ABSTRACT
Emerging evidence indicates that cancer cell-derived exosomes contribute to cancer progression through the modulation of tumor microenvironment, but the underlying mechanisms are not fully elucidated. Here, we reported that hepatocellular carcinoma (HCC)-derived exosomes could remodel macrophages by activating NF-κB signaling and inducing pro-inflammatory factors, and resulted in M2-polarized tumor-associated macrophages. In addition, the expression of IFN-γ and TNF-α was inhibited, while the expression of inhibitory receptors such as PD-1 and CTLA-4 was upregulated in T cells by HCC-derived exosome educated macrophages. Data also revealed that HCC exosomes were enriched with miR-146a-5p and promoted M2-polarization. Further investigation demonstrated that the transcription factor Sal-like protein-4 (SALL4) was critical for regulating miR-146a-5p in HCC exosomes and M2-polarization. Mechanistically, SALL4 could bind to the promoter of miR-146a-5p, and directly controlled its expression in exosomes. Blocking the SALL4/miR-146a-5p interaction in HCC reduced the expression of inhibitory receptors on T cells, reversed T cell exhaustion, and delayed HCC progression in DEN/CCL4-induced HCC mice. In conclusion, identification of a role of the exosomal SALL4/miR-146a-5p regulatory axis in M2-polarization as well as HCC progression provides potential targets for therapeutic and diagnostic applications in liver cancer.
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death in Asia and the fifth most common solid tumor worldwide. It attracts significant attention due to the aggressive nature, high mortality and low response rate to treatments.Citation1 Immune response involving the interplay among macrophages, monocytes, dendritic cells (DCs), neutrophils, and lymphocytes is a physiological response for tissue regeneration, particularly hepatic repair after tissue injury or infection.Citation2 However, in tumor microenvironment, anti-tumor immune response can be switched to a pro-tumor response.Citation3 During tumor progression, T cells are prone to apoptosis, and T cell-mediated anti-tumorigenic response is markedly impaired, characterized by low secretion of IL-2, TNF-α, and IFN-γ and a high expression of inhibitory receptors such as PD-1.Citation4 Clinical data show that PD-1/PD-L1 expression is positively associated with angiogenesis, tumor size, and tumor stage classification in patients with HCC.Citation5 Thus, therapeutic strategy based on improving tumor microenvironment in HCC is gaining attention.
Macrophages, as innate immune cells, perform phagocytic clearance of pathogens and apoptotic cells, and as antigen presenting cells (APC), present antigen to adaptive immune cells. Macrophages, as dominant immune-related stromal cells in the tumor microenvironment, are crucial to tumor progression. Macrophages display diverse phenotypes, including classically activated (M1) or alternatively activated (M2).Citation6 M1 macrophages that differentiated in response to IFN-γ stimulation are involved in Th1-type responses and characterized by elevated expression of major histocompatibility complex (MHC) class II, generation of nitric oxide (NO) and reactive oxygen species, as well as the ability to kill pathogens and infected cells.Citation7-Citation9 M1 macrophages promote abnormal cell apoptosis by producing pro-inflammatory cytokines such as IL-12 and TNF-α, resulting in the inflammation and the exacerbated tissue damage.Citation7,Citation8 In contrast, M2 macrophages are generated under the stimulation of IL-4 or IL-13 and involved in Th2-type responses.Citation10 M2 macrophages are characterized by high levels of scavenging molecules such as mannose receptor CD206 that functions in endocytosis and phagocytosis of the perivascular tumor microenvironment, and scavenger receptor CD163 that involved in anti-inflammatory functions, and releasing chemokines including CCL17, CCL22 (recruiting Th2 cells) and cytokines such as IL-10.Citation8,Citation11-Citation15 Meanwhile, M2 macrophages express the increased level of arginase, an enzyme for the synthesis of collagen, and thereby directly promotes cell division and tissue repair.Citation16 Imbalance of M1 and M2 polarization is often implicated in driving pathophysiological complications.Citation7 Tumor-associated macrophages (TAMs) are generally considered to be more closely resembling M2 phenotypes.Citation17 Especially, tumor-infiltrating macrophages are more M2 tumor-promoting macrophages but not M1 antitumor macrophages.Citation11 Delineating the processes that underlie the phenotypic transition of macrophages will provide a novel and insightful understanding of tumor progression.
Exosomes are extracellular vesicles (30–150 nm) originating from the multivesicular body, and can be isolated from diverse body fluids and cell culture supernatants.Citation18 They contain various types of molecules, including proteins, RNAs, DNAs, and lipids.Citation19,Citation20 A series of studies have identified exosomes as one of the important mediators of the interaction between tumor and surrounding non-tumor cells during cancer progression and metastasis. On the one hand, exosomes can directly modulate tumor cell function. Zakaria Y et al. revealed that tumor-derived exosomes delivered oncogenic nucleic acids and proteins to modulate the activity of normal cells, and accelerate tumorigenesis, metastasis in prostate cancer, breast cancer, and B-cell lymphoma.Citation21-Citation23 On the other hand, exosomes also can indirectly promote tumorigenesis by educating immune cells of the microenvironment, by suppressing T cell activation and natural killer (NK) cell-mediated antitumor activity, or other unknown mechanisms.Citation19 Previous studies have reported that cancer cell-derived miR-1246, miR-21 and miR-222-3p can be delivered into macrophages via exosomes, educating macrophages toward tumor supportive and anti-inflammatory state, to reprogram the tumor microenvironment.Citation6,Citation24,Citation25 We speculated that HCC cell-derived exosomes could remodel the tumor microenvironment by immunosuppression, and facilitate HCC disease progression. However, the potential underlying mechanisms are currently unknown.
In this study, the exosome-educated macrophages were generated by the treatment of HCC-derived exosomes.Citation26 We discovered a mechanism for M2-polarization by exosome-based communication between tumor and macrophages residing in the microenvironment, which form a distinct subpopulation of tumor supportive macrophages. Our findings identified that SALL4, a zinc finger transcription factor that considered as inseparable with malignant proliferation of tumor cells,Citation27 was enriched in tumor tissue, and mediated regulation of miR-146a-5p in HCC-derived exosomes can be potentially exploited for therapeutic and diagnostic applications in liver cancer.
Results
HCC-derived exosomes activated macrophages
The function of T cells was impaired in HCC due to the increased expression of inhibitory receptors and decreased secretion of inflammatory cytokines.Citation28 In this study, HCC mouse model was established after DEN/CCL4 administration for 40 weeks (Figure S1(a,b)). Characterization of the model exhibited up-regulation of inhibitory receptors, including Tim-3, LAG-3, CTLA4, TIGIT, and PD-1, on liver T cells (Figure S1(c)). The direct influence of DEN/CCL4 on T-cell exhaustion was excluded by treatment with DEN/CCL4 in vitro (Figure S1(d)).
To identify whether exosomes derived from HCC contributed to the formation of tumor microenvironment and tumor progression, exosomes were isolated from the supernatant of Hepa1-6 cells by ultracentrifugation. Exosomes were verified as small vesicles of approximately 100 nm in size by transmission electron microscopy (TEM), and with the expression of CD63 and CD81 (). The size distribution of the exosomes was predominantly within the range of 50–150 nm ().
Figure 1. HCC-derived exosomes activated macrophages.
Transmission electron microscopy (TEM) of exosomes isolated from the supernatant of Hepa1-6 cells and the expression of exosome-specific markers CD63 and CD81 analyzed by western blotting (a). Size distribution and concentration of exosomes were measured by Nanoparticle Tracking Analysis (b). The representative phase-contrast images of peritoneal macrophages incubated with DiD-labeled exosomes for 30 min (c). Western blot showed the activation of NF-κB (2 h) and pro-IL-1β (12 h) in PMs co-cultured with exosomes (d). Gene expression of il-1β and tnf-α in PMs treated with exosomes for 24h was measured by qPCR (e). Exo, exosomes. Data were representative of three independent experiments, statistical significance was determined as ***p < 0.001, **p < 0.01 and *p < 0.05 compared with control.
