
HCC-bearing nude mouse model and in vivo treatment
All animal experiments were undertaken in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Scientific Investigation Board of Second Military Medical University, Shanghai. The HCC-bearing male nude mice model SMMC-LTNM were raised and passaged as previously described.Citation35,Citation54 At the third week after subcutaneously inoculation, 10nmol cholesterol-modified MALAT1 antisense oligonucleotide, negative control (synthesized in Ribobio) was delivered by intratumorally injected every 3 days for 2 weeks. Tumor size was measured and serum AFP was detected using ELISA.
Cell culture
Normal liver cell HL-7702 and human HCC cell lines QGY-7701, QGY-7703, BEL-7402, BEL-7404, MHCC-97H, HepG2, huh7, SK-hep1 (all from American Type Culture Collection), SMMC-7704 and SMMC-7721 (maintained by our lab) were cultured in RPMI-1640 or DMEM media containing 10% (v/v) fetal bovine serum (FBS, PAA Laboratories) at 37 °C in a humidified incubator containing 5% CO2.
Plasmid preparation, small interfering RNA (sirna) and plasmids transfection
Plasmid preparation: The human MALAT1 cDNA (Gene ID: 378938) was amplified by RT-PCR using the RNA extracted from QGY-7701 cells, and then cloned into pcDNA3.1 vector (Invitrogen).
siRNA and plasmids transfection: QGY-7701 and QGY-7703 cells were plated at 8 × 10Citation5 cells per well in 6-well plates and incubated overnight. siRNA or plasmids were transfected using INTERFERin (409–10, Polyplus) or jetPRIME (114–15, Polyplus) following protocols provided by the vendors, respectively. siRNA targeted against human MALAT1 were obtained from Ribobio, and siRNA of BRG1 was synthesized by GenePharma (Shanghai, China). The final concentrations of MALAT1 and BRG1 siRNA were 50 nM and 20 nM, respectively. All siRNAs are listed in Supplementary Table 3.
RNA isolation and quantitative real-time RT–PCR (q-PCR)
Total RNAs from HCC tissues and cells were extracted using TRIzol reagent (127401, Invitrogen) according to the manufacturer’s instructions. And then, complementary DNA (cDNA) was performed using the ReverTra Ace -α- (FSK-101, TOYOBO) according to the manufacturer’s instructions. The expression of GAPDH, MALAT1, IL-6 and CXCL8 was measured by q-PCR, which was performed by LightCycler1.5 or LightCycler480II (Roche) with SYBR® Green Real time PCR Master Mix (QPK-201, TOYOBO) according to the manufacturer’s direction. All the samples were normalized to human GAPDH according to the 2−ΔΔCT method. All primers used in this study were synthesized by Sangen Biotech (Shanghai, China) and listed in Supplementary Table 4.
Total/nuclear protein extraction
QGY-7701 and QGY-7703 cells were collected after appropriate treatment. Then, cells were lysed in ice-cold 1M Tris-HCl containing 0.1% Nonidet P-40 (NP40) on ice for 15 min. After centrifugation at 5000 g for 5 min, the supernatant was collected as cytoplasmic protein and the pellets with an additional wash were considered as nuclear fractions. Proteins were then extracted from the nuclear fractions using ice-cold 1M Tris-HCl containing 10% SDS, after sonicate for 5 min, the supernatant was collected as nuclear protein. While total protein was extracted using the cell lysis buffer (10 mM Tris-HCl pH7.4, 10 mM NaCl, 3 mM MgCl2, 0.5% NP-40).
Western blot analysis
Whole cell lysates were prepared using cell lysis buffer (9803, CST) containing Proteinase inhibitor cocktail (539138, Calbiochem) according to the manufacturer’s instructions. Protein concentrations were determined using the BCA protein assay (23225, Thermo Fisher Scientific). Protein samples containing loading buffer were denatured in boiled water for 5 minutes. Then, equal amounts of proteins were loaded to 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (10600002, GE healthcare). Membranes were blocked with 5 % non-fat dry milk in TBST at room temperature for more than 1h and blotted with primary antibodies at 4 °C overnight. Signals were visualized using a horseradish peroxidase (HRP)-labeled secondary antibody and the enhanced chemiluminescent (ECL) detection systems by hemiluminescent Western Blot Scanner (Gene Company, HongKong, China). Primary antibodies used in this study were anti-BRG1 rAb (1:1,000) (21634, Proteintech), anti-NF-kappaB/p65 mAb (1:1,000) (6956S, CST), anti-phospho-p65 rAb (1:1,000) (3033S, CST), anti-β-actin mAb (1:1000) (8H10D10, CST), secondary antibodies anti-rabbit (1:2,000) (7074, CST) and anti-mouse (1:2,000) (7076, CST).
