ABSTRACT
Whereas TLR9 agonists are recognized as powerful stimulators of antitumor immunity, GM-CSF has had mixed reviews. In previously reported randomized trials we assessed the effects of local immune modulation in early-stage melanoma with CpG-B alone or with GM-CSF. Here we discuss the added value of GM-CSF and show sex-related differences.
Introduction
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is an important hematopoietic growth factor that plays multiple roles in the development and differentiation of progenitor cells into granulocytes, macrophages and dendritic cells (DC). Results from clinical trials that evaluated GM-CSF for the treatment of advanced melanoma, extensively reviewed by Hoeller et al., have been ambiguous.Citation1 GM-CSF can enhance anti-tumor responses by stimulating DC but it can also promote myeloid-derived suppressor cell (MDSC) expansion and act as a chemo-attractant for neutrophils which can dampen the immune response and even promote tumor growth and disease progression.Citation1 Much is influenced by the context in which GM-CSF is administeredCitation2 and more research is warranted in order to find optimal dosing, combinations, and administration routes for GM-CSF as a potential treatment for melanoma. For instance, in combination with ipilimumab, an overall survival advantage has been reported, which, beside immune activation, may have been related to lower toxicity rates.Citation3,Citation4 There has also been the further suggestion that clinical benefit may be associated with lower doses, leading to DC activation, rather than with higher doses, which may result in detrimental MDSC mobilization.Citation1 In line with this, localized expression of GM-CSF, encoded by the intratumorally delivered oncolytic virus Talimogene laherparepvec (T-VEC), led to an actual decrease in intratumoral MDSC and regulatory T cell (Treg) rates and increased T cell infiltration.Citation5 In patients with early metastatic melanoma (IIIB/C-IVM1a) impressive clinical outcomes were observed after T-VEC treatment with a best overall response rate and complete response rate of 88.5% and 61.5% respectively.Citation6
In localized melanoma, disease recurrence after resection of the primary tumor is dependent on disease stage, which includes risk factors such as Breslow thickness, tumor ulceration and whether or not the tumor has metastasized to the regional lymphatics.Citation7 As a prognostic measure, a sentinel node biopsy (SNB) is performed to assess lymphogenic metastasis, although so far the treatment implications are very limited after identifying a tumor positive (sentinel) lymph node (stage III disease). A complete lymph node dissection is no longer indicated as this does not improve overall survivalCitation8 and thus far only high-risk stage III patients have an indication for treatment with checkpoint inhibitors. For patients without lymphatic spread or with stage IIIA disease, the only available treatment is surgery followed by a wait-and-see policy, even though the chances of recurrence can be considerable. Our clinical work has clearly demonstrated the potential clinical benefits of local immune modulation prior to SNB.
In three previously reported randomized controlled phase II trials, we intradermally injected GM-CSF alone,Citation9 the TLR9 agonist CpG-B/CPG7909 alone,Citation10,Citation11 or CpG-B/CPG7909 and GM-CSF combinedCitation10 directly adjacent to the excision scar of the primary tumor in early-stage (i.e. localized) melanoma in the week leading up to the SNB. In the first trial, patients received four consecutive daily doses of 3 μg/kg body weight GM-CSF (n = 6) or placebo (n = 6), immediately preceding SNB. In the second trial patients received one dose of 8 mg CpG-B (n = 11) or placebo (n = 13) one week prior to SNB, and in the third trial, patients received 1 mg CpG-B (n = 10), 1 mg CPG-B with 100 µg GM-CSF (n = 9) or placebo (n = 9), 7 and 2 days prior to SNB. We previously reported potential down staging and an improved recurrence-free survival (RFS) in patients who were treated with CpG-B in the second and third trial.Citation12 Of note, 30% of the patients in the intervention group (9/30) of this meta-analysis actually received combinational treatment with CpG-B and GM-CSF. Here, we elaborate on our previous findings from immune monitoring and clinical follow-up studies,Citation10 and, based on previously published data as well as data presented here for the first time, demonstrate and discuss the added benefits of including GM-CSF in CpG-based immune potentiation of the melanoma sentinel lymph node (SLN).
