ABSTRACT
CD40 triggering may result in antitumor effects of potentially high clinical relevance. To gain insights important for patient selection and to identify adequate targeting techniques, we investigated CD40 expression in human cancer tissues and generated a replication-incompetent recombinant vaccinia virus expressing CD40 ligand (rVV40L). Its effects were explored in vitro and in vivo upon direct CD40 targeting on malignant cells or macrophage activation. CD40 expression was analyzed by immunohistochemistry in tumor and stromal cells in a multi-tumor array including 836 specimens from 27 different tumor types. Established tumor cell lines were used to explore the capacity of rVV40L to induce malignant cell apoptosis and modulate functional profiles of polarized macrophages. CD40 expression was detectable in significantly higher numbers of stromal as compared to malignant cells in lung and breast cancers. CD40 ligation following rVV40L infection induced apoptosis in CD40(+) cancer cells, but only in the presence of intact specific signal transduction chain. Importantly, rVV40L infection promoted the induction of TNF-α-dependent antitumor activity of M1-like macrophages directed against CD40(-) targets. CD40-activated M1-like macrophages also displayed enhanced ability to CXCL10-dependently recruit CD8+ T cells and to efficiently present cancer cell intracellular antigens through cross-priming. Moreover, rVV-driven CD40L expression partially “re-educated” M2-like macrophages, as suggested by detectable CXCL10 and IL-12 production. Most importantly, we observed that intra-tumoral injection of rVV40L-infected human macrophages inhibits progression of human CD40(-) tumors in vivo. First evidences of anticancer activity of rVV40L strongly encourage further evaluations.
Introduction
CD40 receptor is a 48-kDa type I transmembrane protein of the TNF receptor (TNFR) superfamily, physiologically expressed by a broad range of different cell types, including endothelial, epithelial, and immune cells. Furthermore, cancer cells from nearly all B-cell malignancies and a variety of solid tumors have also been reported to express CD40.Citation1,Citation2 CD40 ligand (CD40L: CD154), a member of the "TNF ligands family", is expressed on the cell surface of activated helper T-lymphocytes and is released in soluble form by platelets.
Triggering of CD40 receptor expressed by malignant cells has been shown to result in cell growth arrest and apoptosis. These effects have been associated with the recruitment of TNFR-associated factor (TRAF) adapter proteins to the cytoplasmic domain of CD40 receptor, leading to activation of pro-apoptotic JNK-pathway and cleavage of effector caspases.Citation1,Citation3 Recently identified NORE1A protein (RASSF5) has also been proposed as an additional critical mediator of apoptosis and cell growth arrest in CD40-triggered tumor cells.Citation4
Triggering of CD40 on different antigen-presenting cell (APC) types by activated CD4+ T cells expressing CD40L results in their “licensing”, promoting highly efficient induction of cytotoxic T lymphocyte (CTL) responses.Citation5–Citation7 Based on these characteristics, CD40/CD40L interaction has emerged in the past decade as a crucial target for the development of innovative cancer immunotherapy strategies.Citation2,Citation6,Citation7 Monoclonal antibodies (mAbs) targeting CD40 receptor and viral vectors encoding CD40L have been tested in animal models and in cancer patients.Citation6,Citation8–Citation12 Responsiveness to the CD40/CD40L pathway-targeting reagents is associated with direct effects on malignant cells and with the induction of adaptive antitumor immunity.Citation2,Citation6,Citation8–Citation12 Furthermore, T-cell-independent mechanisms, prominently including the activation of tumor-associated macrophages, have also been proposed.Citation8,Citation9,Citation13
Vaccinia virus (VV) is a viral vector, characterized by extranuclear replication and capacity to accommodate large transgenes. Due to these characteristics and its safety, VV has been used in recombinant form (rVV) in a variety of cancer immunotherapy protocols.Citation14–Citation18 In particular, rVVs have successfully been used to induce T-cell responses specific for tumor-associated antigens (TAAs) in patients bearing cancers of different histological origins.Citation15,Citation18 Moreover, rVVs have also been used as oncolytic reagents upon systemic or intratumoral administration.Citation19 An oncolytic rVV expressing CD40L was recently shown to inhibit the growth of CD40+ human tumor cell xenografts in immune-deficient animals.Citation20
In previous investigations, we have shown that a replication-incompetent rVV encoding multiple TAA-derived epitopes and CD80 and CD86 co-stimulatory molecules efficiently induced specific CTL responses in patients with advanced melanoma.Citation18,Citation21 More recently, we constructed an rVV encoding CD40L (rVV40L) and reported its ability to exquisitely promote the expansion of human “central memory” CD8+ T cellsCitation17 in replication-incompetent form. These studies prompted us to explore the possibility of using this non-lytic reagent to elicit the multiplicity of antitumor effects associated with CD40 triggering.
In the present study, we have analyzed CD40 expression in tumor and stromal cells in a large (n: >800) range of human cancers and identified malignancies potentially representing relevant clinical targets. More importantly, we here show that a replication-incompetent rVV40L is able to induce direct and macrophage-mediated apoptosis of tumor cells of different histological origins. Furthermore, we demonstrate the capacity of this reagent to promote macrophage-mediated recruitment of CD8+ T cells and cross-presentation of HLA-class I-restricted epitopes from human intracellular TAA. Most interestingly, we here report that rVV40L-triggered human macrophages are able to inhibit the progression of human CD40(-) tumors in vivo.
Results
CD40 receptor expression in clinical tumor specimens
CD40 receptor has been reported to be expressed in nearly all B-cell malignancies and up to 70% of the solid tumors.Citation2 Triggering of CD40 receptor expressed on transformed cell surfaces has been associated to pro-survival effects or to inhibition of proliferation and/or apoptosis induction.Citation1,Citation5 We analyzed CD40 receptor expression in a tumor microarray (TMA) including 836 specimens from 27 different cancer types. Examples of CD40-specific staining are reported in .
Figure 1. Evaluation of CD40 receptor expression in clinical specimens.
(a) Representative examples of CD40-specific positive and negative immunohistochemical staining. (b) Cumulative results of CD40 expression in tumor specimens included in the TMA. Samples were considered “positive” if ≥10% of the total cell number showed evidence of moderate/strong CD40-specific staining. (c) CD40 expression on tumor and non-malignant stromal cells was differentially analyzed in clinical samples derived from breast, ovarian, and lung cancers. Data refer to percentages of tumor or stromal cells showing evidence of moderate/strong CD40-specific staining.
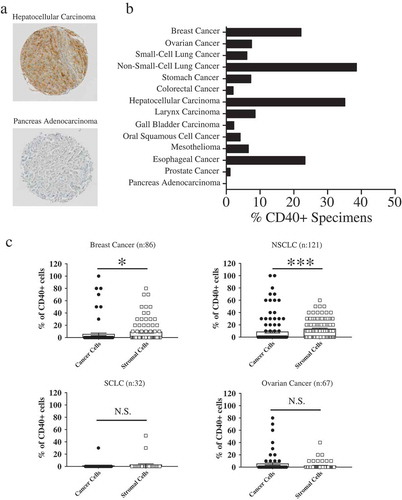
In initial studies, overall CD40 expression, including both tumor and stromal cells, was evaluated. Samples showing moderate/strong CD40-specific staining in ≥10% of all cells were considered positive. According to these criteria, ˃15% of the specimens of breast tumors, non-small-cell lung cancer (NSCLC), hepatocellular carcinoma (HCC), as well as esophageal cancer were considered CD40+ (). Lower percentages of CD40+ specimens, ranging between 5% and 10%, were observed in ovarian, Gastrointestinal tract, head and neck, small-cell lung cancers and mesotheliomas. In contrast, CD40 expression was barely detected in colorectal, gallbladder, prostate cancers (<5% CD40+ specimens) and was undetectable in pancreatic adenocarcinoma (). Importantly, no CD40 expression could be observed in healthy tissues of corresponding histological origin (data not shown).
Differential analysis of CD40 expression in tumor or stromal cells in clinical specimens was then addressed. Moderate/strong CD40-specific staining in malignant cells was only observed in limited numbers of cancer types including breast (8/121), ovarian (7/67), and lung cancers (1/32 Small Cell Lung Cancer (SCLC) and 6/121 NSCLC) (). Most interestingly, however, in NSCLC and breast cancers, CD40 was expressed in significantly higher percentages of stromal as compared to tumor cells (P< 0.0001 and P= 0.02, respectively). In contrast, no significantly different expression in either cell types could be observed in ovarian cancers (P= 0.7) and small-cell lung carcinoma (P= 0.8) ().
