ABSTRACT
Lactate dehydrogenase (LDH) levels are inversely related with response to checkpoint inhibitors. Elevated LDH levels are the product of enhanced glycolytic activity of the tumor and tumor necrosis due to hypoxia, the latter being associated with high tumor burden. In this review, we elucidate the effects of glycolysis and hypoxia on antitumor immunity and set forth ways to improve response to immunotherapy in cancer patients with elevated LDH levels. We discuss the current knowledge on combining immunotherapy with glycolysis inhibitors, anti-acidifying drugs, anti-angiogenic or cytoreductive therapy.
Introduction
In the last decade, immune checkpoint inhibitors have revolutionized cancer treatment. In 2011, ipilimumab, an antibody that inhibits cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), was the first checkpoint inhibitor to receive market approval. Subsequently, antibodies directed against programmed cell death protein-1 (PD-1; nivolumab, pembrolizumab and cemiplimab) and its ligand (PD-L1; atezolizumab, durvalumab and avelumab) came available. The use of checkpoint inhibitors is expanding rapidly. A key benefit of checkpoint inhibitors is that they are able to induce durable responses, which are often maintained after treatment discontinuation.Citation1 This suggests the development of an immunological memory. Unfortunately, only a minority of patients responds.
Elevated lactate dehydrogenase (LDH) levels are associated with poor outcomes in cancer patients. The prognostic value of LDH is most extensively studied in melanoma, where it is incorporated in tumor staging.Citation2 Yet, an association between LDH levels and survival was also found in many other tumor types.Citation3 Additionally, patients with elevated LDH levels seem to benefit less from checkpoint inhibitors as compared to patients with normal LDH levels. Approximately 40% of patients with metastatic melanoma present with LDH levels above the upper limit of normal (ULN).Citation2 Although checkpoint inhibitors are superior to chemotherapy in melanoma patients with elevated LDH levels,Citation4 treatment outcomes following immunotherapy are poor compared to patients with normal LDH levels. Objective response rates (ORR) for the combination of ipilimumab and nivolumab are respectively 44.7% and 37.8% versus 65.3% (LDH 1-2xULN and LDH ≥2xULN versus LDH≤ULN). Progression-free (PFS) and overall survival (OS) are also shorter, with 39% and 28% versus 61% alive after four years.Citation1,Citation5 An extensive overview of outcomes following immunotherapy in melanoma patients with elevated LDH levels was given in a recent meta-analysis.Citation2 Clinical studies on checkpoint inhibitors in other malignancies less commonly report on the outcomes of patients with elevated LDH levels. However, retrospective data support a relationship between LDH levels and clinical outcome following immunotherapy in other tumor types ().Citation6–Citation11
Table 1. Retrospective data on the association between serum LDH levels and outcomes following checkpoint inhibition in other cancer types than melanoma
In this review, we describe mechanisms that may result in elevated LDH levels in cancer patients. Elevated LDH levels are the product of enhanced glycolytic activity of the tumor and tumor necrosis due to hypoxia, the latter being associated with high tumor burden. Additionally, we elucidate the effects of enhanced glycolysis and hypoxia on antitumor immunity and discuss ways to improve response to checkpoint inhibitors in patients with elevated LDH levels. We provide an overview of available evidence in various tumor types. However, most literature on this subject currently focuses on melanoma.
The relationship between LDH levels and tumor burden, glycolytic activity and tumor necrosis
LDH and tumor burden
Elevated serum LDH levels have traditionally been regarded as a marker of high tumor burden, which is a poor prognostic factor in cancer.Citation12 In a recent post-hoc analysis of the KEYNOTE-001, patients with elevated baseline LDH levels had higher tumor burden as compared to patients with normal LDH levels (sum of target lesions 17.3 cm and 6.2 cm, respectively). However, in 27% of patients with elevated LDH levels, tumor burden was below median. In multivariate analyses, LDH levels and tumor burden were independently associated with OS of pembrolizumab-treated patients.Citation13 Others reported a weak to moderate correlation between LDH levels and tumor burden in melanoma (r = 0.36; p-value n/a),Citation14 colorectal cancer (r = 0.52; p < 0,0001)Citation15 and various tumor types (r = 0.49; p < 0,01).Citation16 This suggests that the prognostic attributes of elevated LDH levels encompass more than tumor size alone.
LDH and glycolysis
The enzyme LDH is a major player in glucose metabolism. It is found in all human cells and catalyzes the conversion of pyruvate, which is the end product of glycolysis, to lactate and vice versa. Under aerobic conditions, normal cells transport pyruvate into their mitochondria where it enters the tricarboxylic acid (TCA) cycle and is degraded to CO2 and H2O. In the TCA cycle, NADH is produced, which is reoxidized in the oxidative phosphorylation, producing energy in the form of ATP. In the overall process, metabolism of a single molecule of glucose produces up to 36 molecules of ATP. In hypoxia, pyruvate is converted into lactate by the enzyme LDH, a process known as anaerobic glycolysis, and only 2 molecules of ATP are formed.