Results and discussion
Clinical findings
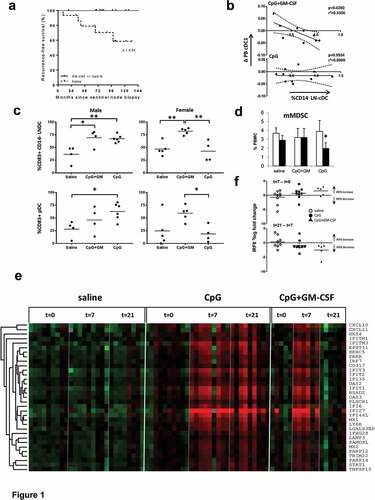
All patients who had been enrolled in one of the two previous randomized trials which included treatment arms with GM-CSF pre-SNB,Citation9,Citation10 alone (n = 5) or combined with CpG-B (n = 9), were combined into one treatment arm. Their recurrence-free survival (RFS) was compared to that of a corresponding combined control group of patients receiving a saline placebo from the same two clinical trials (n = 15). Remarkably, we did not find any loco-regional or distant disease recurrences in the treatment arm after a median follow up of 90 months (). In comparison, we found five recurrences in the combined control groups (n = 15) from the same trials (p = .002). Although we did not find any recurrences in the GM-CSF group, one patient had never attended any follow-up visits and further data could not be retrieved (this patient was therefore not included in the analyses) and one patient died from an unrelated cause at 19 months after treatment (this patient was censored at 19 months). Although these patient groups are too small to draw any firm conclusions, locally administered GM-CSF, either alone or combined with CpG-B, certainly appeared to improve clinical outcome in terms of RFS of patients with early-stage melanoma.
Figure 1. Added local and systemic effects of GM-CSF, co-delivered locally with CpG-B, in early-stage melanoma. Results shown are from patients receiving either a saline placebo (saline), or two administrations of CpG-B (CpG, 1 mg) or CpG combined with GM-CSF (CpG+GM, 1 mg + 100 µg) at day −7 and day −2 before sentinel lymph node biopsy (SNB), or GM-CSF alone (GM-CSF), 4 doses of 3µg/kg divided over the four days leading up to SNB. All were administered at the excision site of the primary tumor. (a) Recurrence-free survival of patients who were treated with GM-CSF with or without CpG-B (n = 14) versus patients that received saline (n = 15, p value is listed). (b) Correlation between changes in BDCA3/CD141+ peripheral blood cDC (cDC1) frequencies (between day −7 and 0) and CD1a−CD11chiCD14− cDC (CD14-LNDC) rates in the SLN of patients who received CpG+GM or CpG. (c) CD14− LNDC and pDC activation (by CD83) in men and women after local treatment with CpG or CpG+GM. (d) Pre- (day −7, open bars) and post-treatment (day 0, closed bars) frequencies of monocytic myeloid derived suppressor cells (mMDSC) in peripheral blood of patients receiving either saline, CpG, or CpG-B+ GM-CSF. (e) Transcriptional profiling reveals post-treatment induction of a type-I Interferon (IFN) response signature in peripheral blood mononuclear cells in patients receiving CpG or CpG+GM. (f) Changes in IRF8 transcript levels (relative to GAPDH) between t = 7 and t = 0 and t = 21 and t = 7 for saline (n = 9); CpG (n = 9); CpG+GM-CSF (n = 5). Statistical significance: * P < .05; ** P < .01; either by One-way ANOVA with Tukey post-hoc test or by paired two-sided student’s T test
Myeloid subset modulation and sex disparities
We have previously identified two skin-derived migratory conventional DC subsets (cDC), i.e. Langerhans cells (LC) and dermal dendritic cells (DDC), and two lymph node resident cDC subsets (LNR-cDC) in melanoma SLN.Citation13 The LNR-cDC subsets differ in their expression of CD14, with the CD14− LNR-cDC phenotypically resembling CD141+ cDC1 with particular cross-presenting abilities. We found locally administered GM-CSF to activate and induce the migration of skin-derived cDC subsets to the SLN.Citation14 The combination of GM-CSF with CpG-B also resulted in superior activation of skin derived cDC.Citation10 These findings are in keeping with our previous observation of increased activation of skin explant-emigrated cDC subsequent to intradermal GM-CSF injection.Citation15 In contrast, no such effect was observed for CpG-B, but rather cDC1 and pDC were mobilized to the deep dermis upon intradermal delivery of CpG-B (Koster et al., manuscript in preparation). We have also reported that the addition of GM-CSF to locally administered CpG-B prior to SNB, resulted in more profound activation of the LNR-cDC subsets, and indeed increased the cross-presentation capacity of SLN-derived leukocyte suspensions.Citation10 We also observed that frequencies of the CD14− LNR-cDC in SLN correlated with the decrease in cDC1 frequencies in PBMC of patients who had received the combination of CpG-B and GM-CSF (, top panel previously publishedCitation10). We now report that this correlation was absent in patients who received CpG-B only (, lower panel). These data indicate that the addition of GM-CSF to CpG-B enhances the mobilization of cDC1 to the SLN as well as their