Taken together, TMA data underline that CD40 receptor is expressed in different cell types present in the tumor microenvironment (TME), but predominantly in stromal cells in sizable percentages of cancers of high epidemiological relevance.
rVV40L infection induces apoptosis/necrosis in CD40+ tumor cell lines
TMA data prompted us to evaluate biological responses induced by replication-incompetent rVV40L infection in a panel of CD40+ and CD40- established tumor cell lines. In particular, CD40+ Na8 melanoma, HCT116 colorectal cancer, MDA-231 breast cancer, PLC, and HepG2 (CD40DIM) HCC cell lines were tested. As controls, we also investigated cell lines of the same histological origin (A375, BT-474, LS180, and HuH-7) with undetectable or negligible expression of CD40 receptor as assessed at mRNA and protein levels (Supplementary Figure 1a, b).
Infection of tumor cells by replication-incompetent rVV40L, irrespective of their histological origin and CD40 receptor status, resulted in surface expression of CD40L in >30% of the tumor cells 24 hours after their incubation with the virus (Supplementary Figure 1c), and in a significant decrease of CD40 expression in CD40+ infected cells (Supplementary Figure 1d).
Importantly, rVV40L infection of CD40+ Na8 melanoma () and MDA-231 breast () resulted in significant increases in percentages of apoptotic/necrotic cells as compared to control cultures. Surprisingly, however, rVV40L infection of CD40+ HCT116 colorectal cancer cells and HepG2 HCC cells did not impact on their survival (), whereas PLC HCC cells underwent apoptosis (). Remarkably, CD40 cross-linking by s40L/enhancer treatment, infection by VV WT, or their combination were totally ineffective (). As expectable, rVV40L infection, s40L/enhancer treatment, or their combination failed to induce apoptosis/necrosis in CD40-cell lines.
Figure 2. CD40L-expressing recombinant vaccinia virus treatment induces cytotoxic effects on CD40+ tumor cells in ‘“vitro’”.
Established tumor cell lines from (a) melanoma (Na8 and A375), (b) breast cancer (MDA-231 and BT-474), (c) colorectal cancer (HCT116 and LS180), and (d) hepatocellular carcinoma (PLC, HepG2 and HuH-7) were left untreated or infected with CD40L-expressing recombinant vaccinia virus (rVV40L) or vaccinia virus wild-type (VV WT) at MOI of 10. Tumor cells were also treated with soluble CD40L recombinant protein (s40L) and oligomerizing enhancer (0.5 and 1 μg/ml, respectively) alone or following VV WT infection (VV WT). On d 4, cells were collected and stained with annexin V and propidium iodide (PI) (a, b, c, and d). Data refer to a representative experiment for each established tumor cell line (upper panels) and report collective results (mean ± SEM) from five different experiments (lower panels). *P < 0.05, **P < 0.01; Mann–Whitney nonparametric test.
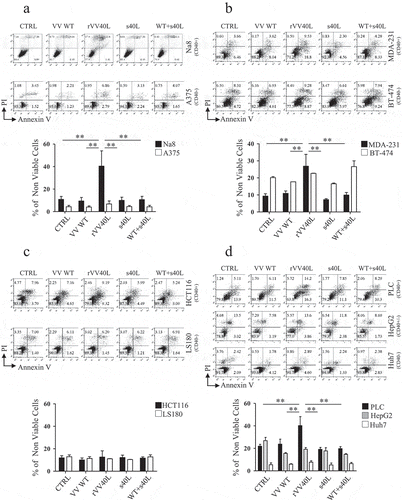
Altogether, VV-mediated CD40L expression sensitized CD40+ tumor cell populations to cell death, with the exception of HCT116 and HepG2 tumor cell lines that appeared resistant.
Impaired CD40 signaling pathway is associated with tumor cell resistance to rVV40L-induced apoptosis/necrosis
CD40 ligation results in receptor clustering, inducing, in turn, recruitment to its cytoplasmic domain, of TNF-receptor-associated factors (TRAFs) mediating intracellular signaling.Citation1 However, only TRAF-1 is regulated at transcription level in response to CD40 ligation and initiates signaling cascades leading to cell death.Citation3 Furthermore, CD40 ligation on tumor cells has recently been reported to result in upregulation of NORE1A (RASSF5) protein, mediating pro-apoptotic JNK pathway and caspase activation, and inducing apoptosis of target cells.Citation4 Thus, we investigated CD40 signaling in tumor cells using TRAF-1 and NORE1A expression as downstream markers.
In apoptosis-responsive CD40+ Na8 and MDA-231 cells, a significant upregulation of TRAF-1 gene expression was observed upon rVV40L infection, whereas s40L/enhancer, alone or in combination with VV-WT, was ineffective (). In sharp contrast, triggering of CD40 receptor expressed on the cellular surface of HCT116 cells by rVV40L infection failed to induce upregulation of TRAF-1 gene expression level (). Instead, both rVV40L and s40L treatment appeared to downregulate CD40 expression in HCT116 CRC cells.
Figure 3. Lack of sensitivity to tumor cell death following rVV40L infection is associated with impaired CD40 signaling pathway.
Established melanoma (Na8 and A375) (a), breast cancer (MDA-231 and BT-474) (b), colorectal cancer (HCT116 and LS180) (c), and hepatocellular carcinoma (PLC, HepG2 and HuH-7) (d) cell lines were left untreated or infected with CD40L-expressing recombinant vaccinia virus (rVV40L) or vaccinia virus wild-type (VV WT) at an MOI of 10. In addition, cells were also treated with soluble CD40L recombinant protein (s40L) and oligomerizing enhancer (0.5 and 1 μg/ml, respectively) alone or following VV WT infection (VV WT), as indicated. After 4 d, TRAF-1 gene expression was evaluated by RT-qPCR. HCT116 (CD40+) colorectal cancer and PLC (CD40+) hepatocellular carcinoma cell lines were similarly treated, and NORE1A gene expression was assessed by RT-qPCR (e). Data are expressed as fold increase as compared to untreated tumor cells (n = 5 A, B, C, D and n = 3 E). *P < 0.05, **P < 0.01; Mann–Whitney nonparametric test.
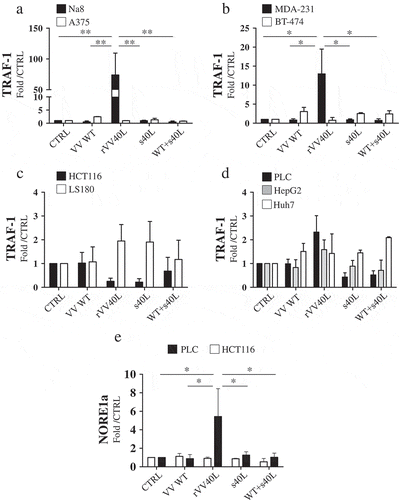
Regarding hepatocellular cell lines (HCC), in PLC CD40+ cells, a trend (P = 0.0671) toward TRAF-1 upregulation was also observed following rVV40L infection in (). This effect was undetectable in CD40+ HepG2 cells. Similarly, expression of NORE1A gene was significantly increased following rVV40L infection but not s40L or WT+ s40L treatment, only in cell death-sensitive CD40+ PLC but not in “insensitive“ CD40+ HCT116 cells (). As expected, no TRAF-1 or NORE1a upregulation was observed in CD40-cell lines.
These data indicate that rVV40L is able to induce apoptosis upon infection of CD40+ cells. However, they also underline that CD40 expression “per se“ is not predictive of sensitivity to rVV40L-mediated cytotoxic effects, and induction of functional adapter proteins is required to elicit apoptosis.Citation3,Citation4
Phenotypic and functional profiles of M1/M2-like “in vitro” generated macrophages
TMA data document the expression of CD40 in tumor-infiltrating stromal cells. Notably, macrophages have been shown to represent a majority of CD40+ tumor-infiltrating cells,Citation2,Citation7,Citation8 and a role of tumor-associated macrophages in the control of tumor progression has repeatedly been reported.Citation22 A key feature of macrophages is represented by their high plasticity with M1/M2 macrophages representing extremes of a continuum of differentiation states, characterized by specific functional attributes.Citation23,Citation24 Therefore, we investigated the possibility that targeting CD40 might condition in vitro differentiation of CD14+ monocytes toward M1/M2 functional profiles.
