ABSTRACT
Glucocorticoids mediate potent anti-inflammatory and immunosuppressive effects. A chronic elevation of the endogenous glucocorticoid tonus subsequent to mental stress, as well as continuous treatment with exogenous glucocorticoids, activate an immunosuppressive transcription factor, TSC22D3, in dendritic cells, causing the subversion of cancer therapy-elicited antineoplastic immune responses and subsequent therapeutic failure.
Synthetic glucocorticoids such as dexamethasone and prednisolone are widely used as anti-inflammatory and immunosuppressive agents to dampen autoimmune and inflammatory diseases. Indeed, they mimic the effects of endogenous glucocorticoids (in humans mostly cortisol) in thus far that they act on glucocorticoid receptors. Endogenous glucocorticoids play a major role in homeostatic responses to stress,Citation1 avoiding unwarranted autoimmunityCitation2 and suppressing potentially lethal inflammatory responses, for instance in the context of septic shock.Citation2,Citation3 In cancer immunotherapy, adverse effects due to the (re-)activation of autoimmune responses are highly frequent and even have been attributed a positive prognostic role with respect to the overall response rate. However, such adverse effects are usually managed by the administration of high-dose glucocorticoids,Citation4 and this type of medication is negatively associated with prognostic feature of immunotherapy, in particular for cancers outside of the central nervous system.Citation5 Although glucocorticoids can favor tumor progression through direct effects on the malignant cells per se,Citation6,Citation7 there are reasons to believe that they favor cancer progression and therapeutic failure through indirect immunological effects as well.
In a recently published study,Citation8 we demonstrated that, in mouse models, both endogenous and exogenous glucocorticoids can inhibit anticancer immune responses, thereby subverting the oncopreventive action of anticancer vaccination with dying-tumor cell preparations, as well as abolishing the tumor growth reducing effects of immunogenic chemotherapy (such as anthracyclines and oxaliplatin) and immunotherapy with PD-1 blockade. These findings were obtained in two models to cause “mental” stress, namely a model of social defeat-induced by repeated male domination (in which C57Bl/6 males are exposed to males from an inherently aggressive CD-1 strain) or repeated restraint stress (in which mice are confined in narrow falcon tubes). Especially in the social defeat model, mice reproducibly developed signs of anxiety and depression, coupled to a chronic elevation of glucocorticoid tonus (in mice mostly corticosterone) that were durable for at least 3.5 weeks. Indeed, administration of mifepristone, a potent glucocorticoid receptor antagonist, abolished the negative effects of mental stress on the efficacy of cancer vaccination or treatment with chemotherapy or immunotherapy. In contrast, administration of dexamethasone or prednisolone mimicked these negative impacts,Citation8 echoing the clinical results obtained in humans.Citation5 Indeed, treatment of human immune cells with exogenous glucocorticoids used at clinically relevant concentrations has immunosuppressive effects on the capacity of dendritic cell to present tumor antigens,Citation9 as well as on T cell activation and tumor-killing activity.Citation10
To investigate the molecular mechanism through which mental stress cause cancer therapy-relevant immunosuppression in the mouse model, we performed extensive analyses of the transcriptome of tumor-infiltrating dendritic cells (TIDCs) and the profile of soluble factors in the peripheral blood, comparing social defeat pre-conditioned mice and non-stressed control mice. These analyses revealed that mental stress caused a state of both local (within tumor microenvironment) and systemic immune suppression, as manifested by the inhibition of myeloid leukocyte differentiation, antigen signaling pathway, neutrophil chemotaxis, type I and II IFN responses. Moreover, the capacity of anthracyclines or PD-1 blockade to elicit interferon- gamma production by tumor-infiltrating T cells was suppressed upon social defeat. Bioinformatics analyses led to the discovery that dendritic cells upregulated Tsc22d3, an immunosuppressive transcription factor.Citation8
Although Tsc22d3 is ubiquitously expressed by most cell types, it turned out that social defeat favored the transcription of Tsc22d3 mostly in TIDCs, but not in other major immune cell types infiltrating the cancer, or in dendritic cells residing in the spleen or in the tumor-draining lymph node.Citation8 Based on this observation, we took advantage of genetically engineered mice which either overexpress Tsc22d3 in dendritic cells (under the control of the CD11c promoter), or lack Tsc22d3 in dendritic cells subsequent to a conditional knockout (in which a CD11c promoter-driven Cre recombinase excises the first exon of the floxed Tsc22d3 gene). The dendritic cell-restricted overexpression of Tsc22d3 was sufficient to mediate immunosuppression and to abolish tumor-preventive immune responses elicited by a prophylactic vaccination or therapeutically relevant immune response elicited by cancer chemotherapy. Conversely, the dendritic cell-targeted knockout of Tsc22d3 was able to abolish the immunosuppressive effects of exogenous glucocorticoids as well as those mediated by social defeat. Thus, the Tsc22d3 knockout reestablished therapeutic responses to immunogenic chemotherapy and immunotherapy with PD-1 blockade in spite of mental stress. Moreover, Tsc22d3 knockout restored the type-I interferon response, the expression of genes involved in MHC class-I or class-II-restricted antigen presentation, as well as the production of interferon-gamma by tumor-infiltrating T cells in the condition of social defeat. Thus, TSC22D3 function within dendritic cells affected the activity of other immune cells, including T lymphocytes, within the tumor bed.Citation8
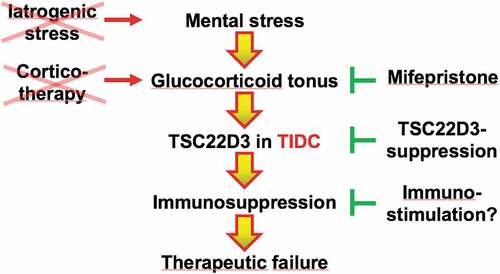
Altogether, these results can be condensed into a cascade linking mental stress to an elevation of endogenous glucocorticoids, TSC22D3 activation in tumor-infiltrating dendritic cells, immunosuppression and subsequent failure of immunogenic chemotherapy or immunotherapy (). Logically, this scheme implies that cancer patients should not be exposed to iatrogenic stress (which would increase the glucocorticoid tonus) and rather might profit from social, psychological or psychopharmacological interventions that reduce their mental stress. Instead of administering synthetic glucocorticoids, alternative strategies for managing side effects (such as administration of tumor necrosis factor antagonists) should be considered. Glucocorticoid antagonists might be prescribed to anxious and depressed patients with elevated cortisol levels. Although, pharmacological TSC22D3 antagonists are currently not available, it might also be attempted to stimulate the antigen-presenting function of tumor-infiltrating dendritic cells by suitable immunomodulators, perhaps by means of local immunotherapy, thus bypassing the immunosuppressive effects of the glucocorticoid-TSC22D3 axis.
