ABSTRACT
Calreticulin (CALR) exposed on the surface of cancer cells succumbing to therapy delivers robust phagocytic signals that support the activation of adaptive anticancer immune responses. Recent data from our group demonstrate that spontaneous CARL exposure on leukemic blasts also supports innate anticancer immunity by natural killer (NK) cells via an indirect mechanism relying on myeloid CD11c+CD14+ cells.
Malignant cells responding to selected stressors, including some chemotherapeutics agents (e.g., anthracyclines, oxaliplatin, cyclophosphamide), radiation therapy, some variants of photodynamic therapy, and high hydrostatic pressure, expose on their surface or release in their microenvironment numerous molecules that deliver a signal of danger to the host immune system.Citation1 These molecules, which are collectively known as “damage-associated molecular patterns” (DAMPs), are responsible for the recruitment and activation of antigen-presenting cells (APCs) or their precursors to sites of cancer cell death, culminating with the local or nodal cross-presentation of tumor-associated antigens to naïve T cells and hence initiation of adaptive anticancer immunity.Citation2 The term “immunogenic cell death” (ICD) has been widely employed to refer to instances of cancer cell death associated with the release of DAMPs in amounts and according to kinetics that are compatible with the initiation of adaptive tumor-targeting immunity.Citation3 Importantly, DAMP emission by cancer cells undergoing ICD occurs downstream of stress responses that are initiated when cells are still alive in support of cellular homeostasis.Citation3
ICD-relevant DAMPs encompass soluble molecules, including ATP, the non-histone chromatin-binding protein high mobility group box 1 (HMGB1) and the cytokine interferon beta 1 (IFNB1), as well as molecules ectopically associated with the plasma membrane, such as the endoplasmic reticulum (ER) chaperone calreticulin (CALR). While soluble DAMPs generally deliver chemotactic and immunostimulatory signals, CALR exposed on the membrane of dying cells and dead cell corpses supports their uptake by APCs in the context of type I IFN production.Citation4,Citation5 Consistent with this notion, elevated levels of CALR on surface of malignant blasts has been linked with improved prognosis in patients with acute myeloid leukemia (AML),Citation6,Citation7 correlating with superior T-cell dependent immunity.Citation6
As the contribution of lymphoid cells other than T cells to anticancer immunity driven by membrane-exposed CALR was unclear, we set to investigate the potential involvement of natural killer (NK) cells, which are gaining considerable momentum as actionable items for cancer immunotherapy.Citation8 Previous findings suggested indeed a link between the exposure of ER chaperones on the surface of malignant blasts and NK cells activation in leukemia.Citation9
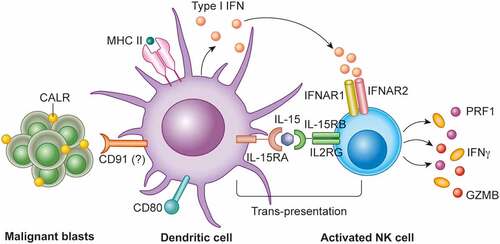
We demonstrated that high levels of membrane-exposed CALR on malignant blasts correlates with increased amounts of circulating NK cells in patients undergoing post-chemotherapy hematopoiesis restoration. Moreover, NK cells from patients with CALR-exposing blasts exhibited increased secretory and cytotoxic functions, which were unrelated to increased expression of NK cell-activatory ligands by cancer cells.Citation10 However, we were unable to document the direct effects of membrane-exposed CALR on the functional and secretory profile of NK cells. Rather, we found that CALR on the surface of malignant blasts favors the accumulation of a population of CD11b+CD14+ myeloid cells exhibiting numerous markers of activation, including CD86 and MHC Class II molecules, chemokine receptors such as C-C motif chemokine receptor 7 (CCR7), membrane-exposed interleukin 15 receptor, alpha chain (IL15RA), and type I IFN secretion.Citation10 Thus, AML patients in whom malignant blasts spontaneously expose CALR on the cell surface manifest the accumulation of an activated APC population with an elevated potential to migrate to lymph nodes (via CCR7)Citation11 and activate NK cells (via IL15RA, which is required for IL15 trans-presentation)Citation12 (). Supporting the clinical relevance of these findings, patients with high levels of CALR on the surface of malignant blasts and high levels of the NK cell activatory receptor (best known as NKGD2)Citation13 on the NK cell surface had superior overall survival as compared to all other patient subgroups.Citation10
Figure 1. Calreticulin and NK cell activation. The exposure of calreticulin (CALR) on malignant blasts from patients with acute myeloid leukemia (AML) favors the accumulation of an activated, poly-functional population of CD11b+CD14+ myeloid cells that support natural killer (NK) cell anticancer functions. CCR7, C-C motif chemokine receptor 7, IL15RA, interleukin 15 receptor, alpha chain (IL15RA); IFN, interferon
In summary, our results suggest that monitoring CALR exposure on malignant blasts and NK cell activation markers may improve current prognostic and predictive assessments in patients with AML. Additional studies are required to elucidate the precise prognostic and predictive value of these parameters.