We generated M1- and M2-like CD14+ monocyte-derived macrophages by culturing peripheral blood CD14+ monocytes in the presence of GM-CSF (M1) or M-CSF (M2).Citation25 Phenotypic characterization of CD14+ monocyte-derived macrophages confirmed a significantly higher expression of CD16 and reduced levels of CD163 and CD204 on M1- as compared to M2-like macrophagesCitation26,Citation27 (Supplementary Figure 2a, b). Accordingly, analysis of cytokine gene expression pattern profiles revealed a significant IL-6 gene expression in M1 macrophages, whereas IL-10 gene expression was significantly higher in M2-like macrophages (Supplementary Figure 2c). Moreover, we observed a significantly higher expression of CD40 receptor in M1-, as compared to M2-like, CD14-derived macrophages ().
Figure 4. rVV40L infection modulates functional profiles of M1-/M2-like CD14-derived macrophages.
(a) Expression of CD40 on surfaces of CD14+ cell-derived M1- or M2-like macrophages was evaluated by flow cytometry. The left panel shows data from one representative experiment, whereas cumulative data from eight experiments with cells from different healthy donors are reported on the right panel. (b) Peripheral blood CD14+ monocytes from healthy donors were infected with rVV40L or with VV WT at MOI of 5 or treated with s40L and enhancer alone or following VV WT infection (WT + s40L). Cells were then cultured in the presence of GM-CSF or M-CSF. Culture supernatants were collected at the indicated time points, and cytokine release was assessed by ELISA. Data refer to cumulative results from eight (a) or four (b) independent experiments. **P < 0.01: Mann–Whitney nonparametric test.
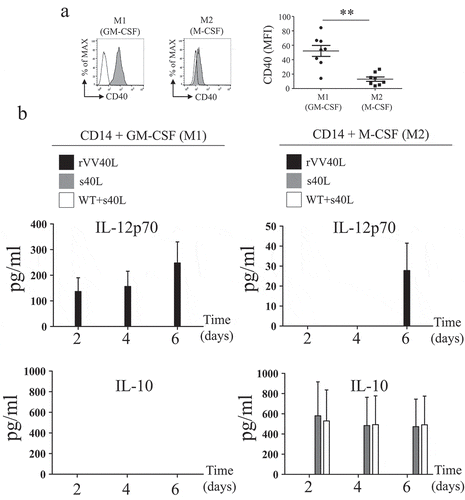
Modulation of M1/M2 functional profiles by rVV40L infection
We evaluated the effects of the treatments under investigation on CD14+ peripheral blood monocytes undergoing cytokine-driven polarization. Infection by rVV40L induced IL-12 production by macrophages undergoing M1-like polarization, whereas s40L/enhancer treatment, alone or in combination with WT infection, was completely ineffective. No IL-10 release could be observed in these conditions. Unexpectedly, however, rVV40L infection also induced minor but detectable IL-12p70 release by macrophages undergoing M2-like polarization, with a delayed kinetic, following 6 d of culture. In contrast, IL-10 production during M2-like polarization was induced by s40L/enhancer treatment alone or in combination with WT infection, but not by rVV40L ().
Co-culture with rVV40L-infected tumor cells promotes cytokine release and antitumor effects in polarized macrophages
We then explored the potential relevance of CD40 receptor ligation in the modulation of effector functions of fully in vitro differentiated M1- or M2-like macrophages. To focus on effects on macrophages, excluding confounding effects possibly related to cancer cell apoptosis and damage-associated molecular pattern expression, we analyzed cytokine production pattern of polarized macrophages upon co-culture with untreated, VV WT, or rVV40L-infected CD40- HuH-7 HCC and LS180 CRC cells.
TNF-α release was only detectable upon co-culture of M1-like differentiated macrophages in the presence of rVV40L-infected LS180 cells. CD40 ligation by infected tumor cells also induced IL-12p70 and IL-6 production in these cells. Interestingly, IL-12p70 and IL-6 were released, albeit to significantly lower extents, also by M2-like cells following exposure to rVV40L-infected LS180 cells. Notably, IL-10 release was observed upon co-culture of M2-like macrophages with LS180 cells, irrespective of viral infection (). Similar results were also observed upon M1- or M2-like macrophage stimulation with rVV40L-infected Huh7 cells. However, these infected cells induced IL-10 release by M2-like macrophages (Supplementary Figure 3a).
Figure 5. Co-culture with rVV40L-infected tumor cells promotes cytokine release and antitumor effects in polarized macrophages.
(a) M1- and M2-like macrophages were co-cultured (1:1 ratio) with LS180 CRC cells infected with VV WT or rVV40L (MOI 10). After 4 d, supernatants were collected and TNF-α, IL-12p70, IL-6, and IL-10 release was evaluated by ELISA. (b) M1- and M2-like macrophages were co-cultured with LS180 CRC cells, treated as described above, at the indicated ratios. On d 4, proliferation of LS180 CRC cells was evaluated by 3H-thymidine incorporation and expressed as percentage of proliferation as compared to untreated tumor cells. (c) Proliferative capacity of LS180 CRC cells, treated as described in (a), upon co-culture with M1- and M2-like macrophages, was evaluated by 3H-thymidine incorporation in the presence of neutralizing of anti-TNF-α mAb at 10 μg/ml concentration. Data refer to cumulative results from four independent experiments. *P < 0.05: Mann–Whitney nonparametric test.
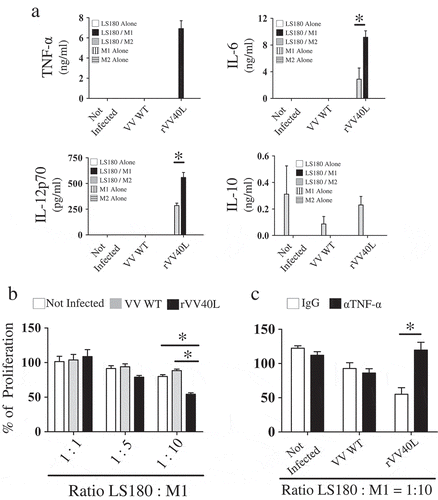
Taken together, these data indicate that CD40 is functional in both M1- and M2-like fully polarized macrophages and suggest that its triggering might partially steer M2 cells toward an M1-like functional profile.
Consistent with their TNF-α production, M1-like macrophages significantly inhibited the proliferation of rVV40L-infected CD40-LS180 () and HuH-7 established tumor cell lines (Supplementary Figure 3b). In contrast, M2-like macrophages were unable to inhibit the proliferation of infected cells (data not shown).
Although macrophages could release a variety of cytotoxic/cytostatic mediators, the pivotal role of TNF-α in the elicitation of these effects was confirmed by its neutralization, resulting in significant inhibition of antiproliferative effects of CD40-stimulated M1-like cells on rVV40L-infected tumor cells ( and Supplementary Figure 3c).
CD40-stimulated M1- and M2-like macrophages promote the recruitment of CD8+ T cells
In a variety of solid tumors, high infiltration by CD8+ T cells is associated with favorable prognosis. Thus, their recruitment might represent a critical step in the elicitation of antitumor immune responses.Citation28–Citation30
CXCR3 chemokine receptor is expressed in CD8+ lymphocytes, and, preferentially, in “central memory” T cells, characterized by marked antitumor potential Citation17,Citation31 (Supplementary Figure 4a). While CXCR3 ligands include CXCL9, CXCL10, and CXCL11, the main role in CD8+ T cells recruitment has been attributed to CXCL10.Citation32
We tested CXCL10 release in supernatants of co-cultures of M1- or M2-like macrophages and LS180 CRC and HuH-7 HCC cell lines. As depicted in , rVV40L but not VV WT infection of tumor cells resulted in significant increases of CXCL10 production by both M1- and M2-like macrophages.
Figure 6. rVV40L induces CXCL10-mediated recruitment of CD8 + T cells and promotes cross-presentation of tumor-associated antigens by M1-like macrophages.
