ABSTRACT
Crizotinib is a tyrosine kinase inhibitor (TKI) approved for the treatment of non-small cell lung cancers (NSLCL) and lymphomas expressing activating translocations or mutations of oncogenic tyrosine kinases (in particular ALK and ROS1). We recently observed that high-dose (final concentration in vivo: ~10 µM) crizotinib can induce immunogenic cell death (ICD) in cancer cells lacking ALK/ROS1 activation through off-target effects that require the inhibition of several other tyrosine kinases. When combined with cisplatin (which alone does not induce ICD), crizotinib sensitizes NSCLC models to subsequent immunotherapy with PD-1 blockade, allowing to cure more than 90% of established orthotopic cancers. Of note, simultaneous treatment of mice with cisplatin, crizotinib and PD-1 blocking antibodies causes acute hepatotoxicity that can be avoided by a sequential regimen involving initial treatment with cisplatin plus crizotinib, followed by PD-1 blockade one week later. It will be important to translate these results obtained in mice into a clinical trial in NSCLC patients.
Non-small cell lung cancer (NSCLC) has become the most frequent lethal human cancer. In spite of the massive implementation of immune checkpoint blockers targeting the PD-1/PD-L1 interaction either in first line or in second line, mostly after cisplatin-based chemotherapies, the objective response rate is low (<30%, even for patient populations selected based on expression of the targeted molecules) and permanent cure is anecdotic, calling for major efforts in research and development. In the end, NSCLC is as lethal as it used to be before the implementation of immune checkpoint blockade (ICB), meaning that, at best, patients can hope for a transient delay of the advancement of the disease, often at the expense of major side effects.
With this major unmet medical need in mind, we reasoned that strategies for improving the treatment of NSCLC should be developed. Chemotherapy is particularly efficient against cancer when it is capable of inducing an antitumor immune response that is often the result of immunogenic cell death (ICD). Indeed, some particularly successful antineoplastic cytotoxicants (such as anthracyclines, cyclophosphamide and oxaliplatin) are able to stress and kill cancer cells in a way that their demise is perceived by the immune system as immunogenic, hence triggering a cytotoxic T lymphocyte (CTL) response against residual neoplastic cells expressing tumor-associated antigens.Citation1 Induction of ICD also sensitizes tumors to subsequent treatment with ICB.Citation2 Nonetheless, chemotherapeutics have major side effects, a fact that has mitigated efforts to develop new treatment schedules for the clinics. We therefore asked the question whether tyrosine kinase inhibitors (TKIs) might induce ICD in therapeutic settings. For this, we took advantage of the ever-more extensive knowledge on the cell biology of ICD, which is a cell death modality preceded by two major premortem stress responses, (i) autophagy favoring the lysosomal secretion of ATPCitation3 and (ii) endoplasmic reticulum (ER) stress leading to the exposure of the ER protein on the cell surface.Citation4 ICD is also accompanied by a type 1 interferon response,Citation5 the release of annexin A1 (ANXA1) from the cytoplasm,Citation6 and the exodus of high molecule group B1 (HMGB1) protein from the nuclei of dying cells.Citation7 Based on this knowledge, we screened several compound libraries for the identification of TKIs that would induce the hallmarks of ICD in vitro, in cultured cells expressing fluorescent biosensors.Citation8 We found that among some 500 distinct TKIs, high-dose (~10 µM) crizotinib stood out by its ability to induce all hallmarks of ICD in several human and mouse cancer cell lines.Citation9 Importantly, this effect was obtained on cells that lack activating translocations or mutations of oncogenic tyrosine kinases (in particular ALK and ROS1), indicating that it has to be considered ‘off-target’. However, the TKI-inhibitory (R) enantiomer of crizotinib was more effective in inducing ICD hallmarks than the (S) chiral compound, and genetic inhibition (with specific small interfering RNAs) of several tyrosine kinases that are inhibited by high-dose crizotinib could mimic at least part of the ICD-inducing activity, suggesting that crizotinib mediates these effects through a multi-kinase inhibitory action.Citation9
In a subsequent round of experiments, we evaluated the capacity of crizotinib to improve the therapeutic efficacy of cisplatin, a classical chemotherapeutic widely used for the treatment of NSCLC, which lacks the capacity to induce ICD.Citation1 In conditions in which cisplatin or crizotinib alone failed to exert significant therapeutic effects, the combination of both agents caused a synergistic tumor growth reduction. This result was obtained in three different models of NSCLC, namely (i) transplantable TC1 cancers developing subcutaneously or orthotopically in the lung, (ii) oncogene (KRAS–induced NSCLC and (iii) carcinogen (urethane)-induced NSCLC. Of note, these effects were accompanied by an increase in the infiltration of cancers by interferon-γ (IFNγ) producing CTLs and the local depletion of regulatory T cells (Tregs), leading to an amelioration of the CTL/Treg ratio. Moreover, the capacity of the combination treatment to eradicate approximately one third of established orthotopic TC1 cancers was suppressed when CD8+ T cells were depleted or IFN-γ was neutralized, proving that the effects are mediated by the immune system. Of note, TC1 cancers failed to respond to the combination of cisplatin plus crizotinib when they developed from cells engineered to lack ANXA1 or HMGB1 (by CRISP/Cas9 technology), supporting the idea that ICD must be induced in malignant cells to stimulate a therapeutically relevant anticancer immune response.Citation9
As mentioned above, an optimal treatment schedule with cisplatin plus crizotinib allowed to cure one third of mice bearing detectable TC1 lung cancers. This cure rate could be elevated to 90–100% when this treatment was followed by PD-1 blockade, yielding mice that remained tumor-free for several months and actually resisted to subcutaneous rechallenge with TC1 cells (but not that with antigenically unrelated tumors), proving the induction of long-term immune memory. Another model of orthotopic lung cancer, LLC3, also was amenable to complete cure when first treated with cisplatin plus crizotinib and then injected with PD-1 blocking antibodies. In both cases (TC1 and LLC3), the PD-1 targeting ICB alone failed to yield any therapeutic effect, meaning that the ICD-inducing combination therapy provides sensitization to PD-1 blockade.Citation9 This sensitization effect may be correlated with the capacity of crizotinib to upregulate PD-L1 expression in tumor cells both in vitro and in vivo. It is important to note that simultaneous treatment with all the agents (cisplatin + crizotinib + PD-1 blockade) or two among them (crizotinib + PD-1 blockade) caused signs of general toxicity (weight loss) and hepatotoxicity (liver inflammation and release of hepatocyte-specific transaminases into the plasma), while the sequential treatment (first cisplatin + crizotinib, then later PD-1 blockade) was devoid of detectable side effects.Citation9 Hence, the preclinical results confirm a prior clinical trial revealing a potentially lethal hepatotoxic effect when crizotinib is combined with PD-1 blockade,Citation10 as they provide a simple strategy to avoid such undesirable effects, consisting in administering sequential rather than simultaneous treatments.