In malignant tumors, commonly a shift in glucose metabolism is seen, a phenomenon known as aerobic glycolysis or the Warburg effect (). Cancer cells predominantly process glucose via the glycolytic pathway, regardless of oxygen availability. A major regulator of glycolytic activity in tumors is the transcription factor hypoxia-inducible factor-1 (HIF-1).Citation17 Despite its low energy yield, the high rate of glycolysis is considered advantageous to highly proliferative cancer cells. Due to the metabolic shift, tumors are less dependent on oxygen availability. Moreover, the increased glycolytic flux leads to the synthesis of substrates for cell membranes, nucleic acids and proteins, which are needed for cancer cell proliferation. Additionally, NADPH is produced, which is essential for the control of redox potential.Citation18
Figure 1. Glucose metabolism in cancer
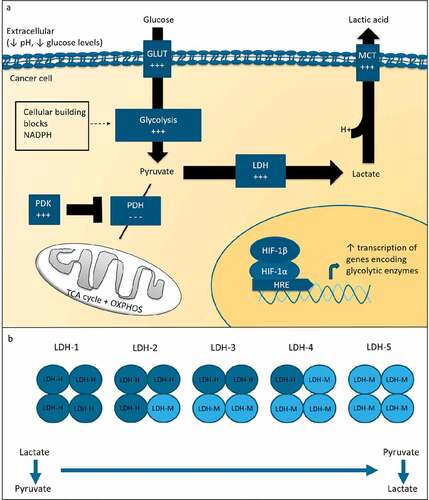
LDH plays a major role in aerobic glycolysis. LDH is a tetrameric molecule composed of LDH-M and LDH-H subunits, which are encoded by the LDH-A and LDH-B gene, respectively. Five isoforms exist. Isoforms consisting predominantly of LDH-M, i.e. LDH-5, preferentially convert pyruvate to lactate, whereas isoforms consisting predominantly of LDH-H preferentially catalyze the reverse reaction (). In serum, LDH isotyping is not regularly performed. Studies evaluating tumor LDH expression, however, commonly analyze LDH-5 protein or LDH-A gene expression. LDH-5 expression is increased in cancer cells as compared to healthy tissue.Citation19 High tumor LDH-5 expression is indicative of a poor prognosis among different tumor types.Citation20
As LDH is a cytosolic enzyme, which only enters the blood stream when the cell membrane is damaged, it is questionable whether serum LDH levels reliably reflect tumor LDH expression. Data on the correlation between serum LDH levels and tumor LDH expression are limited. In a breast cancer study, tumor LDH-A expression was not consistent with serum LDH levels.Citation21 However, high tumor LDH-5 expression was associated with high serum LDH levels in non-small cell lung cancer (NSCLC), but only in patients with tumors greater than 3 cm.Citation22 A recent study in melanoma patients showed that high glucose uptake on FDG-PET was associated, but did not fully coincide, with elevated serum LDH levels.Citation23
LDH and tumor necrosis
Serum LDH is considered an indicator of cell injury and necrosis. Tumor necrosis is thought to result from nutrient and oxygen deprivation, which is caused by an insufficient blood supply in relation to the nutrient and oxygen consumption needed to maintain high tumor cell proliferation. Studies, indeed, show that the expression of hypoxia markers (GLUT1, CAIX) and hypoxia-related genes is higher in tumors with necrotic fractions.Citation24,Citation25
Tumor necrosis may result from rapid tumor growth, poor vascularization or a combination of both. Accordingly, in some studies an association between proliferation rate and tumor necrosis was identified,Citation26,Citation27 whereas other studies could not confirm any association.Citation25 Likewise, many,Citation27,Citation28 but not all studiesCitation29 described a positive correlation between tumor vascularization and necrosis. Hypoxia and necrosis are known to stimulate the production of pro-angiogenic factors such as vascular endothelial growth factor (VEGF), thereby promoting microvessel formation.Citation27 However, the imbalance between pro- and anti-angiogenic factors commonly leads to a highly disorganized and dysfunctional tumor vasculature.Citation30 Therefore, angiogenesis does not necessarily improve blood perfusion in all tumor regions.
Although hypoxia-driven necrosis might occur in patients with limited disease, the prevalence of necrosis is higher in larger tumors, explaining the high LDH levels in patients with high tumor burden.Citation31,Citation32
Immune suppressive effects of glucose deprivation, tumor acidity and hypoxia
As mentioned above, elevated LDH levels are the product of enhanced glycolytic activity of the tumor and tumor necrosis due to hypoxia. In tumors with enhanced glycolytic activity, either aerobic glycolysis or anaerobic glycolysis in case of hypoxia, immune cell function might be hampered by glucose deprivation or tumor acidity. In addition, hypoxia itself, or the overexpression of hypoxia-regulating factors in tumors with high glycolytic activity, might influence antitumor immunity ().
Figure 2. Immune suppressive effects of glucose deprivation, tumor acidity and hypoxia
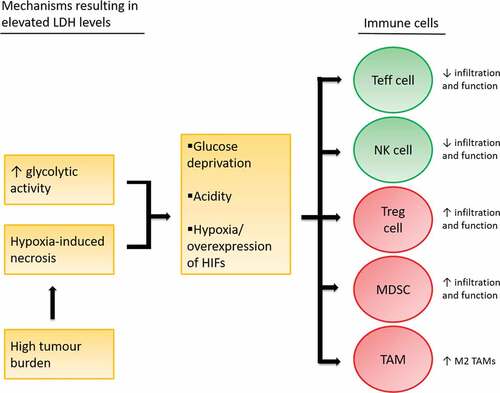
Glucose deprivation
T cells are key players in the antitumor immunity due to their ability to selectively recognize and kill cancer cells. Like cancer cells, effector T cells highly depend on aerobic glycolysis for their function. Aerobic glycolysis in T cells is regulated by the enzyme GAPDH. Besides its metabolic function, GAPDH acts as a regulator of mRNA translation. IFNΥ is a cytokine that plays a central role in antitumor immunity. When T cells are glucose-restricted, GAPDH becomes available to bind IFNΥ mRNA, preventing its translation.Citation33 Chang and colleaguesCitation34 showed that high glucose consumption by tumor cells, restricts murine T cell function in vitro. Glucose restriction led to dampened glycolytic activity in T cells and decreased IFNΥ production. Adding glucose, restored IFNΥ production in a dose-dependent manner. Glucose concentrations in human melanomas were found to be significantly lower than in healthy tissue.Citation35 Although the link between T cell metabolism and effector functions is well established in murine cells, the importance of glycolysis for the effector functions of human T cells is less clear. In vitro studies with human T cells showed that glucose deprivation reduced proliferation, but had no impact on IFNΥ production.Citation36
Regulatory T cells are a subset of T cells with immunosuppressive functions. In contrast to effector T cells, regulatory T cells are less dependent on glycolysis for their energy production, allowing them a metabolic advantage in glucose-deprived environments compared to effector T cells.Citation37
Tumor acidity
In normoxic conditions, tumor cells convert 60 to 80% of glucose to lactate. This is enhanced up to 90% in hypoxia.Citation38 Lactate is secreted from tumor cells along with a proton, together called lactic acid, leading to acidification of the tumor microenvironment. Tumor LDH-A expression correlates well with the presence of lactate.Citation39 Moreover, a significant inverse correlation was found between 18 F-FDG uptake in tumor lesions on PET imaging and tumor pH as assessed by MRI-CEST, confirming that glycolysis is an important contributor to tumor acidity.Citation40
Studies show that acidity influences immune cell function. Brand and colleaguesCitation35 studied the effect of lactic acid on T cells in melanoma. In immunocompetent mice, knockdown of LDH-A increased the number of tumor-infiltrating T and natural killer (NK) cells and reduced tumor growth. In immune compromised mice lacking T and NK cells, on the other hand, knockdown of LDH-A had no impact on tumor growth. When incubating CD8+ T cells with labeled lactic acid, intracellular accumulation of labeled and unlabeled lactate was seen together with a decrease in ATP production.Citation35,Citation41 Taken together, this data indicate that tumor-derived lactic acid can suppress T cells by blocking lactate export. Accordingly, in patients with metastatic melanoma and NSCLC, the expression of LDH-A and other glycolysis-related genes negatively correlates with T cell infiltration.Citation35,Citation42