activation. Remarkably, when we subdivided patients by sex, disparities in terms of DC activation became apparent (see ). Whereas CpG-B monotherapy in men induced significant activation of both CD14− LNR-cDC and pDC in the SLN (by CD83 expression), and in equal measure as when combined with GM-CSF, in women an equivalent activation induction in these subsets required combined CpG-B and GM-CSF activation. Of note, similar observations were made for the CD14+ LNR-cDC subset (data not shown). The role of sex specific hormones and genetics on the immune systemCitation16,Citation17 and cancer progressionCitation18,Citation19 has been well documented, but this has not yet led to cancer treatment implications based on the patient’s sex. Our data suggest that optimal activation of DC and, as a consequence, subsequent priming of antitumor effector T cells in women with early-stage melanoma may require combined administration of GM-CSF and CpG-B. This may be related to sex-dependent differences in TLR9 expression levels as previously observed in a mouse study, wherein increased TLR9 expression levels in males led to improved clearance of a viral infection.Citation20 Moreover, a sex-dependent inflammatory cytokine pattern during melanoma development was also described before in an experimental mouse model.Citation21 Improved pDC activation and IFNα release by GM-CSF has also been previously reported,Citation22 but, as far as we know, has never previously been linked to sex-dependent mechanisms. It is important to note that despite these remarkable differences in DC subset activation, we did not find any differences in RFS between women receiving CpG-B only or CpG-B combined with GM-CSF. However, again, caution is warranted due to the small sample size.
Whereas CpG-B monotherapy led to a significant reduction in circulating monocytic MDSC (mMDSC) rates (defined as CD14hiHLA-DR−) at day 7 after the first injection (i.e. day of SNB, see ), this reduction was abrogated by the addition of GM-CSF. This is consistent with the reported systemic mobilization of MDSC by GM-CSF,Citation1 apparently counteracting the mMDSC-reducing effect of CpG-B. Whereas high doses of CpG and chronic type-I IFN exposure may lead to increased MDSC levels,Citation23,Citation24 local CpG may actually attenuate MDSC development and their suppressive activity by inducing their maturation.Citation25 The latter is consistent with our observation.
IFN response gene expression
Type-I IFN responses have been identified as crucial to the generation of an effective antitumor T cell response,Citation26 inducing the maturation of cDC1 and enhancing their cross-presenting ability, while simultaneously boosting the effector functions of cytotoxic T cells and NK cells alike. We analyzed transcript levels of IFN Response Genes (IRGs) in PBMC before treatment (t = 0), one week after treatment (t = 7) and three weeks after treatment (t = 21). Cluster analysis revealed concerted up-regulation of a group of 33 IRGs at t = 7 in the CpG-treated patient groups (shown in ). Of the in total 47 tested IRGs, 33 IRGs were significantly upregulated at t = 7 as compared to matching baseline values in the CpG-treated patient groups (p values, corrected for multiple testing, ranging from 0.019 to 7.4x10e-6), but none in the saline placebo group. Of these 33 significantly up-regulated genes, 31 were also part of the identified co-regulated cluster of 33 genes shown in . Interestingly, whereas the IRG expression levels remained high on t = 21 in the CpG-B only group, their levels had gone down by then in the combined CpG-B and GM-CSF group (see ). This would fit with reports that GM-CSF may initially enhance pDC-derived type-I IFN responses through up-regulation of IRF8, but at later time points would lead to down-regulation of IRF8 and so attenuate IFN responses.Citation27,Citation28 Analysis of changes in IRF8 transcript levels from t = 0 to t = 7 versus from t = 7 to t = 21 between the treatment groups would indeed seem to support this hypothesis (). Interestingly, these differential expression levels of IRF8 transcripts between CpG-B only and combined GM-CSF/CpG-B may also explain the observed effects on mMDSC levels (), as GM-CSF-induced down-regulation of IRF8 has been implicated in increased MDSC development.Citation29 Of note, although type-I IFN responses favor antitumor immunity and facilitate immune checkpoint blockade, chronic type-I IFN exposure can also have detrimental effects, up-regulating MDSC rates and PD-L1 expression and actually mediating resistance to certain cancer therapies, including immune checkpoint blockade.Citation26,Citation30 Thus, timely attenuation of IRGs by GM-CSF may actually be beneficial.