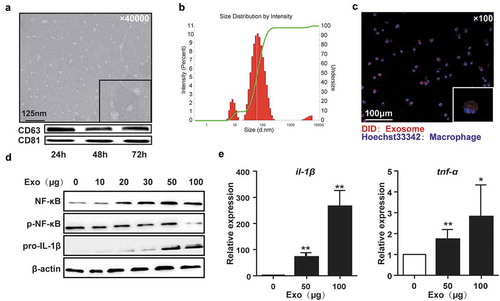
Tumor-associated macrophages (TAMs), which phagocytose multiple cell fragments or proteins, trigger tumorigenic signals to enhance cancer cell growth, invasion, and metastasis during HCC progression.Citation29 TAMs expressed high levels of dysfunctional molecules, which interacted with T cells and mediated T cell exhaustion in HCC.Citation30 Therefore, firstly, we determined whether HCC-derived exosomes were involved in the activation of macrophages. The HCC-derived exosomes were labeled with DiD dye and incubated with peritoneal macrophages (PMs) from healthy mice. Confocal high content analysis showed that the DiD-labeled exosomes were efficiently internalized by PMs within 30 min of incubation (). Moreover, exposure to exosomes activated NF-κB in PMs in a dose-dependent manner, accompanied by the increase in pro-IL-1β, tnf-α, and il-1β (). Similar results were seen in RAW264.7 cells (Figure S2(a)). The dose 50 μg/mL was used in the following in vitro experiments unless otherwise noted. These data indicate that HCC-derived exosomes could be taken up by macrophages resulting in their activation.
HCC-derived exosomes promoted M2 polarization
TAMs exhibit M2 phenotype and promote tumor development.Citation31 To determine whether HCC-derived exosomes regulated macrophage polarization, the populations of M1 and M2 macrophages in PMs incubated with exosomes were analyzed by Flow cytometry. We observed that the proportion of CD11b+F4/80+CD206+ macrophages was significantly increased following exposure to exosomes from Hepa1-6 cells ( left) or serum of DEN/CCL4-treated mice in vitro ( right). Furthermore, mRNA levels of M2 phenotype markers including ccl17, ccl22 and arg-1, as well as the production of ARG-1 in the supernatant were upregulated in PMs treated with HCC-exosomes ().
Figure 2. HCC-derived exosomes promoted macrophages toward M2-polarized tumor-associated macrophages.
PMs incubated with exosomes from Hepa1-6 cells (left) or the serum of DEN/CCL4-induced HCC mice (right) for 24 h. CD206 expression was analyzed by Flow cytometry (a). Gene expression of ccl22, ccl17, and arg-1 was assayed by qPCR and ELISA (b). The activation of STAT3 in PMs was analyzed by western blot (c). The activation of STAT1 in PMs was analyzed by Flow cytometry (d). Representative micrographs of immunofluorescence analysis of ROS in PMs (e). Exo, exosomes. Data were representative of three independent experiments, statistical significance was determined as ***p < 0.001, **p < 0.01 and *p < 0.05 compared with control.
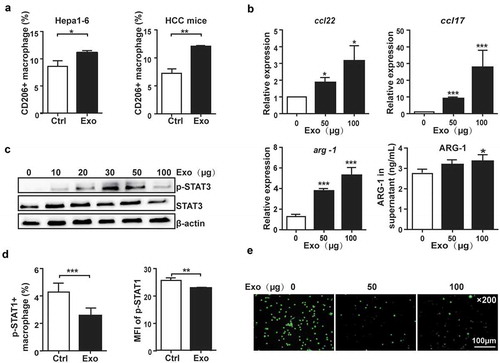
Studies have shown that the activation of STAT3 signaling pathway mediated the M2-type polarization of macrophages,Citation32,Citation33 while STAT1 signaling pathway mediated the polarization of M1-type macrophages.Citation33,Citation34 Here, we observed that STAT3 phosphorylation was elevated in PMs incubated with HCC-exosomes (), while the phosphorylation of STAT1 decreased () accompanied by the reduction of ROS production (). Similar results were observed in RAW264.7 and human THP-1 cells (Figure S2(b–d)). These results indicated that HCC-derived exosomes promoted polarization of macrophages toward M2 tumor-associated macrophages.
Macrophages educated with HCC-derived exosomes inhibited T cell response
While analyzing the phenotype of HCC-exosome “educated” macrophages, we noticed that the proportion of CD11b+F4/80+CD169+ macrophages, which was associated with suppressive activity of macrophages and immune tolerance,Citation35,Citation36 was significantly upregulated in the PMs treated by HCC-exosomes compared with the control group (). However, the antigen presenting MHC-II was downregulated by HCC-exosome treatment, while the ligands related to macrophage dysfunction such as PD-L1 and CD80 were upregulated (). These phenomena were also observed in RAW264.7 cells (Figure S3). Subsequently, we determined whether HCC-exosome “educated” macrophages could affect the function of T cells. We observed that the inhibitory receptors PD-1, TIGIT, and CTLA4 on CD3+T cells were increased by co-culturing with PMs treated with HCC-exosomes compared with T cells co-cultured with untreated PMs (). In addition, the production of IL-2, IFN-γ, and TNF-α in CD3+ T cells was lowered when co-cultured with HCC-exosome “educated” PMs (). No significant difference was observed between HCC-exosome treated and untreated T-cells. Similarly, PMs treated by the exosomes from serum of DEN/CCL4-treated mice upregulated PD-1 on T cells (Figure S4(a)). Human THP-1 treated by exosomes from HepG2 or H7402 cell lines downregulated the inducible co-stimulator ICOS and Granzym B on T cells (Figure S4(b)).
Figure 3. Macrophages educated with HCC-derived exosomes inhibited T cell response.
The expression of CD169 (a), MHC-II, PD-L1 and CD80 (b) on PMs was analyzed by FACS after exosomes treatment for 24 h. Naïve CD3+ T cells co-cultured with exosomes (Exo), PMs (M(Ctrl)) or exosome “educated” PMs (M(Exo)) for 5 d, and the inhibitory receptors PD-1, TIGIT, CTLA4 (c) and cytokines IL-2, IFN-γ and TNF-α (d) of T cells were analyzed by Flow cytometry. BMDMs incubated with exosomes from Hepa1-6 cells for 24 h, and then the expression of CD206 and iNOS was analyzed by Flow cytometry (e). BMDMs (M(Ctrl)) and exosome-“educated” BMDMs (M(Exo)) were, respectively, administrated to mice macrophage deleted, and the inhibitory receptors Tim-3 and TIGIT, and the cytokines IL-2 and TNF-α in T cells from liver were analyzed by Flow cytometry after 5 d (f). Hepatic T lymphocytes were isolated from mice treated as above. The cytolysis assay for T cells against Hepa1-6 cells at different E:T (effector: target) ratios was detected by CFSE/7-AAD Viability Staining Solution (g). After stained with CFSE and incubated with anti-CD3/CD28 for 3 d, T cell proliferation ability was analyzed by Flow cytometry (h). Exo, exosomes. Data were representative of three independent experiments, statistical significance was determined as ***p < 0.001, **p < 0.01 and *p < 0.05 compared with control.