Enzyme-linked immunosorbnent assay (ELISA)
QGY-7701 or QGY-7703 were transfected with specific siRNA for 48 h and then treated with 1μg/ml LPS for 0 h, 6 h, 12 h. The culture supernatants were collected and the level of IL-6 and CXCL8 were measured by ELISA kits which were from Proteintech (KE0007), R&D Systems (D8000C) respectively. AFP collected from mice was measured by ELISA kit from Shanghaijining (SU-B10784) according to the manufacturer’s directions.
Cell proliferation, invasion, cell cycle and apoptosis assay
Cell proliferation assay
Cell-Light EdU DNA Cell Proliferation Kit (C10310-1, RiboBio, China) was used to detect cell proliferation. According to the manufacturer’s instructions, QGY-7701, QGY-7703 cells were transfected with specific siRNA for 48 h and treat with 5ʹ- ethynyl-2ʹ -deoxyuridine (EdU) (50 μM) for an additional 2 h. The cells were then harvested and to fixed cells at room temperature for 30 min with 4% paraformaldehyde, wash with glycine (2 mg/ml). Centrifuge and discard the supernatant, the pellets incubation with reaction buffer (Tris–HCl, pH 8.5,100 mM; CuSO4, 1 mM; Apollo 550 fluorescent azide, 100 mM; ascorbic acid, 100 mM) for 30 min while protecting from light, and then wash with 0.5% Triton X-100 for three times. Finally, adding 500 μl PBS re-suspended cells and a FACSCalibur Flow Cytometer (BD LSRII, San Jose, CA, USA) was used to detect the EdU positive cells.
Invasion assay: Potentiality of cell invasion was measured by in vitro Transwell coated with Matrigel (356234, BD Pharmingen); 2 × 10^5 QGY-7701 or QGY-7703 cells were seeded to the upper chamber in serum-free RPMI-1640, and 15% serum was added to the lower chamber as a chemoattractant. After invasion for 24 h, penetrated cells on the filters were fixed in dried methanol, stained in 0.1% crystal violet, and then photographed and quantified by counting five fields per filter in each group.
Cell cycle assay
QGY-7701, QGY-7703 cells were treatment with specific siRNA for 48 h before harvested, and fixed in cold 75% ethanol overnight at 4 °C. Then, cells were treated with 20 μg/ml RNase A, followed by 25 μg/ml propidium iodide (PI) staining. The percentage of cells at each stage of the cell cycle was determined a flow cytometer.
Apoptosis assay
QGY-7701, QGY-7703 cells were transfected with specific siRNA for 48 h and treated with apoptosis inducers Doxorubicin (A603456, Sangon Biotech) for 24 h. Apoptotic cells were analyzed using FACSCalibur flow cytometry after incubating with reagent containing Annexin V-FITC and Propidium Iodide (556547, BD biosciences) for 15 min in darkness at room temperature.
RNA-pulldown/RIP/Co-IP assays
RNA-pulldown
Biotin RNA Labeling Mix (11685597910, Roche) and T7 RNA polymerase (10881767001, Roche) were applied to synthesize biotin-labeled RNAs, and then purified with RNeasy Mini Kit (74104, Qiagen) after treated with RNase-free DNase I (M610A, Promega). 3 µg of biotinylated RNA was heated to 65°C for 5 min, and then left at room temperature (RT) for 30 min to form proper secondary structure before using. Folded RNA was then mixed with QGY-7701/QGY-7703 nuclear protein extraction (containing 1 mg proteins) in IP buffer and then incubated at 4 °C for overnight. The second day, 50 µl washed Streptavidin agarose beads (11206D, Invitrogen) were added to each binding reaction and incubated at 4 °C for another one hour. Beads were washed softly with RIP buffer for five times and boiled in SDS loading buffer. Then the denatured proteins were detected by western blot or resolved in gradient gel electrophoresis followed by mass spectrometry (MS) identification.