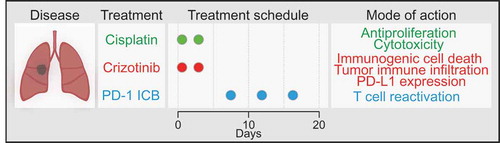
Altogether, the present results reveal a stratagem to obtain therapeutic effects by the combination of low-dose cisplatin, high-dose crizotinib and subsequent PD-1 blockade against NSCLC (). It will be important to design clinical trials that evaluate this approach in NSCLC patients.
Figure 1. A novel therapeutic strategy against non-small cell lung cancer. Shown here are the schedule of the treatment of non-small cell lung cancers (NSCLC) using cisplatin-based chemotherapy, crizotinib-based targeted therapy and immune checkpoint blockade abolishing the PD-1/PD-L1 interaction, as well as the underlying molecular mechanisms. For details see main text.
Conflicts of interest
OK and GK are scientific co-founders of Samsara Therapeutics.
Acknowledgments
GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de lRecherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Fondation Carrefour; Institut National du Cancer (INCa); Inserm (HTE); Inserm Transfert; Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology; the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM).
References
- Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28(6):690–714. doi:10.1016/j.ccell.2015.10.012.
- Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, Yamazaki T, Poirier-Colame V, Newton A, Redouane Y, et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity. 2016;44(2):343–354. doi:10.1016/j.immuni.2015.11.024.
- Martins I, Wang Y, Michaud M, Ma Y, Sukkurwala AQ, Shen S, Kepp O, Metivier D, Galluzzi L, Perfettini JL, et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ. 2014;21(1):79–91. doi:10.1038/cdd.2013.75.
- Bezu L, Sauvat A, Humeau J, Gomes-da-Silva LC, Iribarren K, Forveille S, Garcia P, Zhao L, Liu P, Zitvogel L, et al. eIF2alpha phosphorylation is pathognomonic for immunogenic cell death. Cell Death Differ. 2018;25(8):1375–1393. doi:10.1038/s41418-017-0044-9.
- Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remedios C, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20(11):1301–1309. doi:10.1038/nm.3708.
- Vacchelli E, Ma Y, Baracco EE, Sistigu A, Enot DP, Pietrocola F, Yang H, Adjemian S, Chaba K, Semeraro M, et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science. 2015;350(6263):972–978. doi:10.1126/science.aad0779.
- Liu P, Zhao L, Loos F, Iribarren K, Lachkar S, Zhou H, Gomes-da-Silva LC, Chen G, Bezu L, Boncompain G, et al. Identification of pharmacological agents that induce HMGB1 release. Sci Rep. 2017;7(1):14915. doi:10.1038/s41598-017-14848-1.
- Menger L, Vacchelli E, Adjemian S, Martins I, Ma Y, Shen S, Yamazaki T, Sukkurwala AQ, Michaud M, Mignot G, et al. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Transl Med. 2012;4(143):143ra199. doi:10.1126/scitranslmed.3003807.
- Liu P, Zhao L, Pol J, Levesque S, Petrazzuolo A, Pfirschke C, Engblom C, Rickelt S, Yamazaki T, Irribaren K, et al. Crizotinib-induced immunogenic cell death in non-small cell lung cancer. Nat Commun. 2019;10(1486):1–17. doi:10.1038/s41467-019-09415-3.
- Spigel DR, Reynolds C, Waterhouse D, Garon EB, Chandler J, Babu S, Thurmes P, Spira A, Jotte R, Zhu J, et al. Phase 1/2 study of the safety and tolerability of nivolumab plus crizotinib for the first-line treatment of anaplastic lymphoma kinase translocation - positive advanced non-small cell lung cancer (CheckMate 370). J Thorac Oncol. 2018;13(5):682–688. doi:10.1016/j.jtho.2018.02.022.