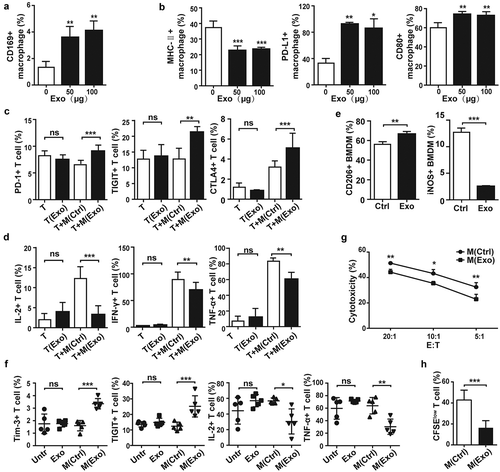
To further confirm the function of HCC-exosome “educated” macrophages in vivo, CD11b+F4/80+ macrophages derived from bone marrow (BMDMs) were prepared (Figure S5(a,b)) and treated with HCC-exosomes in vitro. As shown in , the proportion of CD206+ cells increased, while the iNOS+ cells decreased in the presence of HCC-exosomes, indicating M2 polarization. C57BL/6 mice were administrated with 200 μL of clodronate liposomes by intravenous injection to deplete macrophages (Figure S5(c)), and then transferred with control or HCC-exosome treated BMDMs. Compared with the hepatic T cells from mice transferred with control BMDMs, the inhibitory receptors Tim-3 and TIGIT were upregulated on T cells from mice transferred with HCC-exosome treated BMDMs, while the production of IL-2 and TNF-α was decreased (). Meanwhile, the cytolysis activity of T cells against HCC cells () and the expansion ability of T cells () were suppressed by transferring HCC-exosome treated BMDMs. Thus, HCC-exosome “educated” macrophages displayed immunosuppressive activity by inducing T cell exhaustion.
MiR-146a-5p in HCC-derived exosomes exerted key role on macrophage M2-polarization
MicroRNAs (miRNAs) are selectively packaged into exosomes, which largely dictate the effects of exosomes on recipient cells.Citation37,Citation38 Therefore, several miRNAs involved in immunoregulation were analyzed in exosomes derived from Hepa1-6 cells, including miR-146a-5p, miR-155, miR-26a, and let-7f, which were either up-regulated or down-regulated in HCCCitation39-Citation43 and closely related to macrophage-polarization.Citation44-Citation47 Intriguingly, miR-146a-5p was the most abundant one among the four miRNAs expressed in Hepa1-6, H22, HepG2 and H7402 exosomes (approximately 100-fold higher relative to U6) ( and S6(a)). And, its expression was remarkably higher in Hepa1-6 and H22 exosomes than healthy mouse hepatocytes ( and S6(b)). However, the transfection of miR-146a-5p inhibitors could inhibit CD206+ macrophages significantly ( and S6(c)) and downregulated p-STAT3 and C/EBPβ () in RAW264.7 cells and PMs incubated with HCC-exosomes, which suggested that miR-146a-5p in HCC-exosomes is an important mediator of M2 polarization.
Figure 4. MiR-146a-5p in HCC-derived exosomes exerted key role on macrophage M2-polarization.
The levels of miR146a-5p, miR-155, let-7f and miR-26a in exosomes from Hepa1-6 cells were analyzed by qPCR (a). MiR-146a-5p level in exosomes from Hepa1-6 cells or normal hepatocytes was analyzed by qPCR (b). RAW264.7 cells were transfected with miR-146a-5p inhibitor (146a-inh) or negative control (NC), then co-cultured with the exosomes from Hepa1-6 cells for 24 h, respectively. CD206 expression was analyzed by Flow cytometry (c), and the levels of STAT3 and C/EBPβ in RAW264.7 cells were examined by western blotting (d). Exosomes were isolated from the supernatants of Hepa1-6 cells transfected with miR-146a-5p overexpressing-, miR-146a-5p inhibiting- or empty vector for 48 h, and then co-cultured with RAW264.7 cells. After 24 h, the levels of tnf-α in RAW264.7 cells were measured by qPCR (e), the polarization of RAW264.7 cells was analyzed by Flow cytometry (f) and western blotting (g). HD, healthy mice hepatocytes. HCC, Hepa1-6 cells. Exo, exosomes. ov-146a, miR-146a-5p overexpressing-vector; inh-146a, miR-146a-5p inhibiting-vector; Ctrl, empty vector. Data were representative of three independent experiments, statistical significance was determined as ***p < 0.001, **p < 0.01 and *p < 0.05 compared with control.
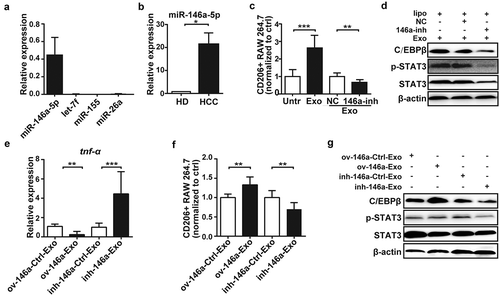
To test this hypothesis, miR-146a-5p was overexpressed in exosomes by miR-146a-5p overexpressing vector (ov-146a) in Hepa1-6 (Figure S6(d)). We found the overexpression of miR-146a-5p in Hepa1-6 cells significantly increased the level of miR-146a-5p in the secreted exosomes (Figure S6(e)), which declined the expression of tnf-α () and promoted the differentiation of CD206+ macrophages ( and S6(f)). In contrast, miR-146a-5p inhibiting vector (inh-146a) decreased the expression of miR-146a-5p in the Hepa1-6-derived exosomes, which up-regulated the level of tnf-α and suppressed the generation of CD206+ macrophages significantly. Meanwhile, the levels of p-STAT3 and C/EBPβ were increased in macrophages incubated with exosomes from HCC overexpressing miR-146a-5p, which was reversed by inhibiting miR-146a-5p (). Similar phenomena were observed in human HCC cell lines (Figure S6(g–i)). These data indicate that delivery of miR-146a-5p by HCC-derived exosomes is involved in M2 macrophage polarization.
Transcription factor SALL4 controlled miR-146a-5p expression in HCC-derived exosomes
SALL4, a zinc finger transcription factor, was recently demonstrated to be highly expressed in HCC, and regulated by STAT3 transcription factors.Citation27 We found SALL4 expression was increased during HCC development accompanied by the increasing number of M2-like macrophages, while silencing SALL4 via hydrodynamic injection of sh-SALL4 vector in DEN/CCL4-induced HCC mice (Figure S7) significantly decreased the number of M2-like macrophages (). So, we speculated that miR-146a-5p was regulated by SALL4.
Figure 5. Transcription factor SALL4 controlled miR-146a-5p levels in HCC-derived exosomes.
Mice were sacrificed after treatment with DEN/CCL4 for 15 weeks, then hepatocytes and the exosomes from serum were isolated. The mRNA level of sall4 in hepatocytes was analyzed by qPCR (a). CD206+ M2 macrophage number in liver was detected by Flow cytometry (b). MiR-146a-5p in exosomes was analyzed by qPCR (c). RAW264.7 cells were co-cultured with exosomes derived from the serum of DEN/CCL4-treated mice for 24 h, CD206 expression was detected by Flow cytometry (d). RAW264.7 cells were co-cultured with exosomes from Hepa1-6 cells transfected with sh-SALL4 or empty vector for 48 h, then CD206+ cells (e), the levels of STAT3 and C/EBPβ (f) were detected by Flow cytometry and western blotting, respectively. The miR-146a-5p level in exosomes derived from Hepa1-6 cells transfected with sh-SALL4 or empty vector was analyzed by qPCR (g). ChIP assay was performed to detect the recruitment of SALL4 in miR-146a-5p promoter (h). 293T cells were co-transfected with pGL-miR146a-Promoter-Luciferase and pcDNA3.1, SALL4 overexpressing plasmid or sh-SALL4 vector, and the Renilla expression vector pRLSV40 was co-transfected to normalize the transfection efficiency. After 24 h (overexpressing SALL4) or 48 h (sh-SALL4), luciferase activity of lysate was detected by dual-G1oTM Luciferase assay system. The ratio of firefly and Renilla luciferase activity associated with pGLSV40-Luciferase transfection was set as 1 (i). Exo, exosomes. Data were representative of three independent experiments, statistical significance was determined as ***p < 0.001, **p < 0.01and *p < 0.05 compared with control.