(a) M1- or M2-like macrophages were co-cultured at 1:1 ratio with HuH-7 hepatocellular (upper panel) or LS180 colorectal cancer (lower panel) cells untreated or infected with VV-WT or rVV40L at MOI 10. After 4 d, supernatants were collected and CXCL10 release was evaluated by ELISA. (b) CD8+ T-cell migration, induced by supernatants from co-cultures of M1-/M2-like macrophages with differentially infected tumor cells, was analyzed in the presence or absence of a neutralizing anti-CXCL10 mAb, as evaluated by flow cytometry. (c) Cross-presentation of MelanA/Mart-127–35 epitope from HLA-A0201(-) CD40(-) HT29 CRC cell lines co-infected with VV WT or rVV40L and with an rVV encoding MelanA/MART-1 full gene (rVVMART1 FG); 24h after infection, HT29 were co-cultured at 1:1 ratio with HLA-A0201+ M1- or M2-like macrophages. After 48 additional hours, 30,000 lymphocytes from a MelanA/Mart-127–35-specific, HLA-A0201-restricted CD8+ T-cell clones (see supplementary Figure 4) were added to the cultures, and IFN-γ release was evaluated by ELISA 48 hours later (*P < 0.05; Mann–Whitney nonparametric test).
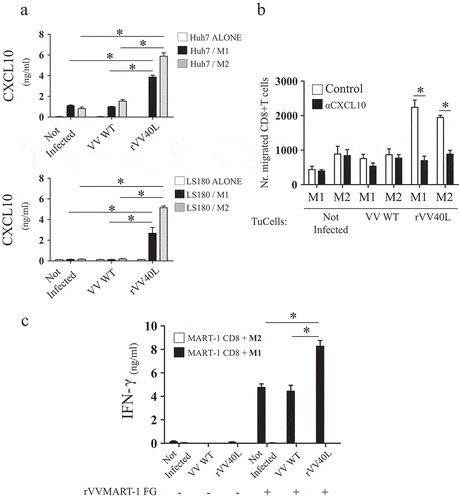
Migration assays were performed to formally demonstrate the ability of CD40-stimulated M1- and M2-like macrophages to promote CXCL10-mediated CD8+ T-cell recruitment. Supernatants from macrophages, CD40L stimulated as described above, induced the migration of CD8+ T cells to significantly higher extents as compared to control supernatants. Importantly, antibody-mediated CXCL10 neutralization from these supernatants abrogated CD8+ T-cell migration (), thus confirming its critical relevance in lymphocyte recruitment.
CD40L-expressing recombinant vaccinia virus promotes M1-mediated cross-presentation of tumor-associated antigens (TAAs) to CD8+ T cells
Macrophages are able to cross-present intracellular MHC-class I-restricted antigens derived from apoptotic cells to specific T cells.Citation33 Cross-presentation-based vaccination strategies have shown promising results for cancer treatment.Citation34,Citation35 Therefore, we evaluated the capacity of rVV40L-stimulated macrophages to promote the activation of tumor-reactive HLA-class I-restricted CD8+ T cells through cross-presentation of intracellular antigens.
HLA-A0201-CD40-HT29 CRC cells (Supplementary Figure 4b) were co-infected with rVV40L and a recombinant vaccinia virus encoding MelanA/MART-1 full gene (rVVMART-1 FG). HT29 CRC cells were then co-cultured with HLA-0201+ M1- or M2-like macrophages and, after 2 d, the ability of macrophages to cross-present MART-127–35 HLA-A0201-restricted epitope was evaluated by measuring IFN-γ release by a specific CD8+ T-cell clone (Supplementary Figure 4b). This clone efficiently responded to antibody-mediated CD3 triggering and to presentation of the target peptide by HLA-0201+ M1- but not HLA-0201+ M2-like APCs, despite a similar HLA-class I expression (Supplementary Figure 4c left panel).
HLA-A0201+ M1-like macrophages exposed to HLA-A0201- tumor cells infected by rVVMART-1 FG were able to cross-present MART-127–35 HLA-A0201-restricted epitope to specific T cells, thereby inducing IFN-γ release. However, M1-like macrophage infection by rVV40L significantly enhanced activation of MART-127–35 reactive CD8+ T cells as compared to control culture conditions (). As expected, T cells were not activated in the presence of HLA-A0201+ M1-like cells previously exposed to tumor cells which were not infected by rVVMART-1 FG, thus confirming the integrity of the experimental design. On the other hand, HLA-A0201- tumor cells infected by the reagents under investigation were unable “per se” to induce IFN-γ production in macrophages alone or in CTLs alone (Supplementary Figure 4c right panel).
CD40L-expressing recombinant vaccinia virus (rVV40L) promotes in vivo tumor regression through macrophage activation
We then addressed the antitumor efficacy of rVV40L in vivo. TMA data indicated that in a large majority of tumors tested CD40 expression was absent or present in very limited numbers of tumor cells (see above). Therefore, in order to exclude effects mediated by triggering of CD40 on model human tumor cells, while focusing on infiltrating immune cells, in these experiments, NSG immunodeficient mice were subcutaneously inoculated with LS180 CRC and HuH-7 HCC CD40- cells. Upon in vivo growth, tumors were first injected with replication-incompetent VV WT or rVV40L followed, after 48 hours, by human M1- or M2-like macrophages.
Control and VV WT-infected tumors rapidly and similarly progressed in the presence or absence of M1- or M2-like cells (). In sharp contrast, consistent with “in vitro” observations, rVV40L infection of Huh-7 HCC and LS180 CRC resulted in a complete inhibition of tumor progression upon intratumor injection of M1-like cells (). Similar effects were also observed upon injection of the positive control Lipopolysaccharides (LPS)-stimulated M1-like cells (data not shown). Injection of M2-like cells also resulted in a modest, nonsignificant inhibition of LS180 CRC tumor progression ().
Figure 7. rVV40L promotes the antitumor activity of M1 macrophages in vivo.
105 LS180 CRC or HuH-7 HCC cells were inoculated subcutaneously (s.c.) into the flanks of NSG mice. Established tumors were then injected with PBS or 107 pfu of replication-incompetent VV WT or rVV40L; 48 hours later, 5 × 105 M1- or M2-like macrophages were injected into the tumor masses. Tumor volume of LS180 (a–c) and Huh7 (b–f) is expressed as fold increases as compared to tumor volumes measured at the time of macrophage inoculation. Data refer to two independent experiments with three mice in each condition. On d 8, mice were sacrificed and histology (H&E) and cleaved caspase 3 expression (green) were evaluated in LS180 (g) and HuH-7 (h) tumors. Data refer to representative stainings out of two tumors per condition displaying similar results (magnification 20x; scale bar 100 µm) (*P < 0.05; Mann–Whitney nonparametric test).
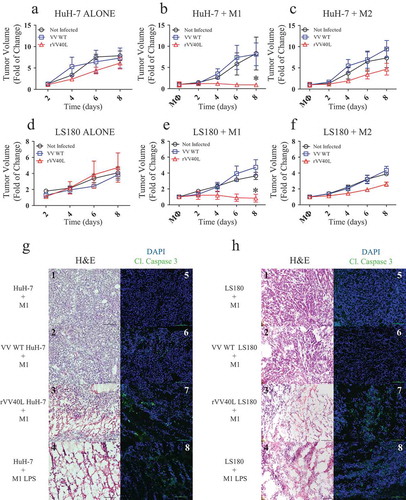
Remarkably, histological analysis of excised tumors revealed that rVV40L-mediated activation of M1-like macrophages resulted in a significant disruption of tumor tissues, comparable to LPS-stimulated M1-like macrophages (). Consistently, immunofluorescence studies provided evidence of significant caspase 3 cleavage in these tumors ().