RNA immunology precipitation (RIP)
Cells in culture were treated with 1% formaldehyde at room temperature for 10 min. The cross-linking reactions were quenched by the addition of glycine at a final concentration of 0.25 M for 5 min. The cells were harvested and lysates were collected. The cross-linked complexes were incubated with the specific BRG1 or rIgG (B900610, Proteintech) coated beads overnight. Formaldehyde cross-links were reversed by incubation with protein K at 65 °C for 4 h. Presence of RNA was purified by TRIzol reagent and then measured by RT-PCR. MALAT1-specific primer pairs for RIP-qPCR detection are 5ʹ- GTTTTCTCAGGTTTTGCTTTT-3ʹ (sense) and 5ʹ- TGAAGACAGATTAGTAGTCAA-3ʹ (antisense).
Co-immunoprecipitation (Co-IP)
Cell pellets were re-suspended in 1 ml cell lysis buffer mixture with protease inhibitor, and then supernatant were collected by centrifugation (500 g, 5 min). A total of 600 mg protein was incubated with the primary Abs of NF-kappaB/p65 or BRG1 at 4 °C overnight. The second day, immune complexes were collected by direct binding to protein A agarose. Protein complex was then denatured in boiled water for 10 minutes with loading buffer and the interaction between two proteins were detected by western blot.
Chromatin immunoprecipitation (CHIP)
In brief, QGY-7701 and QGY-7703 cells were stimulated or not with LPS (1μg/ml) for 1h. Whole cells were cross-linked with a 1% formaldehyde solution for 10 min at room temperature, and then quenched by the addition of glycine at a final concentration of 0.25 M for 5 min. Cells were sonicated (Branson Sonifier 250) for six cycles (output: 1, duty cycles: 50%, time: 10 s on 20 s off). ChIP assays were performed with 2 μg/mg NF-kB/P65 (6956S, CST) or BRG1 (21634, Proteintech) antibody and incubated overnight at 4 °C. Bead-bound DNA was reverse cross-linked and purified with phenol/chloroform reagent. Samples were then analyzed by qPCR using the indicated primers listed in Supplementary Table 1 and 2.
Nanospray liquid chromatography–tandem mass spectrometry
Biotinylated MALAT1 or antisense RNA in vitro transcribed with T7 RNA polymerase (10881767001, Roche) were incubated with QGY-7701/QGY-7703 nuclear protein extraction, targeted by streptavidin agarose beads (11206D, Invitrogen), and washed with 1 ml RIP buffer 5 × 10 min, and associated proteins were resolved in gradient gel electrophoresis.Citation55 Different bands were cut out. Proteins were eluted and digested. Digests were analyzed by nano-ultra-performance liquid chromatography–electrospray ionization tandem mass spectrometry. Data from liquid chromatography–tandem mass spectrometry were processed through the use of ProteinLynx Global Serverversion 2.4 (PLGS 2.4); the resulting peak lists were used for searching the NCBI protein database with the Mascot search engine.
Immunohistochemical staining (IHC)
IL-6 and CXCL8 levels in liver tumors were evaluated by IHC. IHC method was the same as previously reported.Citation36 Monoclonal antibodies used in this study were Ki-67 (9449, Cell Signaling Technology), Bcl-2 (sc-7382, Santa Cruz, CA), MMP9 (13667, CST), CD31 (3528, CST), IL-6 (ab6672, Abcam) and CXCL8 (ab7747, Abcam). Immunostaining was performed using ChemMate DAKO EnVision Detection Kit, Peroxidase/DAB, Rabbit/Mouse (code K 5007, DakoCytomation) according to the manufacturer’s instructions. Immunoreactivity scoring system was based on staining intensity (score 0–3: negative = 0, weak = 1, moderate = 2 and strong = 3) and positive areas (score 1–4: the frequency of positive cells less than 5% = 0, 5%-25% = 1, 26%-50% = 2, 51%-75% = 3 and more than 75% = 4) as described before.Citation56 And the final quantitative value was decided by multiplication of the intensity score and the score for positive stained cells, ranging from 0 to 12.