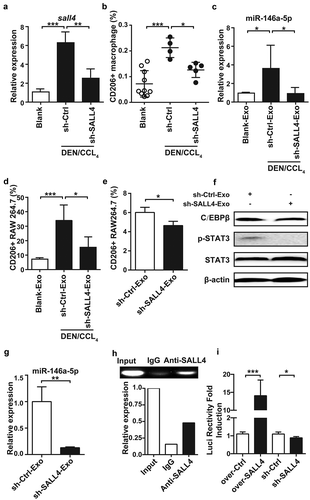
To verify whether SALL4 was involved in exosome-induced M2 polarization by regulating miR-146a-5p, we analyzed the level of miR-146-5p in exosomes from the serum of HCC mice. The results showed that the level of miR-146-5p in the exosomes was elevated by DEN/CCL4 treatment, and prevented by sh-SALL4 (). Meanwhile, compared with exosomes from blank mice, exosomes from DEN/CCL4-treated mice could significantly increase the percentage of CD206+ macrophages, while exosomes from sh-SALL4-tranduced mice did not show significant influence (). Consistently, the ratio of CD206+ RAW264.7 cells was significantly lowered by co-incubation with exosomes derived from sh-SALL4-treated Hepa1-6 cells compared with exosomes derived from sh-control-treated Hepa1-6 cells (), accompanied by downregulation of C/EBPβ and p-STAT3 ().
Finally, we tried to clarify if SALL4 could directly regulate the expression of miR-146a-5p in HCC. We observed that silencing SALL4 decreased miR-146a-5p significantly in HCC cells (Figure S8) and the derived exosomes () in vitro. And, ChIP analysis clearly demonstrated that SALL4 could bind to the miR-146a-5p promoter (). In addition, we found the transcription activity of miR-146a-5p promoter was enhanced by overexpressing of SALL4 but repressed by silencing of SALL4 (). These data indicate that direct binding of SALL4 to the miR-146a-5p promoter regulated M2 polarization.
SALL4 could switch the dysfunction of T cells and promote tumor development in DEN/CCL4-induced HCC
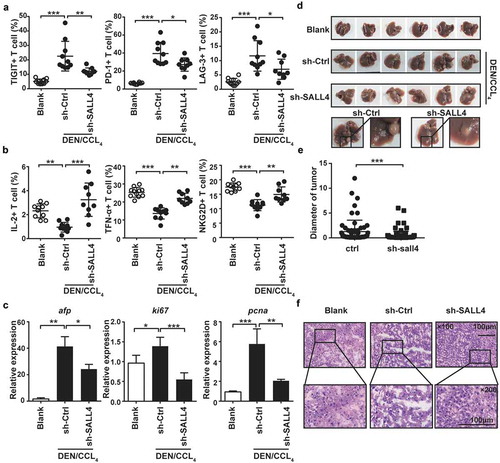
To verify the key role of SALL4 in T cell exhaustion, we analyzed the changes of inhibitory receptors in DEN/CCL4-induced HCC mice treated by sh-SALL4. The results showed that the administration of sh-SALL4 significantly lowered the inhibitory receptors TIGIT, LAG-3 and PD-1 on T cells of DEN/CCL4-treated HCC mice (), but enhanced the production of IL-2 and TNF-α as well as the activatory receptor NKG2D (). Additionally, silencing SALL4 could obviously reduce the expression of afp, ki67 and pcna in hepatocytes () and the tumor diameter (), accompanying with low degree of malignancy after administration for 30 weeks (). These results confirm that SALL4 activation enhances the expression of inhibitory receptors in T cells and promotes T cell exhaustion, accelerating HCC progression.
Figure 6. Blocking SALL4 reversed T cell exhaustion and inhibited HCC progression.
Described as Fig. S7A, C57BL/6 mice were treated with DEN/CCL4, meanwhile SALL4 was silenced by hydrodynamic injection of sh-SALL4 vector. The levels of TIGIT, PD-1, and LAG-3 (a), the production of IL-2 and TNF-α, and the expression of NKG2D (b) in T cells from liver of these mice were detected by Flow cytometry. Tumor-related factors afp, ki67 and pcna in hepatocytes were analyzed by qPCR (c). Morphology (d), diameter of the tumor exposed to the liver (e) and H&E staining (f) were analyzed. Data were representative of three independent experiments, statistical significance was determined as ***p < 0.001, **p < 0.01and *p < 0.05 compared with control.
Discussion
In the present study, we found that HCC cell-secreted miR-146a-5p could be delivered by exosomes into macrophages, switch the cytokine profile and attenuate antigen presentation of macrophages, and promote macrophages toward M2-polarized tumor-associated macrophages. Significantly, macrophages educated with HCC-derived exosomes impaired T cell functions. Furthermore, we found SALL4 could bind to the promoter and promote the expression of miR-146a-5p, while silencing SALL4 inhibited the expression of inhibitory receptors and reversed T cell exhaustion. This work uncovered a novel function of SALL4/miR-146a-5p axis and its clinical significance in HCC.
Recent studies have highlighted the key involvement of exosomes in remodeling of tumor microenvironment via communication between cancer cells, as well as cancer cells and non-malignant bystander cells such as immune cells, fibroblasts, and endothelial cells. K. Al-Nedawi et al. revealed that epidermal growth factor receptor (EGFR) can be transferred between glioma cells by exosomes, leading to the increased growth ability and the expression of anti-apoptotic genes, indicating an exosomal mediated cancer cell proliferation.Citation48 In addition, exosomes derived from highly metastatic ovarian cancer strongly induced metastasis in other tumors via exosomal MMP1 mRNA.Citation49 Furthermore, accumulating evidence supported the oncogenic properties of exosomes in regulating immune cells such as macrophages and neutrophils in several cancer types eg. human head and neck squamous cell carcinoma, mutant p53 cancers, melanoma, and lung cancer.Citation50,Citation51 Meanwhile, Wang X, et al. revealed that overexpressing 14–3-3ζ might be transmitted from HCC cells to T cells through exosomes to impair T cell function.Citation52 It was not surprising that HCC cells are also involved in the process of exosomal transfer. In this study, we confirmed that HCC-derived exosomes can activate macrophages, push macrophages toward M2-like phenotype ( and ), and suppress T cell functions (). We also found the effect of macrophages on T cells was mainly influenced by intercellular contacts (data not shown). Majority of the tumor-associated macrophages exhibit M2-like phenotype, but in our study, exosome-educated macrophages shared features of both M1 and M2 types, appearing high levels of il-1β, tnf-α, ccl17, ccl22, and arg-1. This was in line with previous studies.Citation7,Citation53 Unlike the binary M1/M2 definition, the “educated” macrophages were composed of several distinct populations, but with greater overall similarity to M2-polarized macrophages.