In contrast, the addition of lactate does not affect the suppressive functions of regulatory T cells in vitro, and may even lead to an increase in regulatory T cells.Citation37 Lactate concentrations also affect other immune suppressive cells. Myeloid-derived suppressor cells (MDSCs) are immature myeloid cells that have the ability to potently suppress T cell activity. Tumor-derived lactate increases the number of infiltrating MDSCs.Citation43 Additionally, lactate polarizes tumor-associated macrophages (TAMs), which can have pro-inflammatory (M1) or immune suppressive (M2) phenotypes, into M2 macrophages.Citation44
Hypoxia
In addition to glucose deprivation and acidity, hypoxia also influences immune cells, mainly via HIF-1. Facciabene and colleaguesCitation45 showed that high expression of HIF-1α in cancer cells, the alpha subunit of HIF-1, promotes the recruitment of regulatory T cells via an increased production of the chemokine CCL-28. In hypoxia, T cells themselves also increase HIF-1α levels upon T cell receptor engagement. HIF-1α induces the expression of Foxp3 in T cells, thereby promoting the differentiation toward regulatory T cell.Citation46 The impact of hypoxia on effector T cells is less clear. Notably, Doedens and colleaguesCitation47 showed that HIFs augmented effector T cell function in the context of antigen persistence, indicating that effector T cells might have better antitumor activity in hypoxic conditions. On the other hand, hypoxia induces the expression of CD39 and CD73 on tumor cells and immune cells,Citation48 enzymes involved in the conversion of ATP or ADP into adenosine. Adenosine is an important suppressor of NK and effector T cell function. Besides its effects on T cells, hypoxia also increases the infiltration of TAMs, via chemokines such as VEGF, and supports their polarization into M2 macrophages.Citation49 In addition, hypoxia influences the differentiation and function of MDSCs.Citation50
For a comprehensive overview of the impact of acidity and hypoxia on the various immune cell populations we refer to two recent reviews.Citation17,Citation51 In conclusion, glucose deprivation, acidity and hypoxia all contribute to an immunosuppressive tumor microenvironment. It is likely that checkpoint inhibitors are less effective in this setting. Indeed, previous studies indicate that a decreased ratio of cytotoxic to regulatory T cells and high MDSC counts are associated with poor outcomes following checkpoint inhibition.Citation52–Citation54
Serum LDH: possible roles in treatment stratification and monitoring
LDH isotyping
The effect of tumor metabolism on antitumor immunity is now well-recognized. Targeting tumor metabolism might be an effective strategy to optimize response to immunotherapy in patients with tumors that exhibit high glycolytic activity. Since distinct mechanisms may lead to elevated LDH levels, it appears interesting to study the distribution of LDH isoforms in serum of cancer patients. This might provide additional information on the glycolytic activity of the tumor. Studies reporting serum LDH isoenzyme levels in relation to tumor LDH expression are lacking. However, a previous study did demonstrate that serum LDH-5 levels were elevated in many cancer patients, including patients with normal total serum LDH levels.Citation55
On-treatment LDH levels
Not only baseline LDH levels, but also the changes in LDH levels during the first weeks of checkpoint inhibition appear to relate with treatment outcomes.Citation56–Citation58 A retrospective study in 238 melanoma patients showed that patients responding to pembrolizumab had a marked reduction in LDH levels after 6 weeks of treatment (median: −15.6%; ICR: −23.1% to −1.3%), whereas LDH levels increased in patients with progressive disease (median: + 6.2%, ICR: −12.8% to +44.5%) (p = .0088). Increases in LDH levels of 25% or more were strongly associated with a detrimental OS (HR 10.75; 95% CI 4.62–25.02). In patients treated with anti-PD-1 plus anti-CTLA-4, on-treatment changes in LDH levels also significantly differed between responders (median: +3.2%, IQR −15.3% to +25.4%) and patients with progressive disease (median: +14.2%, IQR: −15.3% to +25.4%)(p = .036). However, the differences were less pronounced, possibly due to a high incidence of immune-related adverse events in these patients, which can also elevate LDH levels.Citation57 At the Radboudumc, we assessed the changes in LDH levels in 58 bladder cancer patients treated with anti-PD-(L)1. Here, we also identified a decline in LDH levels in responding patients after two cycles (median: −10,9%, ICR: −21,4% to +1,1%), whereas LDH levels increased in non-responders (median: +5,1%, ICR: −2,9% to +18,0%) (p = 0,003) [unpublished data]. As LDH levels are associated with tumor burden, it is possible that the changes in LDH levels merely reflect increases or decreases in tumor burden. However, serum LDH levels are easy to obtain and changes in LDH levels seem to have predictive value as early as 6 weeks into the course of checkpoint inhibitor treatment whereas imaging is usually not performed until 9 to 12 weeks after treatment initiation. Therefore, early on-treatment measurement of serum LDH levels might be useful in clinical practice. These findings warrant further investigation.
How to improve response to immunotherapy in patients with elevated LDH levels?
Given the poor clinical outcomes following immunotherapy in patients with elevated LDH levels, new treatment strategies are urgently needed. Below the rationale and clinical evidence for several combination therapies are reviewed, including the combination of checkpoint inhibitors with glycolysis inhibitors, anti-acidity interventions, VEGF inhibitors and cytoreductive therapies.
Combining checkpoint inhibitors with glycolysis inhibitors
As serum LDH levels seem to partially reflect the glycolytic activity of the tumor, patients with elevated LDH levels might benefit from a combination of checkpoint inhibitors and glycolysis inhibitors. Altered tumor metabolism is increasingly being recognized as an important hallmark of cancer,Citation59 leading to renewed interest in therapeutic strategies that target glycolysis. Although glycolysis inhibitors have not yet been approved in clinical practice, several glycolysis inhibitors have been developed and are currently being evaluated in preclinical and early clinical trials.Citation60,Citation61
Given the negative effects of glycolysis on antitumor immunity, there is a clear rationale for combining checkpoint inhibitors with glycolysis inhibitors. Yet, there are concerns regarding the effect of glycolysis inhibitors on T cells, because T cells depend on glycolysis for their function. Unfortunately, there are no clinical data available on the efficacy of treatment strategies that combine glycolysis inhibitors and checkpoint inhibitors. However, the widely used non-steroidal anti–inflammatory drug diclofenac, which functions as an inhibitor of glycolysis,Citation62 was found to have a positive effect on response to checkpoint inhibitors in mice.Citation51 Future studies should assess the added value of glycolysis inhibitors to checkpoint inhibitor therapy and investigate whether such combination treatments are beneficial for patients with elevated LDH levels.