Statistical analysis
All data are presented as the mean ± standard error of the mean, which means of groups were from at least three independent experiment. Statistical analysis was analyzed by SPSS 16.0 software. The comparison between groups was performed using the Student’s t-test. P values < 0.05 was considered statistically significant.
Author Contributions
X. C. designed and supervised the research; M.H., H.W., and X.H., performed the experiments; X. C., H.W., M.H. and X.H. analyzed data and wrote the paper.
Author Information
The authors declare no competing financial interests. Correspondence and requests for materials should be addressed to X. C. ([email protected])
Supplemental Material
Download MS Word (20.2 KB)Supplemental Material
Download MS Word (604 KB)Acknowledgments
This work is supported by grants from the National 135 Mega Program of China (2017ZX10102032-001, 2017ZX10202203-002), National Natural Science Foundation of China (81788101) and CAMS Innovation Fund for Medical Sciences (2016-12M-1-003).
Supplementary:
Supplemental data can be accessed here.
References
- Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431(7007):405–406. PMID:15385993. doi:10.1038/431405a
- Kubes P, Jenne C. Immune responses in the liver. Annu Rev Immunol. 2018;36:247–277. PMID:29328785. doi:10.1146/annurev-immunol-051116-052415
- Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124(4):823–835. PMID:16497591. doi:10.1016/j.cell.2006.02.016
- Nagi RS, Bhat AS, Kumar H. Cancer: a tale of aberrant PRR response. Front Immunol. 2014;5:161. PMID:24782866. doi:10.3389/fimmu.2014.00161
- Wang L, Zhu R, Huang Z, Li H, Zhu H. Lipopolysaccharide-induced toll-like receptor 4 signaling in cancer cells promotes cell survival and proliferation in hepatocellular carcinoma. Dig Dis Sci. 2013;58(8):2223–2236. PMID:23828139. doi:10.1007/s10620-013-2745-3
- Dapito DH, Mencin A, Gwak G-Y, Pradere J-P, Jang M-K, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21(4):504–516. PMID:22516259. doi:10.1016/j.ccr.2012.02.007
- Yu L-X, Yan H-X, Liu Q, Yang W, Wu H-P, Dong W, Tang L, Lin Y, He Y-Q, Zou -S-S, et al. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. 2010;52(4):1322–1333. PMID:20803560. doi:10.1002/hep.23845
- Yeh D-W, Huang L-R, Chen Y-W, Huang C-YF, Chuang T-H. Interplay between inflammation and stemness in cancer cells: the role of toll-like receptor signaling. J Immunol Res. 2016;2016:1–14. PMID:28116318. doi:10.1155/2016/4368101
- Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 2012;56(3):704–713. PMID:22120206. doi:10.1016/j.jhep.2011.09.020
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431(7007):461–466. PMID:15329734. doi:10.1038/nature02924
- Haybaeck J, Zeller N, Wolf MJ, Weber A, Wagner U, Kurrer MO, Bremer J, Iezzi G, Graf R, Clavien P-A, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell. 2009;16(4):295–308. PMID:19800575. doi:10.1016/j.ccr.2009.08.021
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317(5834):121–124. PMID:17615358. doi:10.1126/science.1140485
- Wong VW, Yu J, Cheng AS, Wong GL, Chan HY, Chu ES, Ng EK, Chan FK, Sung JJ, Chan HL. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer. 2009;124(12):2766–2770. PMID:19267406. doi:10.1002/ijc.24281
- David JM, Dominguez C, Hamilton DH, Palena C. The IL-8/IL-8R axis: a double agent in tumor immune resistance. Vaccines (Basel). 2016;4(3): PMID:27348007. doi:10.3390/vaccines4030022
- Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, Sanguinetti M, Morelli D, Paroni Sterbini F, Petito V, et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology. 2018: PMID:29665135. doi:10.1002/hep.30036
- Spira A, Beane JE, Shah V, Steiling K, Liu G, Schembri F, Gilman S, Dumas Y-M, Calner P, Sebastiani P, et al. Airway epithelial gene expression in the diagnostic evaluation of smokers with suspect lung cancer. Nat Med. 2007;13(3):361–366. PMID:17334370. doi:10.1038/nm1556
- Sanmamed MF, Carranza-Rua O, Alfaro C, Oñate C, Martín-Algarra S, Perez G, Landazuri SF, Gonzalez A, Gross S, Rodriguez I, et al. Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins. Clin Cancer Res. 2014;20(22):5697–5707. PMID:25224278. doi:10.1158/1078-0432.CCR-13-3203
- Luo J-H, Ren B, Keryanov S, Tseng GC, Rao UNM, Monga SP, Strom S, Demetris AJ, Nalesnik M, Yu YP, et al. Transcriptomic and genomic analysis of human hepatocellular carcinomas and hepatoblastomas. Hepatology. 2006;44(4):1012–1024. PMID:17006932. doi:10.1002/hep.21328
- Yan X, Hu Z, Feng Y, Hu X, Yuan J, Zhao SD, Zhang Y, Yang L, Shan W, He Q, et al. Comprehensive genomic characterization of long non-coding RNAs across human cancers. Cancer Cell. 2015;28(4):529–540. PMID:26461095. doi:10.1016/j.ccell.2015.09.006
- Zhao J, Sun BK, Erwin JA, Song -J-J, Lee JT. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322(5902):750–756. PMID:18974356. doi:10.1126/science.1163045
- Willingham AT, Orth AP, Batalov S, Peters EC, Wen BG, Aza-Blanc P, Hogenesch JB, Schultz PG. A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science. 2005;309(5740):1570–1573. PMID:16141075. doi:10.1126/science.1115901
- Ji P, Diederichs S, Wang W, Böing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22(39):8031–8041. PMID:12970751. doi:10.1038/sj.onc.1206928
- Li Y, Wu Z, Yuan J, Sun L, Lin L, Huang N, Bin J, Liao Y, Liao W. Long non-coding RNA MALAT1 promotes gastric cancer tumorigenicity and metastasis by regulating vasculogenic mimicry and angiogenesis. Cancer Lett. 2017;395:31–44. PMID:28268166. doi:10.1016/j.canlet.2017.02.035
- Hirata H, Hinoda Y, Shahryari V, Deng G, Nakajima K, Tabatabai ZL, Ishii N, Dahiya R. Long noncoding RNA MALAT1 promotes aggressive renal cell carcinoma through Ezh2 and interacts with miR-205. Cancer Res. 2015;75(7):1322–1331. PMID:25600645. doi:10.1158/0008-5472.CAN-14-2931
- Latorre E, Carelli S, Raimondi I, D’Agostino V, Castiglioni I, Zucal C, Moro G, Luciani A, Ghilardi G, Monti E, et al. The ribonucleic complex HuR-MALAT1 represses CD133 expression and suppresses epithelial-mesenchymal transition in breast cancer. Cancer Res. 2016;76(9):2626–2636. PMID:27197265. doi:10.1158/0008-5472.CAN-15-2018
- Vassallo I, Zinn P, Lai M, Rajakannu P, Hamou M-F, Hegi ME. WIF1 re-expression in glioblastoma inhibits migration through attenuation of non-canonical WNT signaling by downregulating the lncRNA MALAT1. Oncogene. 2016;35(1):12–21. PMID:25772239. doi:10.1038/onc.2015.61
- Lai M-C, Yang Z, Zhou L, Zhu -Q-Q, Xie H-Y, Zhang F, Wu L-M, Chen L-M, Zheng -S-S. Long non-coding RNA MALAT-1 overexpression predicts tumor recurrence of hepatocellular carcinoma after liver transplantation. Med Oncol. 2012;29(3):1810–1816. PMID:21678027. doi:10.1007/s12032-011-0004-z
