ABSTRACT
Immunotherapy is an effective treatment in advanced cancer, although predictors of response are limited. We studied whether excess weight influences the efficacy outcomes of immunotherapy. We have also evaluated the combined prognostic effect of excess weight and immune-related adverse events (irAEs).
Efficacy of anti-PD-1 treatment was evaluated with both objective radiological response (ORR) rate and progression-free survival (PFS), and toxicity with irAEs. We studied the association between excess weight and ORR, PFS or irAEs.
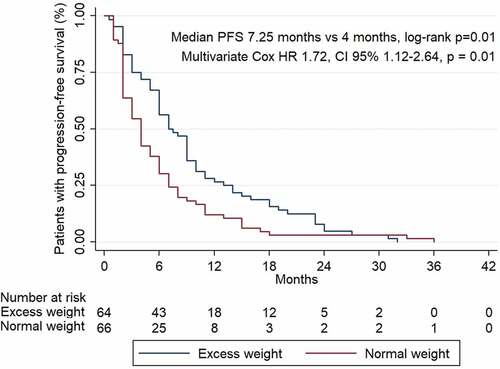
132 patients diagnosed with advanced cancer were included. Median body mass index (BMI) was 24.9 kg/m2. 64 patients had normal weight (BMI<25 kg/m2), and 64 patients had excess weight (BMI≥25 kg/m2). Four patients had underweight and were excluded from further analysis. ORR was achieved in 50 patients (38.0%), median PFS was 6 months. 44 patients developed irAEs (33.3%). ORR was higher in excess weight patients than in patients with normal weight (51.6% vs 25.0%; OR 3.45, p = .0009). PFS was improved in patients with excess weight (7.25 months vs 4 months, HR 1.72, p = .01). The incidence of IrAEs was not different in patients with excess weight (54.5% vs 43.2%, p = .21). When high BMI and irAEs were combined, we observed a marked prognostic trend in ORR rate (87.5% vs 6.2%; OR 161.0, p < .00001), and in PFS (14 months vs 3 months; HR 5.89, p < .0001).
Excess weight patients with advanced cancer that receive single-agent anti-PD-1 antibody therapy exhibit a significantly improved clinical outcome compared with normal BMI patients. This association was especially marked when BMI and irAEs were considered combined.
Introduction
A general adverse impact of excess weight (BMI ≥ 25 kg/m2) in the general population has been recognized in recent years.Citation1–Citation4 Cancer patients with excess weight may also have a worse overall prognosis compared to patients with normal Body Mass Index (BMI), Citation5–Citation11 and some studies have suggested that excess weight may be related to a worse response to conventional therapies, Citation12–Citation14 although this has not always been confirmed.Citation6,Citation15-Citation18
A recent report has suggested that there is a paradoxical effect of obesity on immunotherapy-treated cancer, because there is an improved antitumor efficacy after checkpoint blockade.Citation19 The authors suggested that this might be related to a direct targeting of some of the pathways activated in obesity. In a group of patients with advanced melanoma, a significant improvement of response to checkpoint inhibitors was also reported in patients with BMI above normal.Citation20,Citation21 This surprisingly positive association between obesity and the efficacy of cancer immunotherapy has been recently recognized, and the need for further studies and the understanding of the underlying immunological principles has been highlighted.Citation22
The aim of our study was to evaluate the association between excess weight and cancer immunotherapy outcome and toxicity in patients with advanced solid and hematological tumors treated with anti-PD-1 immune checkpoint inhibitors. We used the objective radiological response (ORR) and progression-free survival (PFS) as efficacy outcomes. Since we have recently reported that immune-related adverse events (irAEs) in patients treated with anti-PD-1 drugs is directly related to the response and an increase in PFS, Citation23 we also evaluated the influence of the development of irAEs in terms of ORR and PFS in the cohort of excess weight or normal weight patients.
Results
Patient characteristics
One hundred and thirty-two patients were studied. Median age was 69 y (32–86 y); 95 patients were male (71.9%) and 103 (79.2%) were smokers at diagnosis. Patients’ malignancies were non-small-cell lung cancer (NSCLC) in 93 cases (56 non-squamous, 33 squamous and 4 non-specified), melanoma in 12, squamous cell carcinoma of head and neck (SCCHN) in 9, clear cell renal carcinoma in 6, urothelial bladder carcinoma in 4, Hodgkin lymphoma (HL) in 3 cases, gastric carcinoma in 2 cases, and gallbladder adenocarcinoma, merkel cell carcinoma and hepatocellular carcinoma, 1 case each. All selected patients had advanced or metastatic disease. ECOG performance status (PS) was 0–1 in 92 patients (75.1%), and 2 in 40 (24.9%). No patient had ECOG PS 3 or 4. Median follow-up was 6 months (0.5–32 months). PD-L1 expression was available for 41 cases, 35 of which had a positive result. Twenty of these positive samples had high PD-L1 expression (PD-L1 ≥ 50%: 48.7%). Patients’ median weight was 70 (42–102) kg and median BMI was 24.9 (14.8–37.1) kg/m2. Sixty-four patients had normal BMI (18.5–25 kg/m2), and 64 patients had excess weight (BMI ≥ 25 kg/m2). Of these, 18 patients were obese (BMI ≥30 kg/m2).