Discussion
Due to its peculiar expression pattern and multifunctional potential, CD40 represents an important target for the development of innovative cancer immunotherapy protocols.Citation2,Citation11
Administration of agonistic anti-CD40 mAbs and CD154 (CD40L)-expressing recombinant adenovirus has been shown to result in tumor regression in experimental models.Citation6,Citation13 These effects have initially been largely attributed to CD40-mediated-APC licensing and activation of T-cell-mediated antitumor immune responses.Citation36 However, data from clinical studies in human pancreatic cancers, typically lacking T-cell infiltration, suggest that elicitation of antitumor effects of therapeutic anti-CD40 agonistic mAbs might rather rely on macrophage activation.Citation8,Citation9,Citation13
The effectiveness of CD40-targeting strategies developed so far appears to be limited. In particular, CD40 expression by tumor-infiltrating immune cells does not effectively predict responsiveness to agonistic anti-CD40 mAbs.Citation8,Citation9 Furthermore, epitope specificity and isotype might critically affect the activity of therapeutic reagents.Citation7,Citation37 Regarding CD40L-recombinant adenovirus, while safety has been reported, convincing evidence of clinical efficacy is still missing.Citation10–Citation12 Potential limitations of adenovirus-mediated anticancer biological approach might be associated with the requirement of specific cell entry pathways and of the administration of high viral titers.Citation10–Citation12
In order to develop a multipotent anticancer biological, we generated a replication-incompetent CD40L recombinant vaccinia virus (rVV40L) targeting multiple functional aspects of tumor–immune system interaction and tested its antitumor effects in vitro and in vivo. In particular, we addressed the ability of rVV40L to directly promote CD40+ tumor cell apoptosis, to modulate functional profiles of differentially polarized macrophages, to recruit immune cell populations of proven anticancer relevance, and to inhibit tumor progression in vivo.
Evaluation of CD40 expression in a large cohort of clinical specimens (n = 836) derived from different tumor types (n = 27) indicates that, with some exceptions, CD40 is expressed on cell surfaces in limited percentages of transformed cells in tumors included in the TMA under investigation. Instead, CD40 expression was frequently detectable in significantly higher percentages of stromal cells. This observation underlines that the effectiveness of CD40-based therapeutic strategies should rely on infiltrating immune cells in addition to direct cytostatic/cytotoxic effects on CD40+ malignant cells. While the use of TMA might represent a limitation of our study, underestimating tumor heterogeneity, the large number of cases investigated powerfully corroborates the clinical relevance of our data.
rVV40L infection effectively induced apoptosis/necrosis in several CD40+ melanoma, HCC, and breast cancer tumor cell lines. Instead, in line with previous reports,Citation3,Citation4 mere CD40 triggering by cross-linked s40L failed to induce cytotoxic effects in tumor cells, although it was able to rapidly promote IL-10 release by M2-like macrophages (). Most importantly however, CD40 expression by tumor cells cannot be considered “per se” a sufficiently predictive signature of the effectiveness of “direct” strategies, including the use of rVV40L, since impaired intracellular signaling due to TRAFs adapter molecule alterations and defective activation of effector proteins might prevent the induction of apoptosis/necrosis following receptor ligation on malignant cells.Citation3,Citation4
In addition to malignant cells, TME includes a variety of different non-transformed cell types with a major impact on clinical course.Citation28–Citation30 For instance, macrophages have been suggested to be able to eliminate transformed cells and to promote adaptive tumor-specific immune responses in situ.Citation38 However, pro-tumorigenic functions, such as supporting tumor-associated angiogenesis, proliferation of malignant cells, and suppression of antitumor T-cell responses, have been convincingly demonstrated in experimental models.Citation39–Citation45 Furthermore, clinical evidence underlines that in a majority of human tumors, high macrophage infiltration is frequently associated with severe prognosis.Citation39–Citation45
Therefore, macrophage infiltration emerges as acritical target of antitumor therapeutic strategies, and the clinical potential of mAbs neutralizing CCL2Citation46 and M-CSF receptorCitation47 is currently being evaluated in specific clinical trials. These strategies point to the prevention of recruitment and differentiation of macrophages.Citation45–Citation47 Alternative approaches focus on re-conditioning of tumor-infiltrating macrophages through the modulation of their functional activityCitation8,Citation9,Citation13 by exogenous signals enhancing their antitumor activity.Citation27,Citation48,Citation49
Co-culture with rVV40L-infected tumor cells induced significant TNF-α release by M1- but not M2-like macrophages. This cytokine appeared to represent a critical mediator of cytostatic/cytotoxic activity exerted by CD40-activated M1-like macrophages on malignant cells.
Macrophages do orchestrate anticancer adaptive immune responses in situ.Citation49 Several studies have underlined their capacity to mediate, through chemokine production, selective recruitment of defined immune cell subsets, such as CD8+ T cells, with a critical antitumor activity,Citation28–Citation30 in malignant tissues.Citation45,Citation50 CXCL10 is the main mediator of CD8+ T lymphocyte recruitment,Citation32 and in our study, rVV40L-driven CD40-stimulation resulted in significant CXCL10 release by both M1- and M2-like polarized macrophages, leading to effective migration of CD8+ T cells.
The elicitation of CD8+ T-cell immune responses is conditioned by cytokines produced by APC.Citation17,Citation36,Citation51 Production of IL-12 or IL-10 has been associated with the ability of tumor-associated macrophages to promote or, respectively, inhibit adaptive anticancer T-cell-mediated immune response.Citation42,Citation49,Citation50 rVV40L infection during macrophage polarization or co-culture with infected tumor cells significantly modulated their cytokine release profile. In particular, it promoted robust production of IL-12 by M1-like macrophages and, more surprisingly, although to significantly lower extents, also by M2-like macrophages. Most importantly, we observed that infection of tumor cells by rVV40L significantly enhanced M1-like-mediated cross-presentation of TAAs to CD8+ T cells.
Importantly, oligomerized sCD40L was ineffective in a large majority of our “in vitro” experiments. Due to its effectiveness in B-cell activation,Citation52–Citation55 the original CD40-mediated functional assay, oligomerized s40L is usually considered as the reference standard for CD40 triggering tests. However, the extent of CD40 polymerization has been shown to critically affect the elicitation of the multiplicity of functional effects induced by its triggering.Citation56–Citation59 Our data suggest that rVV-mediated CD40L expression on infected cells is likely to result in a highly polymerized interaction with CD40, thereby allowing a full display of the effects associated with CD40 stimulation.
In vivo studies critically reinforce our in vitro results. Indeed, we observed a significant inhibition in the progression of CD40-tumors upon rVV40L infection in the presence of adoptively transferred macrophages. In particular, exogenous administration of human M1-like macrophages induced massive destruction of tumor tissue mediated by cleaved caspase 3 activation. Intriguingly, detectable, albeit not significant, effects were also elicited by M2-like macrophages.
Considering the absence of overt toxic effects of macrophage administration in animals bearing rVV40L-infected tumors, and the replication-incompetent nature of our reagent, these data may suggest innovative adoptive cancer immunotherapy protocols. Tumor tissues could be treated with rVV, followed by intra-tumor injection of polarized, patient-derived macrophages which could be easily obtained from peripheral blood cells. In these conditions, T cells might also be attracted to tumor sites, and the generation of adaptive immune responses might ensue. Further studies are warranted to validate this working hypothesis, requiring human autologous sets of cellular reagents, including cancerous cells and T lymphocytes.
Taken together, our data underline the major antitumor potential of rVV40L. Indeed, transduction by this replication-incompetent reagent might lead to direct inhibitory effects on CD40(+) malignant cells. In addition, inhibition of tumor progression could also result from the marked ability of rVV40L to promote M1 activation and, possibly, from a partial re-polarization of M2 macrophages. Most importantly, CD40-activated macrophages are able to induce direct TNF-α-mediated antitumor cytotoxic activity, lymphocyte recruitment, and cross-presentation of cellular antigens to CD8+ T cells.
Materials and methods
Immunohistochemistry
The multi-tumor tissue microarray used in this work has been extensively described in previous studies.Citation60,Citation61 Briefly, tissue cylinders with a diameter of 0.6 mm were punched from morphologically representative areas of >800 blocks from individual tumors of 27 different histological origins and brought into recipient paraffin blocks (30 x 25 mm) by using a semi-automated tissue arrayer. To partially overcome limitations inherent in tumor heterogeneity, punches were derived from the center of each donor tumor tissue block so that each TMA spot consisted of at least 50% tumor cells. Corresponding healthy tissues were also included in the TMA.