Tumor-derived exosomes could impair T cell functions in different ways. Gautam N et al. considered that tumor-derived exosomes inhibited T cell function directly in head and neck cancer, ovarian tumors, breast cancer, and melanoma,Citation50,Citation54-Citation56 but impaired T cell function by dendritic cells in HCC.Citation57 However, here we observed HCC-derived exosomes did not influence T cells directly (), but they could suppress T cell functions by educating macrophages. This inconsistency might be due to the different tumor models or the system and dosage used in our and other studies.Citation58,Citation59 In addition, macrophages scavenge cell fragments or protein except membrane fusing with exosomes compared with T cells, and thus can be the superior recipient of exosomes.
MiR-146a-5p is a well-known anti-inflammatory miRNA and frequently overexpressed in HCC that played a key role in promoting M2-like phenotype.Citation13 In the current study, we observed that HCC generated miR-146a-5p-abundant exosomes that transferred miR-146a-5p to macrophages and modulated their polarization and functions (). This observation extended the understanding of the oncogenic role of miR-146a-5p except the well-established intracellular signaling of miR-146a-5p. Therefore, oncogenic miR-146a-5p affected not only cancer cells but also tumor microenvironment. Although we focused on miR-146a-5p-abundant exosomes, other microRNAs such as miR-18a, −221, −222, −21 and −224 that are enriched in HCC derived exosomes may also contribute to the recruitment or polarization of immunosuppressive macrophages.Citation60
Our previous study suggested that STAT3 could control the expression of miR-146a-5p in human HCC.Citation39 Here, we found another factor, SALL4, which is reactivated in human HCC and correlates with disease progression of human malignancy and treatment status,Citation61 regulated miR-146a-5p. Previous study demonstrated that SALL4 to be inseparable from malignant proliferation of tumor cells, and was regulated by the transcription factor STAT3.Citation27 Here, we found that SALL4 could directly regulate the expression of miR-146a-5p in HCC cells, silencing SALL4 alleviated the generation of miR-146a-5p and M2-polarization (), and markedly improved the outcome of HCC in mice (). SALL4 is downstream of STAT3, and its binding sequences in the miR-146a-5p promoter overlapped with STAT3 binding sequences. In addition, silencing SALL4 and STAT3 showed similar effects on the transcriptional activity of miR-146a-5p promoter (data not shown). These findings indicated the possibility that SALL4-STAT3 control miR-146a-5p expression cooperatively.
In conclusion, our findings elucidated that the tumor-macrophage interplay through communication with exosomes during the development of HCC. We uncovered an important role of SALL4-overexpressing hepatoma cell-secreted miR-146a-5p on tumor progression via remolding tumor microenvironment and provided new findings that could potentially contribute to preventing HCC progress.
Materials and methods
Mice
C57BL/6 mice that were purchased from Beijing HFK Bioscience Co., Ltd., were used for all experiments. All animal experiments were carried out in Shandong University (Jinan, China) according to procedures approved by the institutional ethics committee.
Patient samples
Peripheral blood samples of patients were obtained from Qilu Hospital in accordance with the Ethics Committee of Shandong University, informed consent was acquired from all participants.
Cell lines
Mouse HCC cell lines Hepa1-6 cells (Cell Bank of Type Culture Collection of the Chinese Academy of Sciences) and H22 cells (Institute of Basic Medical Sciences, Shandong Academy of Medical Sciences) were cultured with DMEM and RPMI 1640 medium (GIBCO/BRL), respectively. Mouse macrophage cell line RAW264.7 cells and human monocyte cell line THP-1 cells (Cell Bank of Type Culture Collection of the Chinese Academy of Sciences) were cultured in DMEM and RPMI 1640 medium (HyClone), respectively, and 50 mM 2-mercaptoethanol (Sangon) was supplemented for THP-1 cell culture. Human HCC cell line HepG2 cells (Cell Bank of Type Culture Collection of Chinese Academy of Sciences) and H7402 cells (Institute of Basic Medical Sciences, Shandong Academy of Medical Science) were cultured in DMEM and RPMI 1640 medium (GIBCO/BRL), respectively. All cultures were supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 mg/mL streptomycin, and maintained in a 5% CO2 incubator at 37°C.
Peritoneal macrophages (PMs) were extracted from the abdominal cavity of mice with 1× PBS, and cultured with DMEM medium (GIBCO/BRL) supplemented with 10% FBS for 12 h. Then, these cells were washed with 1× PBS and used in follow-up experiments. For the induction of cell differentiation, human THP-1 cells were induced with 100 ng/mL PMA (Sigma, #P1585) for 12 h and rested for another 12 h. For the generation of “exosome-educated” macrophages, PMs, RAW264.7, BMDMs and differentiated THP-1 cells were incubated with 50 μg/mL of HCC-exosomes for 24 h.Citation26
Exosome isolation
Supernatants from HCC cells with over 95% viability and serum of mice were collected, and exosomes were isolated by ultracentrifugation as previously described.Citation62 Briefly, cell culture medium was sequentially centrifuged at 300g for 10 min, 2000g for 30 min and 10,000g for 70 min at 4°C to remove dead cells and cell debris. Then, the supernatant was filtrated through 0.22 mm filter and further ultracentrifuged at 100,000g for 1.5 h to collect exosomal pellet. The exosome pellets were washed in a large volume of phosphate-buffered saline (PBS) and ultracentrifuged at 100,000g for another 1.5 h at 4°C. The protein content of the concentrated exosomes was measured using the BCA protein assay kit (Beyotime, #P0010S). Each sample was normalized to a concentration of 2 μg/μl with 1× PBS and stored at −80°C until further use.
Electron microscopy
Exosomes were suspended in 1× PBS and spotted onto formvar-carbon-coated grids (200 mesh), and then the grid was fixed in 2% (vol/vol) paraformaldehyde at room temperature for 5 min. Fixation was followed by washing with deionized water, and then the exosomes were negatively stained using uranyl acetate. Exosomes were visualized under a JEM-1011 transmission electron microscope (JEOL, Japan).
Nanoparticle tracking analysis
Nanoparticles were tracked using a Malvern Zetasizer Nano ZS-90 (Malvern, UK) following the manufacturer’s instructions. Exosomes derived from Hepa1-6 and HepG2 cells were diluted with 1× PBS (1:1000). The mean particle size and size distribution were analyzed by Dynamic Light Scattering (DLS) method through Malvern Zetasizer Nano ZS-90 (Malvern, UK).
Western blot analysis
Cells were lysed with RIPA peptide lysis buffer (Beyotime, #P0013B). Protein concentrations were determined by BCA method and separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Then, the proteins were incubated with specific antibodies (1:1000). All antibodies are listed in Supplementary Table 2.
Flow cytometry
Harvested cells were incubated with respective antibodies for 40 min at 4°C after washing twice with 1× PBS. For the detection of intracellular markers, cells were fixed, permeabilized, and then stained with respective antibodies for 1 h at 4°C. For intracellular cytokines staining, the cells were pre-treated with 1 μg/mL ionomycin (Beyotime, #S1672) and 50 ng/mL PMA (Sigma, #P1585) for 5 h in the presence of Brefeldin A (BioLegend, #420601) for the last 4 h. The antibodies utilized are listed in Supplementary Table 2. All stained cells were measured using FACS Calibur (BD Biosciences, USA) or FACS Aria III (BD Biosciences, USA). The data were analyzed with FlowJo software (Treestar Inc., Ashland, OR, USA).
Detection of reactive oxygen species
Reactive oxygen species (ROS) were detected by the DCFH-DA assay.Citation63 Briefly, cells were incubated with 10 μM DCFH-DA (Sigma, #D6883) for 20 min at 37°C. After washing with 1× PBS, ROS production was measured as dichlorofluorescein (DCF) fluorescence intensity by using Olympus IX-71 Inverted microscope (Olympus, Japan).