The distribution of comorbidities between excess weight and normal weight patients is shown in .
Table 1. Characteristics and differences between excess weight and normal BMI
Treatment and clinical outcomes
Ninety-four patients were treated with nivolumab and 38 with pembrolizumab. Twenty-six patients (19.7%) received anti-PD-1 treatment as first-line therapy, 56 patients (42.4%) second line, 26 patients (19.7%) third line, 18 patients (13.6%) fourth line, 4 patients (3.0%) fifth line, 1 patient (0.8%) sixth line and 1 patient received anti-PD-1 treatment as ninth line therapy. All patients with HL had previously received bone marrow transplant, two of them including allogenic bone marrow transplant.
Objective radiological response was observed in 50 patients (38.0%): complete response in 5 cases (3.8%) and partial response in 45 (34.1%). Stable disease was detected in 37 cases (28.0%) and progressive disease in 45 cases (34.1%). Median PFS was 6 months (0.5–36 months).
Forty-four patients (33.3%) developed irAEs, making a total of 60 events occurring at a median time of 6 weeks (range 2–24) from the begining of the treatment. Hypothyroidism was the most frequent irAE observed (n = 22 events, 14 cases grade 1, 4 cases grade 2 and 4 cases grade 3), followed by immune-mediated nephritis (n = 7, 2 cases in grade 1, 2 cases in grade 2 and 3 cases in grade 3), hyperthyroidism (n = 6, 1 case in grade 1 and 5 cases in grade 2), pneumonitis (n = 5, all grade 1), rash (n = 3, all grade 1), immune-mediated hepatitis (n = 3, 1 case in grade 1 and two in grade 4), arthritis (n = 3, 2 case in grade 2 and 1 case in grade 3); panhypopituitarism (n = 2, grade 1), immune-mediated colitis (n = 2, grade 2 and grade 3) hypophysitis (n = 1, grade 1), adrenal insufficiency (n = 2, grade 1), diabetic ketoacidosis (n = 1, grade 4), myositis (n = 2, grade 2) and encephalitis (n = 1, grade 4). Nine of the 44 patients who developed irAEs stopped treatment due to unacceptable toxicity (20.4%): one patient for grade 3 colitis, three patients for grade 3 nephritis, two patients due to hepatitis grade 3 and 4, respectively, one patient with grade 3 arthritis, one patient with grade 4 diabetic ketoacidosis and one patient due to grade 4 encephalitis. Twenty-one patients from the total cohort received treatment with a minimum dose of 0.5–1 mg/kg of methylprednisolone due to severe irAEs development or as anti-inflammatory therapy. None of the patients were not receiving corticosteroids at baseline or during the 2 months before the start of immunotherapy.
Twenty-four of the 44 patients with irAEs had excess weight (54.5%) versus 19 of the 44 patients (43.2%) that had a normal BMI (p = .39); as is the same: 24 patients with excess weight (37.5%) presented an irAE versus 19 patients with normal BMI (29.7%). . Thirty-nine of the 88 patients without treatment-related toxicity were patients with excess weight (44.3%), compared to 46 patients (52.2%) with normal BMI (p = .31).
The clinical characteristics and outcome of the four patients with underweight that were excluded of the analysis were the following. All met criteria of cachexia. All had lung cancer (three non-squamous, one squamous). Their median age was 60 y. Three patients were male. Three patients received treatment with nivolumab and one received pembrolizumab. Two patients developed irAEs (both hypothyroidism) and one patient obtained an objective response, with a median PFS of 5.5 months.
Excess weight and treatment efficacy
Thirty-three of the 64 patients with excess weight presented ORR to anti-PD-1 antibodies (51.6%), compared to 16 of the 64 patients (25.0%) who presented normal BMI (OR 3.45, CI 95% 1.58–7.55, p = .0009). Patients with excess weight had therefore more than 3 times greater probability of obtaining an objective response (ORR) compared to normal weight patients (). We analyzed separately the efficacy in obese or overweight patients: 8/18 obese patients obtained ORR (44.4%) and 25/46 in overweight patients (54.3%) (p = NS).
Table 2. Relationship of objective radiological response (ORR) and excess weight in patients treated with Nivolumab or Pembrolizumab
Regarding progression-free survival, patients with excess weight had higher PFS compared to those with normal BMI: 7.25 vs 4 months of median PFS (long Rank p = .007). Significant differences were still observed even after adjustment for sex, histology, smoking habit, prior treatments received, type of anti-PD-1 antibody received, ECOG functional status and all the clinical and demographic variables studied (HR 3.77, CI 95% 1.33–10.66, p = .01). .