Conclusion
Although GM-CSF can have both adverse and favorable effects on parameters contributing to antitumor immunity, its clearly positive effects on LNR-cDC recruitment and activation and its apparent ability to secure LNR-cDC and pDC activation in women, warrants its further clinical exploration in combination with next-generation CpG oligodeoxynucleotides for the local treatment and conditioning of melanoma SLN in patients with early-stage melanoma. Certainly, the resulting significantly improved clinical outcome, with none of the fourteen GM-CSF-treated patients experiencing recurrences, seems to underline this notion.
Patients and methods
For patient characteristics, we refer to Vuylsteke et al.Citation9 and Sluijter et al.Citation10 Importantly, for both trials, the control groups and treatment groups were balanced in terms of sex, age, ulceration, Breslow thickness, and disease staging. For methods and statistics employed in the flow cytometry analyses, we refer to Sluijter et al.Citation10 and for methods and statistics employed for clinical follow-up analysis, we refer to Koster et al.Citation12 For methods and statistics employed in the flow cytometry analyses, we refer to Sluijter et al.Citation10 and for methods and statistics employed for clinical follow-up analysis, we refer to Koster et al.Citation12 The studies were approved by the Institutional Review Board of the VU University Medical Center, and written informed consent was obtained from each patient before treatment in accordance with the Declaration of Helsinki.
Type-I interferon (IFN) response analysis
PBMC were isolated and total RNA isolated and reverse transcribed as previously described.Citation10 Forty-seven type I IRGs were selected based on significant upregulation in more than 3 experiments published on the Interferome databaseCitation31 (http://www.interferome.org/). Custom-designed TaqMan®assays for each gene were supplied by Applied Biosystems. Quantitative PCR (qPCR) analysis was performed at ServiceXS (ServiceXS B.V., Leiden, The Netherlands) using the 96.96 BioMark™ Dynamic Array for Real-Time PCR (Fluidigm Corporation, San Francisco, CA, USA), according to the manufacturer’s instructions. Thermal cycling and real-time imaging of the BioMark array was done on the BioMark instrument, and cycle threshold (CT) values were extracted using the BioMark Real-Time PCR analysis software. Relative quantities were calculated using the standard curve method, using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a housekeeping gene. Expression levels were 2log-transformed. Cluster analysis was used for categorization of IRGs with respect to their relative expression between treatment arms.Citation32 TreeView was used to visualize the clustering of genes (Eisen Lab, Berkeley, CA, USA). Comparison of IRG expression between time points was assessed using paired t tests. The Benjamini-Hochberg procedure was applied to correct for multiple testing. Corrected P values of <0.05 were considered significant.
Disclosure of Potential Conflicts of Interest
BDK, AJMvdE, and TDdG have received financial research support from Idera Pharmaceuticals.
Acknowledgments
The authors would like to thank Pepijn Wijnands for excellent technical assistance. This work was supported by a grant from the Fritz Ahlquist Foundation.
Additional information
Funding
References
- Hoeller C, Michielin O, Ascierto PA, Szabo Z, Blank CU. Systematic review of the use of granulocyte-macrophage colony-stimulating factor in patients with advanced melanoma. Cancer Immunol Immunother. 2016;65(9):1015–5. doi:10.1007/s00262-016-1860-3.
- Parmiani G, Castelli C, Pilla L, Santinami M, Colombo M, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol. 2006;18(2):226–232. doi:10.1093/annonc/mdl158.
- Hodi FS, Lee S, McDermott DF, Rao UN, Butterfield LH, Tarhini AA, Leming P, Puzanov I, Shin D, Kirkwood JM. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma. JAMA. 2014;312(17):1744. doi:10.1001/jama.2014.13943.
- Luke JJ, Donahue H, Nishino M, Giobbie-Hurder A, Davis M, Bailey N, Ott PA, Hodi FS. Single institution experience of ipilimumab 3 mg/kg with sargramostim (GM-CSF) in metastatic melanoma. Cancer Immunol Res. 2015;3(9):986–991. doi:10.1158/2326-6066.CIR-15-0066.