Combining checkpoint inhibitors with anti-acidifying drugs
Considering the negative effect of acidity on antitumor immunity, another strategy to improve response to immunotherapy in patients with elevated LDH levels might be to combine immunotherapy with anti-acidifying drugs. One possible approach would be to block the export of protons by tumor cells. The export of lactic acid by tumor cells occurs mainly via monocarboxylate transporters (MCTs). MCT inhibitors are currently being tested in phase I clinical trials (NCT01791595). Next to MCTs, there are a number of other transporters that transfer protons out of the tumor cell, such as the vacuolar-type H+-ATPases (V-ATPases). V-ATPases can be blocked by proton pump inhibitors (PPIs), which are widely used in clinical practice for gastric protection. In mouse studies, PPIs were shown to increase tumor pH. The addition of PPIs to adoptive T cell transfer in mice, resulted in an increased number of infiltrating CD44+CD8+IFNγ+ T cells and increased therapeutic efficacy.Citation63 Surprisingly, a retrospective analysis on data of the Checkmate 069 showed that the ORR in melanoma patients treated with ipilimumab plus nivolumab almost halved in patients on PPIs.Citation64 The relation between PPI use and response to checkpoint inhibitors needs further investigation. It is possible that factors other than tumor acidity are responsible for the poor outcomes in patients on PPIs. For example, modulation of the gut microbiome by PPIs might contribute to decreased efficacy of checkpoint inhibitors.Citation65,Citation66 Another possible approach to target tumor acidity is via systemic buffering. In mice, combining anti-PD-1 with bicarbonate therapy significantly reduced tumor size and weight compared to anti-PD-1 monotherapy.Citation67 It is thus far unclear whether such strategies can also be used to improve response to immunotherapy in humans, in particular in patients with elevated LDH levels.
Combining checkpoint inhibitors with VEGF inhibitors
Patients with elevated LDH levels not only benefit less from immunotherapy, but also from many other anticancer therapies such as chemotherapy and targeted therapy.Citation1,Citation68 However, previous studies suggest that patients with high LDH levels benefit more from VEGF (receptor) inhibitors, such as vatalanibCitation69 and bevacizumab,Citation70,Citation71 than patients with normal LDH levels. Two large, randomized controlled trials studied the efficacy of chemotherapy (FOLFOX) plus vatalanib versus FOLFOX alone in patients with colorectal carcinoma. Patients were randomized stratified according to baseline LDH levels (≤ or >1.5xULN). In the overall population, the addition of vatalanib exerted only moderate effects on PFS (HR 0.85, p = .005), whereas a major improvement was seen in patients with high LDH levels (HR 0.65, p < .001).Citation69 It is not surprising that patients with elevated LDH levels benefit most from anti–VEGF therapy, since both glycolysis and hypoxia are associated with active angiogenesis.Citation72,Citation73 Moreover, previous studies found an association between high serum LDH levels and VEGF (receptor) overexpression in various tumors.Citation74
Originally, anti-angiogenic therapies were developed to inhibit angiogenesis and induce tumor cell starvation. However, appropriately dosed anti-angiogenic therapy rather seems to normalize tumor vasculature, thereby temporarily improving tumor oxygenation.Citation75 As a result, anti-angiogenic therapy may reverse the immune suppressive effects of hypoxia. VEGF (receptor) inhibition, indeed, resulted in reduced regulatory T cell and MDSC recruitment to the tumor site and reduced the immune suppressive capacity of MDSCs and macrophages.Citation76,Citation77 Although anti-angiogenic therapy may have a temporarily beneficial effect on antitumor immunity, persistent inhibition of angiogenesis may ultimately increase hypoxia, and consequently hinder effective checkpoint inhibitor therapy.Citation78
Previous studies demonstrated that high pre-treatment levels of VEGF were associated with decreased OS in melanoma patients who were treated with ipilimumab.Citation79 Phase I trials in metastatic melanoma showed promising results for the combination of checkpoint inhibitors and VEGF inhibitors. The combination of ipilimumab and VEGF inhibitor bevacizumab induced partial responses in 17.4% of patients, and disease control in 67.4%.Citation80 In mucosal melanoma, a subtype that usually responds poorly to anti-PD-1, the combination of anti-PD-1 and VEGF inhibitors induced an objective response in 48.3% of patients.Citation81 The results of a randomized phase II study, including 168 melanoma patients, are expected at the end of 2019 (NCT01950390). In metastatic renal cell carcinoma (RCC), VEGF (receptor) inhibitors like bevacizumab and sunitinib are widely used. In 2018, the results of a phase II study, comparing atezolizumab, sunitinib and atezolizumab plus bevacizumab in patients with RCC, were published. ORRs were 25%, 29% and 32%, respectively. Median PFS was 6.1 months (95% CI 5.4–13.6), 8.4 months (95% CI 7.0–14.0) and 11.7 months (95% CI 8.4–17.3).Citation82 Additionally, two recent phase III trials showed that that the combination of anti-PD-(L)1 and VEGF inhibitors is superior to anti–VEGF monotherapy in RCC.Citation83,Citation84 The combination of anti-angiogenic therapy and checkpoint inhibitors is currently also being studied in many other tumor types.Citation75 Previous studies on the combination of VEGF inhibitors and checkpoint inhibitors did not report on LDH levels in relation to response. It appears relevant to specifically study the combination of anti-angiogenic agents and checkpoint inhibitors in patients with elevated LDH levels, considering their responses to anti-angiogenic therapy and the association between serum LDH levels and tumor VEGF (receptor) expression.