Disclosures
JF and RS are supported by Sotio, Prague, Czech Republic. LG provides remunerated consulting to OmniSEQ (Buffalo, NY, USA), Astra Zeneca (Gaithersburg, MD, USA), Inzen (New York, NY, USA) and the Luke Heller TECPR2 Foundation (Boston, MA, USA), and he is member of the Scientific Advisory Committee of OmniSEQ (Buffalo, NY, USA).
References
- Yatim N, Cullen S, Albert ML. Dying cells actively regulate adaptive immune responses. Nat Rev Immunol. 2017;17:262–2. doi:10.1038/nri.2017.9.
- Galluzzi L, Chan TA, Kroemer G, Wolchok JD, Lopez-Soto A. The hallmarks of successful anticancer immunotherapy. Sci Transl Med. 2018;10. doi:10.1126/scitranslmed.aao4496.
- Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. doi:10.1038/nri.2016.107.
- Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi:10.1038/nm1523.
- Chen X, Fosco D, Kline DE, Kline J. Calreticulin promotes immunity and type I interferon-dependent survival in mice with acute myeloid leukemia. Oncoimmunology. 2017;6:e1278332. doi:10.1080/2162402X.2016.1278332.
- Fucikova J, Truxova I, Hensler M, Becht E, Kasikova L, Moserova I, Vosahlikova S, Klouckova J, Church SE, Cremer I, et al. Calreticulin exposure by malignant blasts correlates with robust anticancer immunity and improved clinical outcome in AML patients. Blood. 2016;128:3113–3124. doi:10.1182/blood-2016-08-731737.
- Wemeau M, Kepp O, Tesniere A, Panaretakis T, Flament C, De Botton S, Zitvogel L, Kroemer G, Chaput N. Calreticulin exposure on malignant blasts predicts a cellular anticancer immune response in patients with acute myeloid leukemia. Cell Death Dis. 2010;1:e104. doi:10.1038/cddis.2010.82.
- Chiossone L, Dumas PY, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. 2018;18:671–688. doi:10.1038/s41577-018-0061-z.
- Husak Z, Dworzak MN. CD99 ligation upregulates HSP70 on acute lymphoblastic leukemia cells and concomitantly increases NK cytotoxicity. Cell Death Dis. 2012;3:e425. doi:10.1038/cddis.2012.164.
- Truxova I, Kasikova L, Salek C, Hensler M, Lysak D, Holicek P, et al. Calreticulin exposure on malignant blasts correlates with improved NK cell-mediated cytotoxicity in AML patients. Haematologica. 2019; In press.
- Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi:10.1038/nri2297.
- Castillo EF, Stonier SW, Frasca L, Schluns KS. Dendritic cells support the in vivo development and maintenance of NK cells via IL-15 trans-presentation. J Immunol. 2009;183:4948–4956. doi:10.4049/jimmunol.0900719.
- Lopez-Soto A, Gonzalez S, Smyth MJ, Galluzzi L. Control of Metastasis by NK Cells. Cancer Cell. 2017;32:135–154. doi:10.1016/j.ccell.2017.06.009.