Figure 1. Cascade of events that link mental stress to the failure of anticancer therapies. Mental stress causes an elevation of the glucocorticoid tonus that stimulates TSC22D3 expression by tumor infiltrating dendritic cells (TIDC), causing local immunosuppression and therapeutic failure. Exogenous glucocorticoids (corticotherapy) have a similar immunosuppressive effect. While iatrogenic stress should be avoided, glucocorticoid receptor antagonists exemplified by mifepristone, TSC22D3 inhibition or immunostimulatory agents that bypass the pathway of immunosuppression might be used for avoiding therapeutic failure
Acknowledgement
YM is supported by Natural Science Foundation of China [NSFC, 81722037, 81671630 and 81972701]; China Ministry of Science and Technology (National key research and development program), [2017YFA0506200]; Natural Science Foundation of Jiangsu Province [BK20170006]; CAMS Innovation Fund for Medical Sciences [CIFMS, 2016-I2M-1-005 and 2019-I2M-1-003]; Innovative and Entrepreneurial Team Program in Jiangsu Province. HY is supported by NSFC Grant 81802870 and Natural Science Foundation of Jiangsu Province Grant [BK20170407]. GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Association “Le Cancer du Sein, Parlons-en!”; Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Gustave Roussy Odyssea, the European Union Horizon 2020 Project Oncobiome; Fondation Carrefour; High-end Foreign Expert Program in China [GDW20171100085], Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology [ANR-18-IDEX-0001]; the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and the SIRIC Cancer Research and Personalized Medicine (CARPEM).
References
- Russell G, Lightman S. The human stress response. Nat Rev Endocrinol. 2019;15:525–3. doi:10.1038/s41574-019-0228-0.
- Gonzalo JA, Gonzalez-Garcia A, Martinez C, Kroemer G. Glucocorticoid-mediated control of the activation and clonal deletion of peripheral T cells in vivo. J Exp Med. 1993;177:1239–1246. doi:10.1084/jem.177.5.1239.
- Wick G, Hu Y, Schwarz S, Kroemer G. Immunoendocrine communication via the hypothalamo-pituitary-adrenal axis in autoimmune diseases. Endocr Rev. 1993;14:539–563. doi:10.1210/edrv-14-5-539.
- Williams KJ, Grauer DW, Henry DW, Rockey ML. Corticosteroids for the management of immune-related adverse events in patients receiving checkpoint inhibitors. J Oncol Pharm Pract. 2019;25:544–550. doi:10.1177/1078155217744872.
- Maxwell R, Luksik AS, Garzon-Muvdi T, Hung AL, Kim ES, Wu A, Xia Y, Belcaid Z, Gorelick N, Choi J, et al. Contrasting impact of corticosteroids on anti-PD-1 immunotherapy efficacy for tumor histologies located within or outside the central nervous system. Oncoimmunology. 2018;7:e1500108. doi:10.1080/2162402X.2018.1500108
- Obradovic MMS, Hamelin B, Manevski N, Couto JP, Sethi A, Coissieux -M-M, Münst S, Okamoto R, Kohler H, Schmidt A, et al. Glucocorticoids promote breast cancer metastasis. Nature. 2019;567:540–544. doi:10.1038/s41586-019-1019-4.
- Galluzzi L, Kroemer G. Cancer cells thrive on stress. Trends Cell Biol. 2019;29:447–449. doi:10.1016/j.tcb.2019.03.005.
- Yang H, Xia L, Chen J, Zhang S, Martin V, Li Q, Lin S, Chen J, Calmette J, Lu M, et al. Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced antitumor immunity. Nat Med. 2019;25:1428–1441. doi:10.1038/s41591-019-0566-4.
- Falcon-Beas C, Tittarelli A, Mora-Bau G, Tempio F, Pérez C, Hevia D, Behrens C, Flores I, Falcón-Beas F, Garrido P, et al. Dexamethasone turns tumor antigen-presenting cells into tolerogenic dendritic cells with T cell inhibitory functions. Immunobiology. 2019;224:697–705. doi:10.1016/j.imbio.2019.05.011.
- Draghi A, Borch TH, Radic HD, Chamberlain CA, Gokuldass A, Svane IM, Donia M. Differential effects of corticosteroids and anti-TNF on tumor-specific immune responses: implications for the management of irAEs. Int J Cancer. 2019;145:1408–1413. doi:10.1002/ijc.32080.