Mouse strains and treatments
Six-week-old C57BL/6 male mice (21–23 g) received HCC-derived exosomes (200 μg/20 g) in 100 μL of 1× PBS via the tail vein every other day.Citation64,Citation65 On d 10, liver T cells were harvested for analysis. For macrophage depletion, C57BL/6 mice were injected intravenously with 200 μL of clodronate liposomes (Nicovan RooijenLeb), and depletion efficiency was confirmed by Flow cytometry. BMDMs were transplanted by intravenous administration of 1 × 106 cells, and T cells from liver were harvested 5 d later.
Isolation of primary T cells
Lymphocytes were isolated from spleen or liver by Ficoll gradient centrifugation. Primary T cells were purified by CD3 negative-selection isolation kit (Biolegend, #480031) and cultured in RPMI 1640 supplemented with 100 U/mL human rIL-2 (Changsheng).
T cell proliferation assay
Hepatic T cells isolated from mice transplanted by intravenous administration with HCC-exosomes-treated BMDMs were stained with carboxyfluorescein diacetate succinimidyl ester (CFSE, Beyotime, #C1031) according to the manufacturer’s instruction,Citation57,Citation66,Citation67 then these CFSE–labeled T cells (5 × 105) were incubated with anti-CD3 (5 μg/mL) and CD28 (2 μg/mL) for 3 d followed by Flow cytometry analysis.
Cytolysis assay
CFSE and 7-AAD Viability Staining Solution (Biolegend, #420404) was used to measure the cytolysis activity elicited by T cells against tumor cells.Citation57,Citation68 Hepatic T cells from mice transplanted with HCC-exosome-treated BMDMs were pretreated with anti-CD3 (5 μg/mL) and CD28 (2 μg/mL) for 2 d. CFSE–labeled Hepa1-6 cells (2 × 105) were co-incubated with these T cells at different ratios (1:20, 1:10, or 1:5) for overnight. Then, these co-cultured cells were harvested and stained with 7-AAD Viability Staining Solution according to the manufacturer’s instruction, followed by Flow cytometry analysis. The percent lysis was calculated as follows: %lysis = (%CFSE +7AAD+)/(%total CFSE +) ×100.
Chromatin immunoprecipitation
Chromatin Immunoprecipitation (ChIP) was performed according to the manufacturer‘s description (Millipore, #17–10086). The final lysate was used for immunoprecipitation with anti-SALL4 (Abcam, #ab29112) or normal IgG. After overnight incubation with SALL4 antibody at 4°C, protein A/G agarose beads were added to isolate immune complexes. Finally, the DNA was extracted and analyzed by PCR using primers designed around the binding sites on miR-146a-5p promoter (miR146a-pro, 5ʹ- CCCATGTTGGTGGCTCACAACAAC-3ʹ and 5ʹ- ATCCCTAAACTACATGCTAA -TCAC-3ʹ). The PCR products were assessed on 2% agarose gels.
Luciferase reporter gene assay
For the reporter gene assay, 293T cells were plated at a density of 1 × 104 cells/well in 96-well plates (NEST), and transfected with pGL3-miR-146a-5p-Promoter-Luciferase (200 ng/we11, Addgene, #15091), together with SALL4 overexpressing (over-SALL4) or SALL4 silencing (sh-SALL4) vector in the presence of LipofectamineTM 2000 (Invitrogen, #11668019). The Renilla expression vector pRL-SV40 was co-transfected to normalize the transfection efficiency. After 24 h or 48 h, cells were washed, lysed and a dual-Gloms Luciferase assay system (Promega, #E1910) was used to measure luciferase activity according to the instruction. The ratio of firefly and renilla luciferase activity with pGL3-SV40-Luciferase was set as 1.
Statistical analysis
All data are presented as means ± SD of three or more independent experiments. Statistical comparisons between two groups were performed using a Student’s t-test. One-way ANOVA followed by Tukey‘s post-hoc test was used to compare the differences among more than two groups, followed by the Bonferroni post hoc test. GraphPad Software Prism 6.0 was used for statistical analysis. Statistically significant differences were set at *p < 0.05, **p < 0.01.
Abbreviations
HCC hepatocellular carcinoma
SALL4 Sal-like protein-4
APC antigen presenting cells
TAMs Tumor-associated macrophages
MHC major histocompatibility complex
NO nitric oxide
NK natural killer
PMs peritoneal macrophages
TEM transmission electron microscopy
Disclosure of Potential Conflicts of Interest
The authors declare that they have no competing interests.
Supplemental Material
Download MS Word (2.6 MB)Acknowledgments
We thank the past members (Xiaoxia Sun, Yinli Yang, Zhaohua Hou) in our laboratory for their input on these studies.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Mohs A, Kuttkat N, Reissing J, Zimmermann HW, Sonntag R, Proudfoot A, Youssef SA, de Bruin A, Cubero FJ, Trautwein C. Functional role of CCL5/RANTES for HCC progression during chronic liver disease. J Hepatol. 2017;66:743–753. doi:10.1016/j.jhep.2016.12.011.
- Balmer ML, Slack E, de Gottardi A, Lawson MA, Hapfelmeier S, Miele L, Grieco A, van Vlierberghe H, Fahrner R, Patuto N, et al. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci Transl Med. 2014;6:237ra66–237ra66. doi:10.1126/scitranslmed.3008618.
- Scharping NE, Delgoffe GM. Tumor microenvironment metabolism: a new checkpoint for anti-tumor immunity. Vaccines. 2016;4(4):pii: E46.
- Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MA, Intlekofer AM, Boss JM, Reiner SL, Weinmann AS, et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12:663–671. doi:10.1038/ni.2046.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi:10.1038/nrc3239.
- Ying X, Wu Q, Wu X, Zhu Q, Wang X, Jiang L, Chen X, Wang X. Epithelial ovarian cancer-secreted exosomal miR-222-3p induces polarization of tumor-associated macrophages. Oncotarget. 2016;7:43076–43087. doi:10.18632/oncotarget.9246.
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi:10.1016/j.cell.2010.03.014.
- Patel U, Rajasingh S, Samanta S, Cao T, Dawn B, Rajasingh J. Macrophage polarization in response to epigenetic modifiers during infection and inflammation. Drug Discov Today. 2017;22:186–193. doi:10.1016/j.drudis.2016.08.006.
- Chen D, Xie J, Fiskesund R, Dong W, Liang X, Lv J, Jin X, Liu J, Mo S, Zhang T, et al. Publisher Correction: chloroquine modulates antitumor immune response by resetting tumor-associated macrophages toward M1 phenotype. Nat Commun. 2018;9:1808. doi:10.1038/s41467-018-04169-w.
- Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi:10.1016/j.coi.2010.01.009.
- Chen D, Xie J, Fiskesund R, Dong W, Liang X, Lv J, Jin X, Liu J, Mo S, Zhang T, et al. Chloroquine modulates antitumor immune response by resetting tumor-associated macrophages toward M1 phenotype. Nat Commun. 2018;9:873. doi:10.1038/s41467-018-03225-9.
- Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T, Liu B, Su L, Qiu Z. Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kgamma to promote pancreatic cancer metastasis. Cancer Res. 2018;78:4586–4598. doi:10.1158/0008-5472.CAN-17-3841.
- Vergadi E, Vaporidi K, Theodorakis EE, Doxaki C, Lagoudaki E, Ieronymaki E, Alexaki VI, Helms M, Kondili E, Soennichsen B, et al. Akt2 deficiency protects from acute lung injury via alternative macrophage activation and miR-146a induction in mice. J Immunol. 2014;192:394–406. doi:10.4049/jimmunol.1300959.