TMA slides were pretreated with CC1 reagent (Ventana) for 16 minutes at room temperature (RT). Thereafter, they were incubated for 32 minutes at RT with CD40-specific rabbit polyclonal primary antibodies (Abcam, ab58612) at 1:50 dilution. Specific binding was revealed by using optiView kit with DAB chromogen (Ventana) according to producer’s instructions. Percentages of total, malignant, and tumor-infiltrating CD40+ stromal cells in each punch were evaluated by experienced pathologists blinded to any prior information. The intensity of staining was also analyzed, and samples were classified as negative (0), weakly positive (1), moderately positive (2), and strongly positive (3). Cells were scored positive if they displayed at least a moderate intensity staining.
CD40 ligand-expressing recombinant vaccinia virus (rVV40L)
CD40 ligand-expressing recombinant vaccinia virus (rVV40L) was generated as previously described.Citation14 In order to exclude the cytopathic/lytic effect of replicating a vector and focus on effects of the expressed transgene, viral replication was inactivated by DNA cross-linking by using psoralen (1 µg/ml) and long-wave UV (365 nm) irradiation.Citation14 A similarly inactivated wild-type vaccinia virus (WT VV) was used as a control. Soluble FLAG-tagged CD40 ligand (s40L) and enhancer (Enzo Life Sciences), ensuring receptor multimerization,Citation62,Citation63 were also used as additional controls.
Established tumor cell lines
Established melanoma (A375), HCC (PLC, HepG2, and HuH-7), colorectal (HCT116, LS180, and HT29) and breast (MDA-231 and BT-474) cancer cell lines were purchased from European Collection of Cell Cultures (ECACC). Na-8 melanoma cell line was a gift from Dr Jotereau (Nantes, France). Breast and colorectal cancer cell lines were cultured in RPMI-1640 medium supplemented with glutamine, non-essential amino acids, sodium pyruvate, HEPES buffer, and Kanamycin sulfate (Gibco-Life Technologies), thereafter referred to as complete medium (CM) and 10% Fetal Bovine Serum (FBS). Melanoma and HCC, HepG2, and HuH-7 cells were cultured in D-MEM CM, 10% FBS. PLC cells were cultured in ALPHA-MEM CM supplemented with 10% FBS. When specific established tumor cell lines were required for the indicated experiments, early passage cells were thawed and maintained in culture for less than 2 months.
In vitro generation of CD14+-derived macrophages
CD14+ monocytes were isolated from peripheral blood mononuclear cells from healthy donors to a >95% purity by using anti-CD14 magnetic beads (Miltenyi Biotech). Magnetically isolated cells were cultured in RPMI-CM 10% FBS in the presence of 12.5 ng/ml GM-CSF (R&D Systems) to generate polarized M1-like macrophages or M-CSF (R&D Systems) for M2-like macrophages. On d 6, cells were collected and stained with anti-CD16, anti-CD163, anti-CD204, and anti-CD40 receptor-specific, fluorochrome-conjugated mAbs, and specific fluorescence was evaluated by flow cytometry (see below).
CD40 triggering “in vitro”
Tumor cell lines were infected with rVV40L or VV WT at Multiplicity of Infection (MOI) of 10 for 1 hour at 37°C in 500 μl of their specific culture medium. Cells were then washed and cultured in specific media (see above). When indicated, cultures were supplemented with s40L recombinant protein and oligomerizing enhancer (0.5 and 1 μg/ml, respectively, Enzo Life Sciences, see above) alone or following VV WT infection (WT + s40L). Cells from different cultures were collected after 4 d, and their viability was assessed by Annexin V apoptosis detection kit (Becton Dickson).
CD14+ monocytes from peripheral blood of healthy donors were infected in 500 μl RPMI-1640 CM for 1 hour at 37°C with rVV40L or with VV WT at MOI of 5. In addition, s40L and enhancer were also used alone or following VV WT infection (WT + s40L), as described above. Cells were then cultured in RPMI-1640 CM 10% FBS in the presence of either GM-CSF or M-CSF (see above). At the indicated time points, supernatants from different culture conditions were collected, and cytokine release was assessed by ELISA.
Co-culture of M1- and M2-like macrophages with established tumor cell lines
LS180 and HuH-7 established tumor cell lines were left untreated or infected with rVV40L or with VV WT at MOI of 10. After 24 hours, 3000 VV WT or rVV40L-infected or untreated tumor cells were co-cultured together with M1- or M2-like macrophages at different effector: target ratios in 96 well-plates. On d 4, cytokine and chemokine release in supernatants from different culture conditions was evaluated by ELISA. Cytostatic activity of macrophages on tumor cells was evaluated by adding 1 μCi of 3H-thymidine for the last 18 hours of culture. Cells were then harvested, and tracer incorporation was measured by scintillation counting. TNF-α neutralization was achieved by adding to cultures anti-human TNF-α mAb (BioLegend) at 10 μg/ml final concentration.
Cross-presentation assays
MelanA/Mart127–35-specific HLA-A0201-restricted CD8+ T cell clones were generated as described previously.Citation64 Overall, 3000 cells from CD40- HLA-A0201- HT29 cell line were left untreated or infected with VV WT or rVV40L and co-infected with a recombinant VV encoding MART-1 full gene, inducing the production of the entire protein in infected cells, at MOI of 10. After 24 hours, differentially infected HT29 cells were cultured alone or in the presence of HLA-A0201+ M1- or M2-like polarized macrophages at 1:1 ratio. Two days later, 30,000 cells of a MelanA/Mart-127-35-specific, HLA-A0201-restricted CD8+ T cell clones were added to the different cultures. After 48 hours, the presence of IFN-γ in the supernatants was assessed by ELISA.
Migration assays
Migration of CD8+ T cells toward supernatants from co-cultures of polarized macrophages and tumor cells was assessed in 96-well trans-well plates (5 μm pore size; Corning Costar) upon 60 min culture at 37°C. When indicated, anti-CXCL10 neutralizing mAb (10 μg/ml; R&D System) was also added to culture supernatants. Cell migration was quantified by flow cytometry.
In vivo experiments
In vivo experiments were approved by the Basel Cantonal Veterinary Office (License Number 2266). NSG mice from Charles River Laboratories were bred and maintained under specific pathogen-free conditions in the animal facility of the Department of Biomedicine of the University of Basel. Eight- to 10-week-old mice were injected subcutaneously (s.c.) in the flank with tumor cells (1 05 cells/mouse), resuspended in 1:1 growth factor reduced Matrigel (BD Biosciences)/ Phosphate Buffered Saline (PBS) solution. Tumor formation was monitored twice weekly by palpation and caliper measurements. Once tumor masses reached an approximate diameter of 5 mm (1 × 106 tumor cells), 20 µl of virus solution (107 eq. pfu of VV WT or rVV40L) or PBS was injected in the tumor tissues. After 48 hours, PBS or 5 × 105 M1- or M2-like macrophages were also injected intratumorally. Tumor size changes were followed every day by caliper measurements. One week after, all mice were sacrificed and tumors were harvested. Tumor volumes (in mm3) were determined according to the formula (length x width2)/2.Citation65 Samples from all tissues were harvested for subsequent histological examination.
Gene expression analysis
Total cellular RNA was extracted by using the RNeasyVR Mini Kit (Qiagen) and reverse transcribed according to manufacturer’s instructions (Invitrogen-Life Technologies). Human TRAF-1 and NORE1A (RASSF5) gene expression was evaluated by quantitative RT-PCR (RT-qPCR) using specific primer sets (TaqMan® Assays, Applied Biosystems-Life Technologies) and normalized to human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene expression.
Flow cytometry
Expression of specific markers and induction of apoptosis were evaluated by flow cytometry (FACScalibur; Becton Dickinson). In particular, fluorochrome-labeled mAbs recognizing CD163, CD204, CD154 (CD40L), CD3, CD4, CD8, CD45RO, CD62L, CD183 (CXCR3), and HLA-A0201 were obtained from Becton Dickinson. An mAb recognizing CD16 was obtained from BioLegend, whereas an mAb recognizing CD40 receptor (Clone 5C3) was obtained from eBioscience. The viability of tumor cells was measured by Annexin V apoptosis detection kit (Becton Dickson). Data were analyzed by FlowJo software (Tree Star).
ELISA assays
Interleukin-10 (IL-10), interleukin-12 (IL-12p70), and interferon-γ (IFN-γ) release in culture supernatants was measured by using ELISA kits (Becton Dickinson). Tumor necrosis factor-α (TNF-α) release was measured by using an ELISA kit from BioLegend, whereas C-X-C motif chemokine 10 (CXCL10, IP-10) release was evaluated by using a kit from R&D System.