Combining checkpoint inhibitors with cytoreductive therapy
Considering the association between tumor burden and serum LDH levels, another possible treatment approach would be to reduce tumor burden prior to initiation of checkpoint inhibition. Previous studies describe a negative correlation between baseline tumor size and clinical outcomes following checkpoint inhibition in melanoma and NSCLC.Citation13,Citation85 In urothelial cancer, there are also indications for an association between tumor burden and response to immunotherapy, with much higher response rates to pembrolizumab in patients with metastatic disease limited to the lymph nodes compared to patients with visceral metastases (47% vs 23%).Citation86
As described above, elevated LDH levels in patients with large tumor burden are a result of hypoxia-induced necrosis. Hypoxia negatively influences antitumor immunity. Cytoreduction, either by surgery or systemic therapy, might induce a more permissive tumor microenvironment, thereby possibly enhancing checkpoint inhibitor efficacy. Several studies describe a correlation between tumor size and tumor-infiltrating lymphocytes, with lower numbers of effector T cells in larger tumors.Citation87–Citation90
In urothelial cancer, checkpoint inhibitors are registered as first-line treatment for patients who are cisplatin-ineligible and have high tumor PD-L1 expression, and as second-line treatment for patients who progressed on chemotherapy. A large, phase III trial is now investigating the role of avelumab as maintenance treatment following completion of first-line chemotherapy in urothelial cancer (NCT02603432). This trial will hopefully give more insight in the efficacy of checkpoint inhibitors following cytoreductive chemotherapy. Not only systemic therapy, but also surgery can be used to reduce tumor burden. A phase I trial in RCC showed promising effects of cytoreductive surgery in combination with checkpoint inhibitor therapy.Citation91
We are currently conducting a phase II trial in patients with metastatic melanoma investigating the role of cytoreductive therapy prior to checkpoint inhibition in patients with elevated LDH levels. Combined BRAF and MEK inhibition is a first-line treatment option for patients with a BRAF-mutant advanced melanoma, a mutation present in approximately 50% of melanomas. Although there is an evident association between elevated LDH levels and a reduced survival in melanoma patients treated with BRAF and MEK inhibitors, the treatment is able to induce at least a short-term response in most patients with elevated LDH levels.Citation68 BRAF and MEK inhibitors have been shown to decrease glycolytic activity in BRAF-mutated melanomaCitation92 and to normalize LDH levels.Citation93 Our data from patients with elevated baseline LDH levels indicate that 74% of patients attain LDH normalization within 8 weeks, with a median time to LDH normalization of 25 days[unpublished data]. In addition, BRAF and MEK inhibitors induce a more permissive microenvironment with an increase in tumor-infiltrating effector T cells and increased antigen expression,Citation94 indicating that the treatment is also able to enhance antitumor immunity. In previous studies, the combination of BRAF and MEK inhibitors with ipilimumab caused severe toxicities.Citation95 Sequential administration of both treatment modalities, however, seems to be safeCitation96[own unpublished data]. A short, 6-week induction treatment with combined BRAF and MEK inhibition will normalize LDH levels and reduce tumor burden in most patients and may therefore improve response to immunotherapy. To test this hypothesis, we are currently conducting a phase II, randomized controlled trial in patients with advanced melanoma to investigate whether a 6-week induction treatment with combined BRAF and MEK inhibition increases response rates to combination therapy with ipilimumab and nivolumab in patients with elevated LDH levels (NCT02968303).
Conclusion
Patients with elevated LDH levels benefit less from immunotherapy. As reviewed in this paper, elevated LDH levels are the result of increased glycolytic activity of the tumor and tumor necrosis due to hypoxia, the latter being associated with high tumor burden. Both glycolysis and hypoxia contribute to an immune suppressive microenvironment. Serum LDH isotyping may prove an easily available and noninvasive approach to gain additional information on the tumor metabolic state, and may help identifying patients that benefit from glycolysis inhibitors and/or anti-acidity interventions. Other promising treatment strategies for patients with elevated LDH levels might be to combine checkpoint inhibition with VEGF inhibitors or cytoreductive therapies. In BRAF-mutated melanoma the efficacy of a 6-week induction treatment with combined BRAF and MEK inhibition prior to immunotherapy is currently being investigated. Further research is needed to optimize treatment outcomes in cancer patients with high LDH levels.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
References
- Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob -J-J, Rutkowski P, Cowey CL, Lao CD, Schadendorf D, Wagstaff J, Dummer R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480–11. doi:10.1016/S1470-2045(18)30700-9.
- Petrelli F, Ardito R, Merelli B, Lonati V, Cabiddu M, Seghezzi S, Barni S, Ghidini A. Prognostic and predictive role of elevated lactate dehydrogenase in patients with melanoma treated with immunotherapy and BRAF inhibitors: a systematic review and meta-analysis. Melanoma Res. 2019;29:1–12. doi:10.1097/CMR.0000000000000520.
- Petrelli F, Cabiddu M, Coinu A, Borgonovo K, Ghilardi M, Lonati V, Barni S. Prognostic role of lactate dehydrogenase in solid tumors: A systematic review and meta-analysis of 76 studies. Acta Oncol (Madr). 2015;54:961–970. doi:10.3109/0284186X.2015.1043026.
- Larkin J, Minor D, D’Angelo S, Neyns B, Smylie M, Miller WH, Gutzmer R, Linette G, Chmielowski B, Lao CD, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in checkMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol. 2018;36:383–390. doi:10.1200/JCO.2016.71.8023.
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Hogg D, Hill A, Carlino MS, et al. 3303 efficacy and safety in key patient subgroups of nivolumab (NIVO) alone or combined with ipilimumab (IPI) versus IPI alone in treatment-naïve patients with advanced melanoma (MEL) (CheckMate 067). Eur J Cancer. 2015;51:S664–S665. doi:10.1016/S0959-8049(16)31822-6.
- Taniguchi Y, Tamiya A, Isa S-I, Nakahama K, Okishio K, Shiroyama T, Suzuki H, Inoue T, Tamiya M, Hirashima T, et al. Predictive factors for poor progression-free survival in patients with non-small cell lung cancer treated with nivolumab. Anticancer Res. 2017;37:5857–5862.
- Inomata M, Hirai T, Seto Z, Tokui K, Taka C, Okazawa S, Kambara K, Ichikawa T, Imanishi S, Yamada T, et al. Clinical parameters for predicting the survival in patients with squamous and non-squamous-cell NSCLC receiving PD-1 inhibitor therapy. Pathol Oncol Res. 2018;474:569–575.
- Oya Y, Yoshida T, Kuroda H, Mikubo M, Kondo C, Shimizu J, Horio Y, Sakao Y, Hida T, Yatabe Y. Predictive clinical parameters for the response of nivolumab in pretreated advanced non-small-cell lung cancer. Oncotarget. 2017;8:103117–103128.
- Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, Ponce S, Ares LP, Leroy L, Audigier-Valette C, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4:351–357.
- Bigot F, Castanon E, Baldini C, Hollebecque A, Carmona A, Postel-Vinay S, Angevin E, Armand J-P, Ribrag V, Aspeslagh S, et al. Prospective validation of a prognostic score for patients in immunotherapy phase I trials: the Gustave Roussy Immune Score (GRIm-Score). Eur J Cancer. 2017;84:212–218. doi:10.1016/j.ejca.2017.07.027.
- Cona MS, Lecchi M, Cresta S, Damian S, Del Vecchio M, Necchi A, Poggi MM, Raggi D, Randon G, Ratta R, et al. Combination of Baseline LDH, performance status and age as integrated algorithm to identify solid tumor patients with higher probability of response to anti PD-1 and PD-L1 monoclonal antibodies. Cancers (Basel). 2019;11:223. doi:10.3390/cancers11020223.
- Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545:60–65. doi:10.1038/nature22079.
- Joseph RW, Elassaiss-Schaap J, Kefford R, Hwu W-J, Wolchok JD, Joshua AM, Ribas A, Hodi FS, Hamid O, Robert C, et al. Baseline tumor size is an independent prognostic factor for overall survival in patients with melanoma treated with pembrolizumab. Clin Cancer Res. 2018;24:4960–4967. doi:10.1158/1078-0432.CCR-18-3340.