- Staples KJ, Hinks TS, Ward JA, Gunn V, Smith C, Djukanovic R. Phenotypic characterization of lung macrophages in asthmatic patients: overexpression of CCL17. J Allergy Clin Immunol. 2012;130:1404–1412 e1407. doi:10.1016/j.jaci.2012.07.023.
- Wu XQ, Dai Y, Yang Y, Huang C, Meng XM, Wu BM, Li J. Emerging role of microRNAs in regulating macrophage activation and polarization in immune response and inflammation. Immunology. 2016;148:237–248. doi:10.1111/imm.12608.
- Dzik JM. Evolutionary roots of arginase expression and regulation. Front Immunol. 2014;5:544. doi:10.3389/fimmu.2014.00544.
- Takai H, Ashihara M, Ishiguro T, Terashima H, Watanabe T, Kato A, Suzuki M. Involvement of glypican-3 in the recruitment of M2-polarized tumor-associated macrophages in hepatocellular carcinoma. Cancer Biol Ther. 2009;8:2329–2338. doi:10.4161/cbt.8.24.9985.
- Lin LY, Du LM, Cao K, Huang Y, Yu PF, Zhang LY, Li FY, Wang Y, Shi YF. Tumour cell-derived exosomes endow mesenchymal stromal cells with tumour-promotion capabilities. Oncogene. 2016;35:6038–6042. doi:10.1038/onc.2016.131.
- Liu Y, Gu Y, Cao X. The exosomes in tumor immunity. Oncoimmunology. 2015;4:e1027472. doi:10.1080/2162402X.2015.1008371.
- Tomiyama T, Yang GX, Zhao M, Zhang W, Tanaka H, Wang J, Leung PS, Okazaki K, He XS, Lu Q, et al. The modulation of co-stimulatory molecules by circulating exosomes in primary biliary cirrhosis. Cell Mol Immunol. 2017;14:276–284. doi:10.1038/cmi.2015.86.
- Abd Elmageed ZY, Yang Y, Thomas R, Ranjan M, Mondal D, Moroz K, Fang Z, Rezk BM, Moparty K, Sikka SC, et al. Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem Cells. 2014;32:983–997. doi:10.1002/stem.1619.
- Melo SA, Sugimoto H, O‘Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–721. doi:10.1016/j.ccell.2014.09.005.
- Koch R, Demant M, Aung T, Diering N, Cicholas A, Chapuy B, Wenzel D, Lahmann M, Guntsch A, Kiecke C, et al. Populational equilibrium through exosome-mediated Wnt signaling in tumor progression of diffuse large B-cell lymphoma. Blood. 2014;123:2189–2198. doi:10.1182/blood-2013-08-523886.
- Cooks T, Pateras IS, Jenkins LM, Patel KM, Robles AI, Morris J, Forshew T, Appella E, Gorgoulis VG, Harris CC. Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246. Nat Commun. 2018;9:771. doi:10.1038/s41467-018-03224-w.
- Hsieh CH, Tai SK, Yang MH. Snail-overexpressing cancer cells promote M2-Like polarization of tumor-associated macrophages by delivering MiR-21-abundant exosomes. Neoplasia. 2018;20:775–788. doi:10.1016/j.neo.2018.06.004.
- Chamberlain CS, Clements AB, Kink JA, Choi U, Baer GS, Halanski MA, Hematti P, Vanderby R. Extracellular vesicle-educated macrophages promote early Achilles tendon healing. Stem Cells. 2019. doi:10.1002/stem.2988.
- Sun C, Lan P, Han Q, Huang M, Zhang Z, Xu G, Song J, Wang J, Wei H, Zhang J, et al. Oncofetal gene SALL4 reactivation by hepatitis B virus counteracts miR-200c in PD-L1-induced T cell exhaustion. Nat Commun. 2018;9:1241. doi:10.1038/s41467-018-03584-3.
- Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi:10.1038/nri3862.
- Zhou SL, Hu ZQ, Zhou ZJ, Dai Z, Wang Z, Cao Y, Fan J, Huang XW, Zhou J. miR-28-5p-IL-34-macrophage feedback loop modulates hepatocellular carcinoma metastasis. Hepatology. 2016;63:1560–1575. doi:10.1002/hep.28445.
- Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, Liu J, Shi L, Liu C, Wang G, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342–1351. doi:10.1002/hep.25777.
- Roh-Johnson M, Shah AN, Stonick JA, Poudel KR, Kargl J, Yang GH, Di Martino J, Hernandez RE, Gast CE, Zarour LR, et al. Macrophage-dependent cytoplasmic transfer during melanoma invasion in vivo. Dev Cell. 2017;43:549–562 e546. doi:10.1016/j.devcel.2017.11.003.
- Solis-Martinez R, Cancino-Marentes M, Hernandez-Flores G, Ortiz-Lazareno P, Mandujano-Alvarez G, Cruz-Galvez C, Sierra-Diaz E, Rodriguez-Padilla C, Jave-Suarez LF, Aguilar-Lemarroy A, et al. Regulation of immunophenotype modulation of monocytes-macrophages from M1 into M2 by prostate cancer cell-culture supernatant via transcription factor STAT3. Immunol Lett. 2018;196:140–148. doi:10.1016/j.imlet.2018.02.009.
- Kwon YC, Meyer K, Peng G, Chatterjee S, Hoft DF, Ray R. Hepatitis C virus E2 envelope glycoprotein induces an immunoregulatory phenotype in macrophages. Hepatology. 2018. doi:10.1002/hep.29843.
- Baer C, Squadrito ML, Laoui D, Thompson D, Hansen SK, Kiialainen A, Hoves S, Ries CH, Ooi CH, de Palma M. Suppression of microRNA activity amplifies IFN-gamma-induced macrophage activation and promotes anti-tumour immunity. Nat Cell Biol. 2016;18:790–802. doi:10.1038/ncb3371.
- Black LV, Saunderson SC, Coutinho FP, Muhsin-Sharafaldine MR, Damani TT, Dunn AC, McLellan AD. The CD169 sialoadhesin molecule mediates cytotoxic T-cell responses to tumour apoptotic vesicles. Immunol Cell Biol. 2016;94:430–438. doi:10.1038/icb.2015.111.
- Chavez-Galan L, Olleros ML, Vesin D, Garcia I. Much more than M1 and M2 macrophages, there are also CD169(+) and TCR(+) macrophages. Front Immunol. 2015;6:263.
- Song Y, Dou H, Li X, Zhao X, Li Y, Liu D, Ji J, Liu F, Ding L, Ni Y, et al. Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1beta-primed mesenchymal stem cells against sepsis. Stem Cells. 2017;35:1208–1221. doi:10.1002/stem.2564.
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi:10.1038/ncb1596.
- Sun X, Zhang J, Hou Z, Han Q, Zhang C, Tian Z. miR-146a is directly regulated by STAT3 in human hepatocellular carcinoma cells and involved in anti-tumor immune suppression. Cell Cycle. 2015;14:243–252. doi:10.4161/15384101.2014.977112.
- Wang B, Majumder S, Nuovo G, Kutay H, Volinia S, Patel T, Schmittgen TD, Croce C, Ghoshal K, Jacob ST. Role of microRNA-155 at early stages of hepatocarcinogenesis induced by choline-deficient and amino acid-defined diet in C57BL/6 mice. Hepatology. 2009;50:1152–1161. doi:10.1002/hep.23100.
- Shimizu S, Takehara T, Hikita H, Kodama T, Miyagi T, Hosui A, Tatsumi T, Ishida H, Noda T, Nagano H, et al. The let-7 family of microRNAs inhibits Bcl-xL expression and potentiates sorafenib-induced apoptosis in human hepatocellular carcinoma. J Hepatol. 2010;52:698–704. doi:10.1016/j.jhep.2009.12.024.