- Wu Y, Lu W, Xu J, Shi Y, Zhang H, Xia D. Prognostic value of long non-coding RNA MALAT1 in cancer patients. Tumour Biol. 2016;37(1):897–903. PMID:26254614. doi:10.1007/s13277-015-3870-8
- Schmidt LH, Spieker T, Koschmieder S, Schäffers S, Humberg J, Jungen D, Bulk E, Hascher A, Wittmer D, Marra A, et al. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J Thorac Oncol. 2011;6(12):1984–1992. PMID:22088988. doi:10.1097/JTO.0b013e3182307eac
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. PMID:12490959. doi:10.1038/nature01322
- Jing -Y-Y, Han Z-P, Sun K, Zhang -S-S, Hou J, Liu Y, Li R, Gao L, Zhao X, Zhao Q-D, et al. Toll-like receptor 4 signaling promotes epithelial-mesenchymal transition in human hepatocellular carcinoma induced by lipopolysaccharide. BMC Med. 2012;10:98. PMID:22938142. doi:10.1186/1741-7015-10-98
- Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465(7301):1033–1038. PMID:20577206. doi:10.1038/nature09144
- Rapicavoli NA, Qu K, Zhang J, Mikhail M, Laberge R-M, Chang HY. A mammalian pseudogene lncRNA at the interface of inflammation and anti-inflammatory therapeutics. Elife. 2013;2:e00762. PMID:23898399. doi:10.7554/eLife.00762
- Tian W, Xu H, Fang F, Chen Q, Xu Y, Shen A. Brahma-related gene 1 bridges epigenetic regulation of proinflammatory cytokine production to steatohepatitis in mice. Hepatology. 2013;58(2):576–588. PMID:23281043. doi:10.1002/hep.26207
- Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong Q, Qin L, Wu X, Zheng Y, Yang Y, et al. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell. 2011;19(2):232–243. PMID:21316602. doi:10.1016/j.ccr.2011.01.001
- Ridder K, Sevko A, Heide J, Dams M, Rupp A-K, Macas J, Starmann J, Tjwa M, Plate KH, Sültmann H, et al. Extracellular vesicle-mediated transfer of functional RNA in the tumor microenvironment. Oncoimmunology. 2015;4(6):e1031440. PMID:26451302. doi:10.1080/2162402X.2015.1008371
- Hu G, Gong A-Y, Wang Y, Ma S, Chen X, Chen J, Su C-J, Shibata A, Strauss-Soukup JK, Drescher KM, et al. LincRNA-Cox2 promotes late inflammatory gene transcription in macrophages through modulating SWI/SNF-mediated chromatin remodeling. J Immunol. 2016;196(6):2799–2808. PMID:26880762. doi:10.4049/jimmunol.1502146
- Liu J-Y, Yao J, Li X-M, Song Y-C, Wang X-Q, Li Y-J, Yan B, Jiang Q. Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis. 2014;5:e1506. PMID:25356875. doi:10.1038/cddis.2014.466
- Zhao G, Su Z, Song D, Mao Y, Mao X. The long noncoding RNA MALAT1 regulates the lipopolysaccharide-induced inflammatory response through its interaction with NF-κB.. FEBS Lett. 2016;590(17):2884–2895. PMID:27434861. doi:10.1002/1873-3468.12315
- Ramirez-Carrozzi VR, Nazarian AA, Li CC, Gore SL, Sridharan R, Imbalzano AN, Smale ST. Selective and antagonistic functions of SWI/SNF and Mi-2beta nucleosome remodeling complexes during an inflammatory response. Genes Dev. 2006;20(3):282–296. PMID:16452502. doi:10.1101/gad.1383206
- Bergmann J, Müller M, Baumann N, Reichert M, Heneweer C, Bolik J, Lücke K, Gruber S, Carambia A, Boretius S, et al. IL-6 trans-signaling is essential for the development of hepatocellular carcinoma in mice. Hepatology. 2017;65(1):89–103. PMID:27770462. doi:10.1002/hep.28874
- Yang X, Liang L, Zhang X-F, Jia H-L, Qin Y, Zhu X-C, Gao X-M, Qiao P, Zheng Y, Sheng -Y-Y, et al. MicroRNA-26a suppresses tumor growth and metastasis of human hepatocellular carcinoma by targeting interleukin-6-Stat3 pathway. Hepatology. 2013;58(1):158–170. PMID:23389848. doi:10.1002/hep.26305