Excess weight, irAEs and treatment efficacy
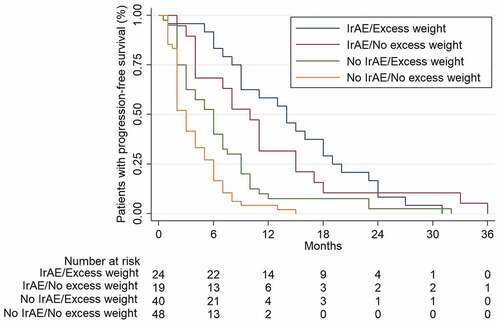
Twenty-one of the 24 patients who presented both irAEs and excess weight simultaneously presented ORR (87.5%), compared to only 3 of the 48 patients (6.2%) who did not present either toxicity or excess weight (OR 161.0, CI 95% 4.95–5.23e + 03, p < .00001) or to 11 of the 39 patients with excess weight who did not develop irAEs (28.2%) (OR 9.03, CI 95% 1.67–48.71, p = .001).
Excess weight patients who also developed irAEs had a benefit in terms of PFS, compared to those who did not have irAEs and who had normal BMI: 14 vs 3 months (log-rank p < .00001). Differences were still statistically significant even after adjusting for sex, histology, smoking habit, prior lines treatment, type of anti-PD-1 antibody received, ECOG functional status and all the clinical and demographic variables studied (HR 5.89, CI 95% 2.77–13.05, p < .0001). .
Multivariate analysis showed significant differences when comparing patients with excess weight who developed irAEs versus those without excess weight who also developed irAEs, with higher PFS for the first group: 14 vs 10 months (HR 12.29, CI 95% 2.8–53.8 p = .01). .
For patients who did not develop irAEs, PFS was 6 months for those with excess weight versus 3 months in individuals with normal BMI (log-rank 0.007). Multivariate analysis in this setting showed a trend toward significance (HR 2.41, CI 95% 0.70–8.31, p = .16).
Discussion
Our study confirms the observation that there is an association between excess weight and anti-PD-1 immunotherapy outcome in patients with advanced cancer, and also expands it by finding that combining BMI with irAEs identifies distinctive predictive groups. We had recently shown that irAEs were associated with a favorable outcome in cancer patients treated with immune-checkpoint inhibitors (ICIs), Citation23 and now we show that those patients with excess weight who also develop irAEs have a dramatic probability of achieving a response and having longer PFS compared to those patients with normal BMI and no irAEs. Very relevantly, the development of irAEs and the presence of excess weight were independent predictors of clinical benefit to anti-PD-1 immune checkpoint inhibitors, and in our cohort, we also observed that excess weight did not influence the development of irAEs.
The BMI data analysis in our series confirms the results of recent studies, in which the benefit and efficacy of treatment with immune checkpoint inhibitors was related to BMI.Citation20,Citation21 In contrast to our study, these published studies either considered a mixed use of immunotherapy drugs (that included anti-CTLA-4 antibodies) or studied patients with a single type of malignancy. One of these studies is a retrospective series published with a total of 76 patients from three hospitals in Austria.Citation20 Patients were diagnosed with melanoma and were treated with a single anti-CTLA-4 drug (ipilimumab). In this study, patients were stratified into two cohorts according to their BMI. They observed that those with a BMI≥25 kg/m2 presented high response rates (p = .024, chi-square), although only with a significant trend in the multivariate analysis (OR 2.75, CI 95% 0.87–8.66, p = .084). They found no significant differences in PFS (p = .924, log-rank), or OS (p = .056, log-rank).Citation20 Another study analyzed BMI in an extensive cohort of patients diagnosed with advanced melanoma, treated with the immunotherapy drugs ipilimumab, pembrolizumab, nivolumab or atezolizumab.Citation21 Although associations were observed, the multivariate analysis found that excess weight were associated to a better PFS or OS only in male patients. A similar finding was also observed in patients treated with targeted therapy, but not in those treated with chemotherapy. These authors did not evaluate ORR.
Murphy and LongoCitation22 have suggested that obesity could be considered as a positive prognostic factor for treatment with immune checkpoint inhibitors (ICIs) due to its mechanism of action. ICIs are drugs that boost our immune system, so in cases where obesity preexists, their action may be favored by the proinflammatory status of these obese patients. In our study, excess weight patients had a better immunotherapy effect, and this was more pronounced in those patients that developed irAEs, and these two clinical characteristics have been associated with a greater proinflammatory response.