- Zhu K, Pan Q, Zhang X, Kong LQ, Fan J, Dai Z, Wang L, Yang XR, Hu J, Wan JL, et al. MiR-146a enhances angiogenic activity of endothelial cells in hepatocellular carcinoma by promoting PDGFRA expression. Carcinogenesis. 2013;34:2071–2079. doi:10.1093/carcin/bgt160.
- Kota J, Chivukula RR, O‘Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi:10.1016/j.cell.2009.04.021.
- Bhatt K, Lanting LL, Jia Y, Yadav S, Reddy MA, Magilnick N, Boldin M, Natarajan R. Anti-inflammatory role of MicroRNA-146a in the pathogenesis of diabetic nephropathy. J Am Soc Nephrol. 2016;27:2277–2288. doi:10.1681/ASN.2015010111.
- Sahu SK, Kumar M, Chakraborty S, Banerjee SK, Kumar R, Gupta P, Jana K, Gupta UD, Ghosh Z, Kundu M, et al. MicroRNA 26a (miR-26a)/KLF4 and CREB-C/EBPbeta regulate innate immune signaling, the polarization of macrophages and the trafficking of Mycobacterium tuberculosis to lysosomes during infection. PLoS Pathog. 2017;13:e1006410. doi:10.1371/journal.ppat.1006410.
- Moore CS, Rao VT, Durafourt BA, Bedell BJ, Ludwin SK, Bar-Or A, Antel JP. miR-155 as a multiple sclerosis-relevant regulator of myeloid cell polarization. Ann Neurol. 2013;74:709–720. doi:10.1002/ana.23967.
- Kumar M, Sahu SK, Kumar R, Subuddhi A, Maji RK, Jana K, Gupta P, Raffetseder J, Lerm M, Ghosh Z, et al. MicroRNA let-7 modulates the immune response to Mycobacterium tuberculosis infection via control of A20, an inhibitor of the NF-kappaB pathway. Cell Host Microbe. 2015;17:345–356. doi:10.1016/j.chom.2015.01.007.
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. doi:10.1038/ncb1725.
- Yokoi A, Yoshioka Y, Yamamoto Y, Ishikawa M, Ikeda SI, Kato T, Kiyono T, Takeshita F, Kajiyama H, Kikkawa F, et al. Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer. Nat Commun. 2017;8:14470. doi:10.1038/ncomms14470.
- Ludwig S, Floros T, Theodoraki MN, Hong CS, Jackson EK, Lang S, Whiteside TL. Suppression of lymphocyte functions by plasma exosomes correlates with disease activity in patients with head and neck cancer. Clin Cancer Res. 2017;23:4843–4854. doi:10.1158/1078-0432.CCR-16-2819.
- Maybruck BT, Pfannenstiel LW, Diaz-Montero M, Gastman BR. Tumor-derived exosomes induce CD8(+) T cell suppressors. J Immunother Cancer. 2017;5:65. doi:10.1186/s40425-017-0269-7.
- Wang X, Shen H, Zhangyuan G, Huang R, Zhang W, He Q, Jin K, Zhuo H, Zhang Z, Wang J, et al. 14-3-3zeta delivered by hepatocellular carcinoma-derived exosomes impaired anti-tumor function of tumor-infiltrating T lymphocytes. Cell Death Dis. 2018;9:159. doi:10.1038/s41419-018-1111-y.
- Ojalvo LS, Whittaker CA, Condeelis JS, Pollard JW. Gene expression analysis of macrophages that facilitate tumor invasion supports a role for Wnt-signaling in mediating their activity in primary mammary tumors. J Immunol. 2010;184:702–712. doi:10.4049/jimmunol.0902360.
- Shenoy GN, Loyall J, Maguire O, Iyer V, Kelleher RJ Jr., Minderman H, Wallace PK, Odunsi K, Balu-Iyer SV, Bankert RB. Exosomes associated with human ovarian tumors harbor a reversible checkpoint of T-cell responses. Cancer Immunol Res. 2018;6:236–247. doi:10.1158/2326-6066.CIR-17-0113.
- Wen SW, Sceneay J, Lima LG, Wong CS, Becker M, Krumeich S, Lobb RJ, Castillo V, Wong KN, Ellis S, et al. The biodistribution and immune suppressive effects of breast cancer-derived exosomes. Cancer Res. 2016;76:6816–6827. doi:10.1158/0008-5472.CAN-16-0868.
- Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–386. doi:10.1038/s41586-018-0392-8.
- Rao Q, Zuo B, Lu Z, Gao X, You A, Wu C, Du Z, Yin H. Tumor-derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and humans in vitro. Hepatology. 2016;64:456–472. doi:10.1002/hep.28549.
- Liu ZM, Wang YB, Yuan XH. Exosomes from murine-derived GL26 cells promote glioblastoma tumor growth by reducing number and function of CD8+T cells. Asian Pac J Cancer Prev. 2013;14:309–314.
- Soderberg A, Barral AM, Soderstrom M, Sander B, Rosen A. Redox-signaling transmitted in trans to neighboring cells by melanoma-derived TNF-containing exosomes. Free Radic Biol Med. 2007;43:90–99. doi:10.1016/j.freeradbiomed.2007.03.026.
- Sato K, Meng F, Glaser S, Alpini G. Exosomes in liver pathology. J Hepatol. 2016;65:213–221. doi:10.1016/j.jhep.2016.03.004.
- Zhang X, Yuan X, Zhu W, Qian H, Xu W. SALL4: an emerging cancer biomarker and target. Cancer Lett. 2015;357:55–62. doi:10.1016/j.canlet.2014.11.037.
- Yang Y, Han Q, Hou Z, Zhang C, Tian Z, Zhang J. Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cell Mol Immunol. 2017;14:465–475. doi:10.1038/cmi.2016.24.
- Zhang Q, Lin S, Shi S, Zhang T, Ma Q, Tian T, Zhou T, Cai X, Lin Y. Anti-inflammatory and antioxidative effects of tetrahedral DNA nanostructures via the modulation of macrophage responses. ACS Appl Mater Interfaces. 2018;10:3421–3430. doi:10.1021/acsami.7b17928.
- Li Z, Zhou X, Wei M, Gao X, Zhao L, Shi R, Sun W, Duan Y, Yang G, Yuan L. In vitro and in vivo RNA inhibition by CD9-HuR functionalized exosomes encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2019;1:19–28.
- Cheng Q, Shi X, Han M, Smbatyan G, Lenz HJ, Zhang Y. Reprogramming exosomes as nanoscale controllers of cellular immunity. J Am Chem Soc. 2018;140:16413–16417. doi:10.1021/jacs.8b10047.
- Zappasodi R, Budhu S, Hellmann MD, Postow MA, Senbabaoglu Y, Manne S, Gasmi B, Liu C, Zhong H, Li Y, et al. Non-conventional inhibitory CD4(+)Foxp3(-)PD-1(hi) T cells as a biomarker of immune checkpoint blockade activity. Cancer Cell. 2018;33:1017–1032 e1017. doi:10.1016/j.ccell.2018.05.009.
- Wang R, Xu A, Zhang X, Wu J, Freywald A, Xu J, Xiang J. Novel exosome-targeted T-cell-based vaccine counteracts T-cell anergy and converts CTL exhaustion in chronic infection via CD40L signaling through the mTORC1 pathway. Cell Mol Immunol. 2017;14:529–545. doi:10.1038/cmi.2016.23.
- Lau J, Cheung J, Navarro A, Lianoglou S, Haley B, Totpal K, Sanders L, Koeppen H, Caplazi P, McBride J, et al. Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat Commun. 2017;8:14572. doi:10.1038/ncomms14572.