- Agarwala SS, Keilholz U, Gilles E, Bedikian AY, Wu J, Kay R, Stein CA, Itri LM, Suciu S, Eggermont AMM. LDH correlation with survival in advanced melanoma from two large, randomised trials (Oblimersen GM301 and EORTC 18951). Eur J Cancer. 2009;45:1807–1814. doi:10.1016/j.ejca.2009.04.016.
- Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Trarbach T, Folprecht G, Shi MM, Lebwohl D, Jalava T, Laurent D, et al. Prognostic and predictive role of lactate dehydrogenase 5 expression in colorectal cancer patients treated with PTK787/ZK 222584 (Vatalanib) antiangiogenic therapy. Clin Cancer Res. 2011;17:4892–4900. doi:10.1158/1078-0432.CCR-10-2918.
- Dercle L, Ammari S, Champiat S, Massard C, Ferté C, Taihi L, Seban R-D, Aspeslagh S, Mahjoubi L, Kamsu-Kom N, et al. Rapid and objective CT scan prognostic scoring identifies metastatic patients with long-term clinical benefit on anti-PD-1/-L1 therapy. Eur J Cancer. 2016;65:33–42. doi:10.1016/j.ejca.2016.05.031.
- Labiano S, Palazon A, Melero I. Immune response regulation in the tumor microenvironment by hypoxia. Semin Oncol. 2015;42:378–386. doi:10.1053/j.seminoncol.2015.02.009.
- Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science (80-). 2010;330:1340–1344. doi:10.1126/science.1193494.
- Kayser G, Kassem A, Sienel W, Schulte-Uentrop L, Mattern D, Aumann K, Stickeler E, Werner M, Passlick B, zur Hausen A. lactate-dehydrogenase 5 is overexpressed in non-small cell lung cancer and correlates with the expression of the transketolase-like protein 1. Diagn Pathol. 2010;5:22. doi:10.1186/1746-1596-5-22.
- Koukourakis MI, Giatromanolaki A, Sivridis E, Bougioukas G, Didilis V, Gatter KC, Harris AL. Tumour and angiogenesis research group: lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer. 2003;89:877–885. doi:10.1038/sj.bjc.6601205.
- Dong T, Liu Z, Xuan Q, Wang Z, Ma W, Zhang Q. Tumor LDH-A expression and serum LDH status are two metabolic predictors for triple negative breast cancer brain metastasis. Sci Rep. 2017;7:6069. doi:10.1038/s41598-017-06378-7.
- Danner BC, Didilis VN, Wiemeyer S, Stojanovic T, Kitz J, Emmert A, Füzesi L, Schöndube FA. Long-term survival is linked to serum LDH and partly to tumour LDH-5 in NSCLC. Anticancer Res. 2010;30:1347–1351.
- de Heer EC, Brouwers AH, Boellaard R, Sluiter WJ, Diercks GFH, Hospers GAP, de Vries EGE, Jalving M. Mapping heterogeneity in glucose uptake in metastatic melanoma using quantitative 18F-FDG PET/CT analysis. EJNMMI Res. 2018;8:101. doi:10.1186/s13550-018-0453-x.
- Shan X, Wang D, Chen J, Xiao X, Jiang Y, Wang Y, Fan Y. Necrosis degree displayed in computed tomography images correlated with hypoxia and angiogenesis in breast cancer. J Comput Assist Tomogr. 2013;37:22–28. doi:10.1097/RCT.0b013e318279abd1.
- Eustace A, Irlam JJ, Taylor J, Denley H, Agrawal S, Choudhury A, Ryder D, Ord JJ, Harris AL, Rojas AM, et al. Necrosis predicts benefit from hypoxia-modifying therapy in patients with high risk bladder cancer enrolled in a phase III randomised trial. Radiother Oncol. 2013;108:40–47. doi:10.1016/j.radonc.2013.05.017.
- Bachmann IM, Ladstein RG, Straume O, Naumov GN, Akslen LA. Tumor necrosis is associated with increased alphavbeta3 integrin expression and poor prognosis in nodular cutaneous melanomas. BMC Cancer. 2008;8:362. doi:10.1186/1471-2407-8-362.
- Bredholt G, Mannelqvist M, Stefansson IM, Birkeland E, Bø TH, Øyan AM, Trovik J, Kalland K-H, Jonassen I, Salvesen HB, et al. Tumor necrosis is an important hallmark of aggressive endometrial cancer and associates with hypoxia, angiogenesis and inflammation responses. Oncotarget. 2015;6:39676–39691. doi:10.18632/oncotarget.v6i37.
- Lewis JS, Landers RJ, Underwood JC, Harris ALLC:. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;192:150–158. PubMed - NCBI. doi:10.1002/(ISSN)1096-9896.
- Suzuki J, Kojima M, Aokage K, Sakai T, Nakamura H, Ohara Y, Tane K, Miyoshi T, Sugano M, Fujii S, et al. Clinicopathological characteristics associated with necrosis in pulmonary metastases from colorectal cancer. Virchows Arch. 2019;474:569–575. doi:10.1007/s00428-019-02535-7.
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi:10.1038/35025220.
- Milross CG, Tucker SL, Mason KA, Hunter NR, Peters LJ, Milas L. The effect of tumor size on necrosis and polarographically measured pO2. Acta Oncol. 1997;36:183–189. doi:10.3109/02841869709109228.
- Serganova I, Rizwan A, Ni X, Thakur SB, Vider J, Russell J, Blasberg R, Koutcher JA. Metabolic imaging: a link between lactate dehydrogenase A, lactate, and tumor phenotype. Clin Cancer Res. 2011;17:6250–6261. doi:10.1158/1078-0432.CCR-11-0397.
- Chang C-H, Curtis JD, Maggi LB, Faubert B, Villarino AV, O’Sullivan D, Huang S-C-C, van der Windt GJW, Blagih J, Qiu J, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–1251. doi:10.1016/j.cell.2013.05.016.
- Chang C-H, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJW, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–1241. doi:10.1016/j.cell.2015.08.016.
- Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016;24:657–671. doi:10.1016/j.cmet.2016.08.011.
- Renner K, Geiselhöringer A-L, Fante M, Bruss C, Färber S, Schönhammer G, Peter K, Singer K, Andreesen R, Hoffmann P, et al. Metabolic plasticity of human T cells: preserved cytokine production under glucose deprivation or mitochondrial restriction, but 2-deoxy-glucose affects effector functions. Eur J Immunol. 2015;45:2504–2516. doi:10.1002/eji.v45.9.
- Angelin A, Gil-de-Gómez L, Dahiya S, Jiao J, Guo L, Levine MH, Wang Z, Quinn WJ, Kopinski PK, Wang L, et al. Foxp3 Reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 2017; 25:1282–1293.e7.