- Kim SJ, Uehara H, Karashima T, Mccarty M, Shih N, Fidler IJ. Expression of interleukin-8 correlates with angiogenesis, tumorigenicity, and metastasis of human prostate cancer cells implanted orthotopically in nude mice. Neoplasia. 2001;3(1):33–42. PMID:11326314. doi:10.1038/sj/neo/7900124
- Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170(6):3369–3376. PMID:12626597
- Li A, Varney ML, Valasek J, Godfrey M, Dave BJ, Singh RK. Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis. Angiogenesis. 2005;8(1):63–71. PMID:16132619. doi:10.1007/s10456-005-5208-4
- Xiao P, Long X, Zhang L, Ye Y, Guo J, Liu P, Zhang R, Ning J, Yu W, Wei F, et al. Neurotensin/IL-8 pathway orchestrates local inflammatory response and tumor invasion by inducing M2 polarization of Tumor-Associated macrophages and epithelial-mesenchymal transition of hepatocellular carcinoma cells. Oncoimmunology. 2018;7(7):e1440166. PMID:29900041. doi:10.1080/2162402X.2018.1440166
- Schraufstatter IU, Trieu K, Zhao M, Rose DM, Terkeltaub RA, Burger M. IL-8-mediated cell migration in endothelial cells depends on cathepsin B activity and transactivation of the epidermal growth factor receptor. J Immunol. 2003;171(12):6714–6722. PMID:14662875
- Kyriakakis E, Cavallari M, Pfaff D, Fabbro D, Mestan J, Philippova M, De Libero G, Erne P, Resink TJ. IL-8-mediated angiogenic responses of endothelial cells to lipid antigen activation of iNKT cells depend on EGFR transactivation. J Leukoc Biol. 2011;90(5):929–939. PMID:21807744. doi:10.1189/jlb.0211097
- Schinke C, Giricz O, Li W, Shastri A, Gordon S, Barreyro L, Barreryo L, Bhagat T, Bhattacharyya S, Ramachandra N, et al. IL8-CXCR2 pathway inhibition as a therapeutic strategy against MDS and AML stem cells. Blood. 2015;125(20):3144–3152. PMID:25810490. doi:10.1182/blood-2015-01-621631
- Tsai M-C, Spitale RC, Chang HY. Long intergenic noncoding RNAs: new links in cancer progression. Cancer Res. 2011;71(1):3–7. PMID:21199792. doi:10.1158/0008-5472.CAN-10-2483
- Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson EG, Teruya-Feldstein J, Bell GW, Weinberg RA. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol. 2010;28(4):341–347. PMID:20351690. doi:10.1038/nbt.1618
- Smaldone MC, Davies BJ. BC-819, a plasmid comprising the H19 gene regulatory sequences and diphtheria toxin A, for the potential targeted therapy of cancers. Curr Opin Mol Ther. 2010;12(5):607–616. PMID:20886393
- Hanna N, Ohana P, Konikoff FM, Leichtmann G, Hubert A, Appelbaum L, Kopelman Y, Czerniak A, Hochberg A. Phase 1/2a, dose-escalation, safety, pharmacokinetic and preliminary efficacy study of intratumoral administration of BC-819 in patients with unresectable pancreatic cancer. Cancer Gene Ther. 2012;19(6):374–381. PMID:22498722. doi:10.1038/cgt.2012.10
- Chen T, Yang M, Yu Z, Tang S, Wang C, Zhu X, Guo J, Li N, Zhang W, Hou J, et al. Small GTPase RBJ mediates nuclear entrapment of MEK1/MEK2 in tumor progression. Cancer Cell. 2014;25(5):682–696. PMID:24746703. doi:10.1016/j.ccr.2014.03.009
- Tsai M-C, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329(5992):689–693. PMID:20616235. doi:10.1126/science.1192002
- Pickering T, Hamm M, Page AF, Wuestner S, Hess O. Cavity-free plasmonic nanolasing enabled by dispersionless stopped light. Nat Commun. 2014;5:3733. PMID:24821572. doi:10.1038/ncomms5972