- Kaufman HL, Ruby CE, Hughes T, Slingluff CL. Current status of granulocyte–macrophage colony-stimulating factor in the immunotherapy of melanoma. J Immunother Cancer. 2014;2(1):11. doi:10.1186/2051-1426-2-11.
- Franke V, Berger DMS, Klop WMC, Hiel B, Wiel BA, Meulen S, Wouters MWJM, Houdt WJ, Akkooi ACJ. High response rates for T‐VEC in early metastatic melanoma (stage IIIB/C‐IVM1a). Int J Cancer. 2019;145(4):974–978. doi:10.1002/ijc.32172.
- Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, Lazar AJ, Faries MB, Kirkwood JM, McArthur GA, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA. Cancer J Clin. 2017;67(6):472–492. doi:10.3322/caac.21409.
- Leiter U, Stadler R, Mauch C, Hohenberger W, Brockmeyer N, Berking C, Sunderkötter C, Kaatz M, Schulte K-W, Lehmann P, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17(6):757–767. doi:10.1016/S1470-2045(16)00141-8.
- Vuylsteke RJCLM, Molenkamp BG, Gietema HA, van Leeuwen PAM, Wijnands PGJTB, Vos W, van Diest PJ, Scheper RJ, Meijer S, de Gruijl TD. Local administration of granulocyte/macrophage colony-stimulating factor increases the number and activation state of dendritic cells in the sentinel lymph node of early-stage melanoma. Cancer Res. 2004;64(22):8456–8460. doi:10.1158/0008-5472.CAN-03-3251.
- Sluijter BJR, van den Hout MFCM, Koster BD, van Leeuwen PAM, Schneiders FL, van de Ven R, Molenkamp BG, Vosslamber S, Verweij CL, van den Tol MP, et al. Arming the melanoma sentinel lymph node through local administration of CpG-B and GM-CSF: recruitment and activation of BDCA3/CD141(+) dendritic cells and enhanced cross-presentation. Cancer Immunol Res. 2015;3(5):495–505. doi:10.1158/2326-6066.CIR-14-0165.
- Molenkamp BG, van Leeuwen PAM, Meijer S, Sluijter BJR, Wijnands PGJTB, Baars A, van den Eertwegh AJM, Scheper RJ, de Gruijl TD. Intradermal CpG-B activates both plasmacytoid and myeloid dendritic cells in the sentinel lymph node of melanoma patients. Clin Cancer Res. 2007;13(10):2961–2969. doi:10.1158/1078-0432.CCR-07-0050.
- Koster BD, Van Den Hout MFCM, Sluijter BJR, Molenkamp BG, Vuylsteke RJCLM, Baars A, Van Leeuwen PAM, Scheper RJ, Petrousjka van den Tol M, Van Den Eertwegh AJM, et al. Local adjuvant treatment with low-dose CpG-B offers durable protection against disease recurrence in clinical stage I–II melanoma: data from two randomized phase II trials. Clin Cancer Res. 2017;23(19):5679–5686. doi:10.1158/1078-0432.CCR-17-0944.
- van de Ven R, van den Hout MFCM, Lindenberg JJ, Sluijter BJR, van Leeuwen PAM, Lougheed SM, Meijer S, van den Tol MP, Scheper RJ, de Gruijl TD. Characterization of four conventional dendritic cell subsets in human skin-draining lymph nodes in relation to T-cell activation. Blood. 2011;118(9):2502–2510. doi:10.1182/blood-2011-03-344838.
- Molenkamp BG, Vuylsteke RJCLM, van Leeuwen PAM, Meijer S, Vos W, Wijnands PGJTB, Scheper RJ, de Gruijl TD. Matched skin and sentinel lymph node samples of melanoma patients reveal exclusive migration of mature dendritic cells. Am J Pathol. 2005;167(5):1301–1307. doi:10.1016/S0002-9440(10)61217-5.
- de Gruijl TD, Sombroek CC, Lougheed SM, Oosterhoff D, Buter J, van den Eertwegh AJM, Scheper RJ, Pinedo HM. A postmigrational switch among skin-derived dendritic cells to a macrophage-like phenotype is predetermined by the intracutaneous cytokine balance. J Immunol. 2006;176(12):7232–7242. doi:10.4049/jimmunol.176.12.7232.
- Trigunaite A, Dimo J. Suppressive effects of androgens on the immune system. Cell Immunol. 2015;294(2):87–94. doi:10.1016/J.CELLIMM.2015.02.004.