- Ratnikov BI, Scott DA, Osterman AL, Smith JW, Ronai ZA. Metabolic rewiring in melanoma. Oncogene. 2017;36:147–157. doi:10.1038/onc.2016.198.
- Rizwan A, Serganova I, Khanin R, Karabeber H, Ni X, Thakur S, Zakian KL, Blasberg R, Koutcher JA. Relationships between LDH-A, lactate, and metastases in 4T1 breast tumors. Clin Cancer Res. 2013;19:5158–5169. doi:10.1158/1078-0432.CCR-12-3300.
- Longo DL, Bartoli A, Consolino L, Bardini P, Arena F, Schwaiger M, Aime S. In vivo imaging of tumor metabolism and acidosis by combining PET and MRI-CEST pH imaging. Cancer Res. 2016;76:6463–6470. doi:10.1158/0008-5472.CAN-16-0825.
- Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi:10.1182/blood-2006-07-035972.
- Cascone T, McKenzie JA, Mbofung RM, Punt S, Wang Z, Xu C, Williams LJ, Wang Z, Bristow CA, Carugo A, et al. Increased tumor glycolysis characterizes immune resistance to adoptive T cell therapy. Cell Metab. 2018;27:977–987.e4. doi:10.1016/j.cmet.2018.02.024.
- Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013;191:1486–1495. doi:10.4049/jimmunol.1202702.
- Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi:10.1038/nature13490.
- Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang L-P, Gimotty PA, Gilks CB, Lal P, Zhang L, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi:10.1038/nature10169.
- Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P, de Zoeten EF, Cambier JC, Stenmark KR, Colgan SP, et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci. 2012;109:E2784–E2793. doi:10.1073/pnas.1202366109.
- Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, Johnson RS, Goldrath AW. Hypoxia-inducible factors enhance the effector responses of CD8+ T cells to persistent antigen. Nat Immunol. 2013;14:1173–1182. doi:10.1038/ni.2714.
- Hatfield SM, Sitkovsky M. A2A adenosine receptor antagonists to weaken the hypoxia-HIF-1α driven immunosuppression and improve immunotherapies of cancer. Curr Opin Pharmacol. 2016;29:90–96. doi:10.1016/j.coph.2016.06.009.
- Laoui D, Van Overmeire E, Di Conza G, Aldeni C, Keirsse J, Morias Y, Movahedi K, Houbracken I, Schouppe E, Elkrim Y, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res. 2014;74:24–30. doi:10.1158/0008-5472.CAN-13-1196.
- Corzo CA, Condamine T, Lu L, Cotter MJ, Youn J-I, Cheng P, Cho H-I, Celis E, Quiceno DG, Padhya T, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi:10.1084/jem.20100587.
- Lacroix R, Rozeman EA, Kreutz M, Renner K, Blank CU. Targeting tumor-associated acidity in cancer immunotherapy. Cancer Immunol Immunother. 2018;67:1331–1348.
- Kim H, Kwon HJ, Han YB, Park SY, Kim ES, Kim SH, Kim YJ, Lee JS, Chung JH. Increased CD3+ T cells with a low FOXP3+/CD8+ T cell ratio can predict anti-PD-1 therapeutic response in non-small cell lung cancer patients. Mod Pathol. 2019;32:367–375.
- Martens A, Wistuba-Hamprecht K, Geukes Foppen MH, Yuan J, Postow MA, Wong P, Romano E, Khammari A, Dreno B, Capone M, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res. 2016;22:2908–2918.
- Weber J, Gibney G, Kudchadkar R, Yu B, Cheng P, Martinez AJ, Kroeger J, Richards A, Mccormick L, Moberg V, et al. Phase I/II study of metastatic melanoma patients treated with nivolumab who had progressed after ipilimumab. Cancer Immunol Res. 2016;4:345–353.
- Wood DC, Varela V, Palmquist M, Weber F. Serum lactic dehydrogenase and isoenzyme changes in clinical cancer. J Surg Oncol. 1973;5:251–257.
- Diem S, Kasenda B, Spain L, Martin-Liberal J, Marconcini R, Gore M, Larkin J. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer. 2016;114:256–261.
- Wagner NB, Forschner A, Leiter U, Garbe C, Eigentler TK. S100B and LDH as early prognostic markers for response and overall survival in melanoma patients treated with anti-PD-1 or combined anti-PD-1 plus anti-CTLA-4 antibodies. Br J Cancer. 2018;119:339–346.
- Simeone E, Gentilcore G, Giannarelli D, Grimaldi AM, Caracò C, Curvietto M, Esposito A, Paone M, Palla M, Cavalcanti E, et al. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol Immunother. 2014;63:675–683.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674.
- Katt WP, Cerione RA. Inhibition of cancer metabolism: a patent landscape. Pharm Pat Anal. 2019;8:117–138.
- Zhang S-L, He Y, Tam KY. Targeting cancer metabolism to develop human lactate dehydrogenase (h LDH)5 inhibitors. Drug Discov Today. 2018;23:1407–1415.
- Gottfried E, Lang SA, Renner K, Bosserhoff A, Gronwald W, Rehli M, Einhell S, Gedig I, Singer K, Seilbeck A, et al. New aspects of an old drug – diclofenac targets MYC and glucose metabolism in tumor cells. PLoS One. 2013;8(7):e66987. doi:10.1371/journal.pone.0066987.
- Calcinotto A, Filipazzi P, Grioni M, Iero M, De Milito A, Ricupito A, Cova A, Canese R, Jachetti E, Rossetti M, et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 2012;72:2746–2756.
- Homicsko K, Richtig G, Tuchmann F, Tsourti Z, Hanahan D, Coukos G, Wind-Rotolo M, Richtig E, Zygoura P, Holler C, et al. LBA2Proton pump inhibitors negatively impact survival of PD-1 inhibitor based therapies in metastatic melanoma patients. Ann Oncol. 2018:29(Supp 10). doi:10.1093/annonc/mdy511.001.
- Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, Tigchelaar EF, Jankipersadsing SA, Cenit MC, Harmsen HJM, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–748.
- Gong J, Chehrazi-Raffle A, Placencio-Hickok V, Guan M, Hendifar A, Salgia R. The gut microbiome and response to immune checkpoint inhibitors: preclinical and clinical strategies. Clin Transl Med. 2019;8(1):9.
- Pilon-Thomas S, Kodumudi KN, El-Kenawi AE, Russell S, Weber AM, Luddy K, Damaghi M, Wojtkowiak JW, Mul JJ, Ibrahim-Hashim A, et al. Microenvironment and immunology neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Res. 2016;76(6):1381–1390.