Histological evaluation
Cryo-sections embedded in Optimal Cutting Temperature (OCT) compound (Leica) were cut (10 μm) from each tumor and fixed in formalin. Sections were either stained for hematoxylin and eosin using a Continuous Linear Stainer COT 20 (Medite) or incubated with a rabbit anti-cleaved caspase 3 reagent (Cell Signalling), followed by secondary species-specific Alexa Fluor 488-conjugated antibody (Invitrogen) and DAPI for nuclei counterstaining. Sections were examined by using a Nikon TI fluorescence microscope (Nikon Switzerland), and images were captured with 20x magnification using a digital camera and NIS-Elements software.
Statistical analysis
Statistical analysis software SPSS (Version 14.0, SPSS Inc.) was used for statistical analyses. Skewness, kurtosis, distribution parameters, and respective standard errors were used to test the normality of the concerned populations. Mann–Whitney test was used for the analysis of non-parametric data with non-Gaussian distribution of the test population. All reported P-values were considered to be statistically significant at P≤ 0.05.
Disclosure of Potential Conflicts of Interest
No conflict of interest to be disclosed.
Supplemental Material
Download MS Power Point (332.8 KB)Supplemental material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van KC, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi:10.1146/annurev.iy.12.040194.004313.
- Vonderheide RH. Prospect of targeting the CD40 pathway for cancer therapy. Clin Cancer Res. 2007;13:1083–1088. doi:10.1158/1078-0432.CCR-06-1893; 13/4/1083 [pii].
- Elmetwali T, Young LS, Palmer DH. CD40 ligand-induced carcinoma cell death: a balance between activation of TNFR-associated factor (TRAF) 3-dependent death signals and suppression of TRAF6-dependent survival signals. J Immunol. 2010;184:1111–1120. doi:10.4049/jimmunol.0900528; jimmunol.0900528 [pii].
- Elmetwali T, Salman A, Palmer DH. NORE1A induction by membrane-bound CD40L (mCD40L) contributes to CD40L-induced cell death and G1 growth arrest in p21-mediated mechanism. Cell Death Dis. 2016;7:e2146. doi:10.1038/cddis.2016.52; cddis201652 [pii].
- Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi:10.1111/j.1600-065X.2009.00782.x; IMR782 [pii].
- Ullenhag G, Loskog AS. AdCD40L – crossing the valley of death? Int Rev Immunol. 2012;31:289–298. doi:10.3109/08830185.2012.692844.
- Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res. 2013;19:1035–1043. doi:10.1158/1078-0432.CCR-12-2064; 19/5/1035 [pii].
- Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi:10.1126/science.1198443; 331/6024/1612 [pii].
- Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, Troxel AB, Sun W, Teitelbaum UR, Vonderheide RH, et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19:6286–6295. doi:10.1158/1078-0432.CCR-13-1320; 1078-0432.CCR-13-1320 [pii].
- Hangalapura BN, Timares L, Oosterhoff D, Scheper RJ, Curiel DT, de Gruijl TD. CD40-targeted adenoviral cancer vaccines: the long and winding road to the clinic. JGene Med. 2012;14:416–427. doi:10.1002/jgm.1648.
- Loskog A, Maleka A, Mangsbo S, Svensson E, Lundberg C, Nilsson A, Krause J, Agnarsdottir M, Sundin A, Ahlstrom H, et al. Immunostimulatory AdCD40L gene therapy combined with low-dose cyclophosphamide in metastatic melanoma patients. Br J Cancer. 2016;114:872–880. doi:10.1038/bjc.2016.42; bjc201642 [pii].
- Malmstrom PU, Loskog AS, Lindqvist CA, Mangsbo SM, Fransson M, Wanders A, Gardmark T, Totterman TH. AdCD40L immunogene therapy for bladder carcinoma – the first phase I/IIa trial. Clin Cancer Res. 2010;16:3279–3287. doi:10.1158/1078-0432.CCR-10-0385; 1078-0432.CCR-10-0385 [pii].
- Vonderheide RH, Bajor DL, Winograd R, Evans RA, Bayne LJ, Beatty GL. CD40 immunotherapy for pancreatic cancer. Cancer Immunol Immunother. 2013;62:949–954. doi:10.1007/s00262-013-1427-5.
- Feder-Mengus C, Schultz-Thater E, Oertli D, Marti WR, Heberer M, Spagnoli GC, Zajac P. Nonreplicating recombinant vaccinia virus expressing CD40 ligand enhances APC capacity to stimulate specific CD4+ and CD8+ T cell responses. Hum Gene Ther. 2005;16:348–360. doi:10.1089/hum.2005.16.348.
- Gulley JL, Madan RA, Tsang KY, Jochems C, Marte JL, Farsaci B, Tucker JA, Hodge JW, Liewehr DJ, Steinberg SM, et al. Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer Immunol Res. 2014;2:133–141. doi:10.1158/2326-6066.CIR-13-0108; 2326-6066.CIR-13-0108 [pii].
- Kim JW, Gulley JL. Poxviral vectors for cancer immunotherapy. Expert Opin Biol Ther. 2012;12:463–478. doi:10.1517/14712598.2012.668516.
- Trella E, Raafat N, Mengus C, Traunecker E, Governa V, Heidtmann S, Heberer M, Oertli D, Spagnoli GC, Zajac P. CD40 ligand-expressing recombinant vaccinia virus promotes the generation of CD8(+) central memory T cells. Eur J Immunol. 2016;46:420–431. doi:10.1002/eji.201545554.
- Zajac P, Oertli D, Marti W, Adamina M, Bolli M, Guller U, Noppen C, Padovan E, Schultz-Thater E, Heberer M, et al. Phase I/II clinical trial of a nonreplicative vaccinia virus expressing multiple HLA-A0201-restricted tumor-associated epitopes and costimulatory molecules in metastatic melanoma patients. Hum Gene Ther. 2003;14:1497–1510. doi:10.1089/104303403322495016.
- Kirn DH, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9:64–71. doi:10.1038/nrc2545.
- Parviainen S, Ahonen M, Diaconu I, Hirvinen M, Karttunen A, Vaha-Koskela M, Hemminki A, Cerullo V. CD40 ligand and tdTomato-armed vaccinia virus for induction of antitumor immune response and tumor imaging. Gene Ther. 2014;21:195–204. doi:10.1038/gt.2013.73; gt201373 [pii].
- Adamina M, Rosenthal R, Weber WP, Frey DM, Viehl CT, Bolli M, Huegli RW, Jacob AL, Heberer M, Oertli D, et al. Intranodal immunization with a vaccinia virus encoding multiple antigenic epitopes and costimulatory molecules in metastatic melanoma. Mol Ther. 2010;18:651–659. doi:10.1038/mt.2009.275; mt2009275 [pii].
- Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. doi:10.1038/nrclinonc.2016.217; nrclinonc.2016.217 [pii].
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. S1471490602023025 [pii].
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122: 787-95.59643 [pii]. doi:10.1172/JCI59643.
- Verreck FA, de BT, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de Waal-Malefyt R, Ottenhoff TH. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci USA. 2004;101:4560–4565. doi:10.1073/pnas.0400983101; 0400983101 [pii].
- Nebiker CA, Han J, Eppenberger-Castori S, Iezzi G, Hirt C, Amicarella F, Cremonesi E, Huber X, Padovan E, Angrisani B, et al. GM-CSF production by tumor cells is associated with improved survival in colorectal cancer. Clin Cancer Res. 2014;20:3094–3106. doi:10.1158/1078-0432.CCR-13-2774; 1078-0432.CCR-13-2774 [pii].
- Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462–472. doi:10.1016/j.ccell.2015.02.015; S1535-6108(15)00065-3 [pii].
- Fridman WH, Galon J, Dieu-Nosjean MC, Cremer I, Fisson S, Damotte D, Pages F, Tartour E, Sautes-Fridman C. Immune infiltration in human cancer: prognostic significance and disease control. Curr Top Microbiol Immunol. 2011;344:1–24. doi:10.1007/82_2010_46.
- Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12: 298-306.nrc3245 [pii]. doi:10.1038/nrc3245.