- Souyris M, Cenac C, Azar P, Daviaud D, Canivet A, Grunenwald S, Pienkowski C, Chaumeil J, Mejía JE, Guéry J-C. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol. 2018;3(19):eaap8855. doi:10.1126/sciimmunol.aap8855.
- Yuan Y, Liu L, Chen H, Wang Y, Xu Y, Mao H, Li J, Mills GB, Shu Y, Li L, et al. Comprehensive characterization of molecular differences in cancer between male and female patients. Cancer Cell. 2016;29(5):711–722. doi:10.1016/j.ccell.2016.04.001.
- Folkerd EJ, Dowsett M. Influence of sex hormones on cancer progression. J Clin Oncol. 2010;28(26):4038–4044. doi:10.1200/JCO.2009.27.4290.
- Traub S, Demaria O, Chasson L, Serra F, Desnues B, Alexopoulou L. Sex bias in susceptibility to MCMV infection: implication of TLR9. Peterson KE, editor. PLoS One. 2012;7(9):e45171. doi:10.1371/journal.pone.0045171.
- Surcel M, Constantin C, Caruntu C, Zurac S, Neagu M. Inflammatory cytokine pattern is sex-dependent in mouse cutaneous melanoma experimental model. J Immunol Res. 2017;2017:1–10. doi:10.1155/2017/9212134.
- Leonard D, Eloranta M-L, Hagberg N, Berggren O, Tandre K, Alm G, Rönnblom L. Activated T cells enhance interferon-α production by plasmacytoid dendritic cells stimulated with RNA-containing immune complexes. Ann Rheum Dis. 2016;75(9):1728–1734. doi:10.1136/annrheumdis-2015-208055.
- Taleb K, Auffray C, Villefroy P, Pereira A, Hosmalin A, Gaudry M, Le Bon A. Chronic Type I IFN is sufficient to promote immunosuppression through accumulation of myeloid-derived suppressor cells. J Immunol. 2017;198(3):1156–1163. doi:10.4049/jimmunol.1502638.
- Chen J, Deng C, Shi Q, Jiang J, Zhang Y, Shan W, Sun W. CpG oligodeoxynucleotide induces bone marrow precursor cells into myeloid-derived suppressor cells. Mol Med Rep. 2013;8(4):1149–1154. doi:10.3892/mmr.2013.1655.
- Zoglmeier C, Bauer H, Norenberg D, Wedekind G, Bittner P, Sandholzer N, Rapp M, Anz D, Endres S, Bourquin C. CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clin Cancer Res. 2011;17(7):1765–1775. doi:10.1158/1078-0432.CCR-10-2672.
- Budhwani M, Mazzieri R, Dolcetti R. Plasticity of Type I interferon-mediated responses in cancer therapy: from anti-tumor immunity to resistance. Front Oncol. 2018;8:322. doi:10.3389/fonc.2018.00322.
- Pelka K, Latz E. IRF5, IRF8, and IRF7 in human pDCs - the good, the bad, and the insignificant? Eur J Immunol. 2013;43(7):1693–1697. doi:10.1002/eji.201343739.
- Sichien D, Scott CL, Martens L, Vanderkerken M, Van Gassen S, Plantinga M, Joeris T, De Prijck S, Vanhoutte L, Vanheerswynghels M, et al. IRF8 transcription factor controls survival and function of terminally differentiated conventional and plasmacytoid dendritic cells, respectively. Immunity. 2016;45(3):626–640. doi:10.1016/j.immuni.2016.08.013.
- Waight JD, Netherby C, Hensen ML, Miller A, Hu Q, Liu S, Bogner PN, Farren MR, Lee KP, Liu K, et al. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest. 2013;123(10):4464–4478. doi:10.1172/JCI68189.
- Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint Victor C, Cucolo L, Lee DSM, Pauken KE, Huang AC, et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell. 2016;167(6):1540–1554.e12. doi:10.1016/j.cell.2016.11.022.
- Rusinova I, Forster S, Yu S, Kannan A, Masse M, Cumming H, Chapman R, Hertzog PJ. Interferome v2.0: an updated database of annotated interferon-regulated genes. Nucleic Acids Res. 2013;41(Database issue):D1040–D1046. doi:10.1093/nar/gks1215.
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci. 1998;95(25). doi:10.1073/pnas.95.25.14863.