- Long GV, Grob -J-J, Nathan P, Ribas A, Robert C, Schadendorf D, Lane SR, Mak C, Legenne P, Flaherty KT, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol. 2016;17:1743–1754.
- Major P, Trarbach T, Lenz H, Kerr D, Pendergrass K, Douillard J, Chen B, Laurent D. Jacques C CE van: A meta-analysis of two randomized, double-blind, placebo-controlled, phase III studies in patients (pts) with metastatic colorectal cancer (mCRC) receiving FOLFOX4 and PTK/ZK to determine clinical benefit on progression-free survival (PFS) in high LDH pts. J Clin Oncol. 2006;24(Supp 18):3529.
- Scartozzi M, Giampieri R, Maccaroni E, Del Prete M, Faloppi L, Bianconi M, Galizia E, Loretelli C, Belvederesi L, Bittoni A, et al. Pre-treatment lactate dehydrogenase levels as predictor of efficacy of first-line bevacizumab-based therapy in metastatic colorectal cancer patients. Br J Cancer. 2012;106:799–804.
- Yin C, Jiang C, Liao F, Rong Y, Cai X, Guo G, Qiu H, Chen X, Zhang B, He W, et al. Initial LDH level can predict the survival benefit from bevacizumab in the first-line setting in Chinese patients with metastatic colorectal cancer. Onco Targets Ther. 2014;7:1415–1422.
- Mizukami Y, Li J, Zhang X, Zimmer MA, Iliopoulos O, Chung DC. Hypoxia-inducible factor-1-independent regulation of vascular endothelial growth factor by hypoxia in colon cancer. Cancer Res. 2004;64:1765–1772.
- Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL. Tumour angiogenesis research group: lactate dehydrogenase 5 expression in operable colorectal cancer: strong association with survival and activated vascular endothelial growth factor pathway—a report of the tumour angiogenesis research group. J Clin Oncol. 2006;24:4301–4308.
- Faloppi L, Del Prete M, Gardini AC, Santini D, Silvestris N, Bianconi M, Giampieri R, Valgiusti M, Brunetti O, Bittoni A, et al. The correlation between LDH serum levels and clinical outcome in advanced biliary tract cancer patients treated with first line chemotherapy. Sci Rep. 2016;6:24136.
- Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. 2019;18:60.
- Horikawa N, Abiko K, Matsumura N, Hamanishi J, Baba T, Yamaguchi K, Yoshioka Y, Koshiyama M, Konishi I. Expression of vascular endothelial growth factor in ovarian cancer inhibits tumor immunity through the accumulation of myeloid-derived suppressor cells. Clin Cancer Res. 2017;23:587–599.
- Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR, Vianello F, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109:17561–17566.
- Lin Z, Zhang Q, Luo W. Angiogenesis inhibitors as therapeutic agents in cancer: challenges and future directions. Eur J Pharmacol. 2016;793:76–81.
- Yuan J, Zhou J, Dong Z, Tandon S, Kuk D, Panageas KS, Wong P, Wu X, Naidoo J, Page DB, et al. Pretreatment serum VEGF is associated with clinical response and overall survival in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res. 2014;2:127–132.
- Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou J, Sasada T, Zeng W, Giobbie-Hurder A, Atkins MB, Ibrahim N, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res. 2014;2:632–642.
- Sheng X, Yan X, Chi Z, Si L, Cui C, Tang B, Li S, Mao L, Lian B, Wang X, et al. Axitinib in combination with toripalimab, a humanized immunoglobulin g4 monoclonal antibody against programmed cell death-1, in patients with metastatic mucosal melanoma: an open-label Phase IB trial. J Clin Oncol. 2019. doi:10.1200/JCO.19.00210.
- McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, Fong L, Joseph RW, Pal SK, Reeves JA, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24:749–757.
- Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–1127.
- Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S, Uemura M, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–1115.
- Katsurada M, Nagano T, Tachihara M, Kiriu T, Furukawa K, Koyama K, Otoshi T, Sekiya R, Hazama D, Tamura D, et al. Baseline tumor size as a predictive and prognostic factor of immune checkpoint inhibitor therapy for non-small cell lung cancer. Anticancer Res. 2019;39:815–825.
- Balar AV, Castellano D, O’Donnell PH, Grivas P, Vuky J, Powles T, Plimack ER, Hahn NM, de Wit R, Pang L, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:1483–1492.
- Zhang D, He W, Wu C, Tan Y, He Y, Xu B, Chen L, Li Q, Jiang J. Scoring system for tumor-infiltrating lymphocytes and its prognostic value for gastric cancer. Front Immunol. 2019;10:71.
- König L, Mairinger FD, Hoffmann O, Bittner A-K, Schmid KW, Kimmig R, Kasimir-Bauer S, Bankfalvi A. Dissimilar patterns of tumor-infiltrating immune cells at the invasive tumor front and tumor center are associated with response to neoadjuvant chemotherapy in primary breast cancer. BMC Cancer. 2019;19:120.
- Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795.
- Wang B, Wu S, Zeng H, Liu Z, Dong W, He W, Chen X, Dong X, Zheng L, Lin T, et al. CD103 + tumor infiltrating lymphocytes predict a favorable prognosis in urothelial cell carcinoma of the bladder. J Urol. 2015;194:556–562.
- Gao J, Karam JA, Tannir NM, Campbell MT, Slack Tidwell R, Ahrar K, Rao P, Ng CS, Jonasch E, Matin SF, et al. A pilot randomized study evaluating nivolumab (nivo) or nivo + bevacizumab (bev) or nivo + ipilimumab (ipi) in patients with metastatic renal cell carcinoma (MRCC) eligible for cytoreductive nephrectomy, metastasectomy or post-treatment biopsy (Bx). J Clin Oncol. 2019;37:4501.
- Parmenter TJ, Kleinschmidt M, Kinross KM, Bond ST, Li J, Kaadige MR, Rao A, Sheppard KE, Hugo W, Pupo GM, et al. Response of BRAF-mutant melanoma to BRAF inhibition is mediated by a network of transcriptional regulators of glycolysis. Cancer Discov. 2014;4:423–433.
- Schadendorf D, Long GV, Stroiakovski D, Karaszewska B, Hauschild A, Levchenko E, Chiarion-Sileni V, Schachter J, Garbe C, Dutriaux C, et al. Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur J Cancer. 2017;82:45–55.
- Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, Mitra D, Boni A, Newton LP, Liu C, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19:1225–1231.
- Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368:1365–1366.
- Amin A, Lawson DH, Salama AKS, Koon HB, Guthrie T, Thomas SS, O’Day SJ, Shaheen MF, Zhang B, Francis S, et al. Phase II study of vemurafenib followed by ipilimumab in patients with previously untreated BRAF-mutated metastatic melanoma. J Immunother Cancer. 2016;4:44.