- Fridman WH, Dieu-Nosjean MC, Pages F, Cremer I, Damotte D, Sautes-Fridman C, Galon J. The immune microenvironment of human tumors: general significance and clinical impact. Cancer Microenviron. 2013;6:117–122. doi:10.1007/s12307-012-0124-9.
- Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211: 214-24.IMR391 [pii]. doi:10.1111/j.0105-2896.2006.00391.x.
- Yue C, Shen S, Deng J, Priceman SJ, Li W, Huang A, Yu H. STAT3 in CD8+ T cells inhibits their tumor accumulation by downregulating CXCR3/CXCL10 Axis. Cancer Immunol Res. 2015;3:864–870. doi:10.1158/2326-6066.CIR-15-0014; 2326-6066.CIR-15-0014 [pii].
- Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19: 47-64.19/ 1/47[pii]. doi:10.1146/annurev.immunol.19.1.47.
- Zwaveling S, Ferreira Mota SC, Nouta J, Johnson M, Lipford GB, Offringa R, van der Burg SH, Melief CJ. Established human papillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. J Immunol. 2002;169:350–358.
- Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–383. doi:10.1016/j.immuni.2008.08.004; S1074-7613(08)00375-0 [pii].
- Ridge JP, Di RF, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi:10.1038/30989.
- Richman LP, Vonderheide RH. Role of crosslinking for agonistic CD40 monoclonal antibodies as immune therapy of cancer. Cancer Immunol Res. 2014;2:19–26. doi:10.1158/2326-6066.CIR-13-0152.
- Fidler IJ, Poste G. Macrophage-mediated destruction of malignant tumor cells and new strategies for the therapy of metastatic disease. Springer Semin Immunopathol. 1982;5:161–174.
- Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi:10.1002/path.1027; [pii].
- Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, Bottazzi B, Doni A, Vincenzo B, Pasqualini F, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood. 2006;107:2112–2122. doi:10.1182/blood-2005-01-0428; 2005-01-0428 [pii].
- Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol. 2013;35:585–600. doi:10.1007/s00281-013-0367-7.
- Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi:10.1016/j.coi.2010.01.009; S0952-7915(10)00010-5 [pii].
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi:10.1016/j.immuni.2014.06.010; S1074-7613(14)00230-1 [pii].
- Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi:10.1038/nrc1256; nrc1256 [pii].
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi:10.1016/j.cell.2010.03.014; S0092-8674(10)00287-4 [pii].
- Sandhu SK, Papadopoulos K, Fong PC, Patnaik A, Messiou C, Olmos D, Wang G, Tromp BJ, Puchalski TA, Balkwill F, et al. A first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Cancer Chemother Pharmacol. 2013;71:1041–1050. doi:10.1007/s00280-013-2099-8.
- Cassier PA, Italiano A, Gomez-Roca CA, Le TC, Toulmonde M, Cannarile MA, Ries C, Brillouet A, Muller C, Jegg AM, et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study. Lancet Oncol. 2015;16:949–956. doi:10.1016/S1470-2045(15)00132-1; S1470-2045(15)00132-1 [pii].
- Beatty GL. Macrophage-based immunotherapy for the treatment of pancreatic ductal adenocarcinoma. Oncoimmunology. 2013;2:e26837. doi:10.4161/onci.26837; 2013ONCOIMM0297 [pii].
- Mills CD, Lenz LL, Harris RA, Breakthrough: a macrophage-directed cancer immunotherapy. Cancer Res. 2016;76:513–516. doi:10.1158/0008-5472.CAN-15-1737; 0008-5472.CAN-15-1737 [pii].
- Panni RZ, Linehan DC, DeNardo DG. Targeting tumor-infiltrating macrophages to combat cancer. Immunotherapy. 2013;5:1075–1087. doi:10.2217/imt.13.102.
- Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi:10.1111/j.0105-2896.2006.00382.x; IMR382 [pii].
- Elmetwali T, Young LS, Palmer DH. CD40 ligand-induced carcinoma cell death: a balance between activation of TNFR-associated factor (TRAF) 3-dependent death signals and suppression of TRAF6-dependent survival signals. J Immunol. 2010;184:1111–1120. doi:10.4049/jimmunol.0900528.
- Szodoray P, Stanford SM, Molberg O, Munthe O, Bottini N, Nakken B. T-helper signals restore B-cell receptor signaling in autoreactive anergic B cells by upregulating CD45 phosphatase activity. J Allergy Clin Immunol. 2016;138:839–851. doi:10.1016/j.jaci.2016.01.035.
- Carroll VA, Lafferty MK, Marchionni L, Bryant JL, Gallo RC, Garzino-Demo A. Expression of HIV-1 matrix protein p17 and association with B-cell lymphoma in HIV-1 transgenic mice. Proc Natl Acad Sci USA. 2016;113:13168–13173. doi:10.1073/pnas.1615258113.
- Dedobbeleer O, Stockis J, van der Woning B, Coulie PG, Lucas S. Cutting edge: active TGF-β1 released from GARP/TGF-β1 complexes on the surface of stimulated human B lymphocytes increases class-switch recombination and production of IgA. J Immunol. 2017;199:391–396. doi:10.4049/jimmunol.1601882.
- Kehri MR, Castle BE. Regulation of CD40 ligand expression and use of recombinant CD40 ligand for studying B cell growth and differentiation. Semin Immunol. 1994;6:287–294. doi:10.1006/smim.1994.1037.
- Fanslow WC, Srinavasan S, Paxton R, Gibson MG, Spriggs MK, Armitage RJ. Structural characteristics of CD40 ligand that determine biological function. Semin Immunol. 1994;6:267–278. doi:10.1006/smim.1994.1035.
- Pullen SS, Labadia ME, Ingraham RH, McWhirter SM, Alber T, Crute JJ, Kehry MR. High-affinity interactions of tumor necrosis factor receptor-associated factors (TRAFs) and CD40 require TRAF trimerization and CD40 multimerization. Biochemistry. 1999;38:10168–10177. doi:10.1021/bi9909905.
- Elgueta R, Benson MJ, de Vries VC, Vasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi:10.1111/j.1600-065X.2009.00782.x.
- Schultz-Thater E, Piscuoglio S, Iezzi G, Le MC, Zajac P, Carafa V, Terracciano L, Tornillo L, Spagnoli GC. MAGE-A10 is a nuclear protein frequently expressed in high percentages of tumor cells in lung, skin and urothelial malignancies. Int J Cancer. 2011;129:1137–1148. doi:10.1002/ijc.25777.
- Andreozzi M, Quagliata L, Gsponer JR, Ruiz C, Vuaroqueaux V, Eppenberger-Castori S, Tornillo L, Terracciano LM. VEGFA gene locus analysis across 80 human tumour types reveals gene amplification in several neoplastic entities. Angiogenesis. 2014;17:519–527. doi:10.1007/s10456-013-9396-z.
- Svensson A, Patzi CM, Schluter K, Lind L, Eriksson K. Maturation-dependent expression of AIM2 in human B-cells. PLoS One. 2017;12:e0183268. doi:10.1371/journal.pone.0183268; PONE-D-17-11260 [pii].
- Wyzgol A, Muller N, Fick A, Munkel S, Grigoleit GU, Pfizenmaier K, Wajant H. Trimer stabilization, oligomerization, and antibody-mediated cell surface immobilization improve the activity of soluble trimers of CD27L, CD40L, 41BBL, and glucocorticoid-induced TNF receptor ligand. J Immunol. 2009;183:1851–1861. doi:10.4049/jimmunol.0802597; jimmunol.0802597 [pii].
- Schütz A, Oertli D, Marti WR, Noppen C, Padovan E, Spagnoli GC, Heberer M, Zajac P. Immunogenicity of nonreplicating vaccinia expressing targeted or complete MART-1/Malna-A antigen. Cancer Gene Ther. 2001;8:655–661. doi:10.1038/sj.cgt.7700351.
- Pierrillas PB, Tod M, Amiel M, Chenel M, Henin E. Improvement of parameter estimations in tumor growth inhibition models on xenografted animals: a novel method to handle the interval censoring caused by measurement of smaller tumors. Aaps J. 2016;18:404–415. doi:10.1208/s12248-015-9862-1; 10.1208/s12248-015-9862-1 [pii].