Wang et al.Citation19 reported an analysis of a prospective cohort of 250 patients diagnosed for multiple cancers receiving treatment with anti-PD-1. They showed improved PFS (8 vs 4.7 months, p = .003) and overall survival (17.4 vs 12 months, p = .04) in patients with obesity, although no data for ORR were reported. They attributed their findings to a significant increase in the expression of PD-1 in obese patients diagnosed with melanoma in another arm evaluated in this work, Citation19 and suggested that the proinflammatory state of obesity create an excess production of leptin, entails an aging of T lymphocytes and leads to this overexpression of PD-1, allowing a greater beneficial response to anti-PD-1 therapies.Citation22 Our study included patients with several types of malignancies, and we evaluated a single class of immune checkpoint inhibitors, i.e. anti-PD-1, allowing possibly a more consistent interpretation of the findings observed.
To our knowledge, this is the first time in which excess weight is explicitly found to be a predictor of better outcome in patients with several types of tumors receiving treatment with anti-PD-1, and that this effect is enhanced in the presence of irAEs. IrAEs have been related to anti-PD-1 outcome,Citation23 but, again, there is no previous research that shows that the association between irAEs and excess weight further enhances the effectiveness of anti-PD-1 immune checkpoint inhibitors, supporting the synergistic effect that has been seen in our study. We believe that our findings have particular clinical relevance because we demonstrate that the benefit to the same type of anti-PD-1 treatment is increased in all types of advanced cancer when patients present the two characteristics studied in our work. It might be considered that including in the study two patients diagnosed with HL treated with nivolumab and one patient diagnosed with Merkel cell carcinoma treated with pembrolizumab might be a limitation due to better outcome of these histologies in the pivotal studies.Citation24,Citation25 Although the 2 HL patients were in a worse disease stage than patients at the clinical trial.
Conclusion
We show that excess weight is associated with a greater probability of a favorable treatment outcome in patients with advanced cancer who receive immunotherapy single-agent anti-PD-1 antibodies nivolumab or pembrolizumab, and that when irAEs develop in excess weight population the observed therapeutic benefit is further enhanced. Developing a prospective evaluation to confirm our findings is necessary. Such a prospective study should include more precise methods to measure of excess weight, like the hip circumference.
Material and methods
Study design and patients
We performed an observational cohort study where we followed patients starting treatment with anti-PD-1 drugs pembrolizumab or nivolumab. Thus, we carried out a retrospective review of all clinical records of patients diagnosed with advanced cancer and treated with single-agent anti-PD-1 drugs pembrolizumab or nivolumab, from January 2016 to December 2018, at the Hospital Universitario de La Princesa. Patients weight was classified according to WHO and NIH criteriaCitation26,Citation27 using BMI. Underweight was BMI less than 18.5; normal weight, BMI 18.5 to 24.9; overweight, BMI 25 to 29.9; and obese, BMI 30 or more. Overweight and obese patients were considered together and defined as excess weight patients. Underweight patients met criteria of cachexia in all cases and were excluded from the study due to reports of poorer response detected in previous studies.Citation28
Treatment efficacy was measured using ORR (immune RECIST criteria) and PFS.Citation29 IrAEs were defined according to criteria reported in previous studies,Citation23 and their grade was established following to the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE-4.0).
All patients were followed from the start date of treatment until 31st of March of 2019 or until patient death or last date of follow-up. A baseline laboratory test was performed according to routine clinical practice. After treatment was started, clinical and laboratory tests were carried out every 2 weeks in patients receiving Nivolumab or every 3 weeks in patients receiving Pembrolizumab, prior to drug administration. Body CT-scans were performed every 8–12 weeks or as clinically indicated. The dose of pembrolizumab was 200 mg as fixed dose every 21 d if it was a first-line treatment, or 2 mg/kg every 21 d if it was prescribed as second line or beyond. The dose of nivolumab was 3 mg/kg every 14 d in all cases. The study was approved by the Ethics and Research Committee of Hospital Universitario de la Princesa. All patients signed a written informed consent before being included in this study.
Statistical analysis
Descriptive data are reported as relative frequencies for discrete variables. Continuous variables are reported as mean ± standard deviation (SD) or median and interquartile range (IQR) for normal and not normally distributed variables, respectively. To determine the association between weight and ORR, Chi-square Test and Odds Ratio were performed. To determine the relationship between the presence of association of adverse immune-mediated events in patients with excess weight and the achievement of ORR, Chi-square Test and Odds Ratio were also performed. The association between excess weight alone and excess weight plus irAEs development with the PFS, were analyzed with log-rank test and multivariate Cox regression analysis, adjusted by sex, histology, smoking habit, prior lines treatment, type of anti-PD-1 antibody received, functional status as measured by the scale of the Eastern Cooperative Oncology Group (ECOG) and all the clinical and demographics variables studied. Kaplan–Meier curves showing PFS according to development or not of irAEs depending on the presence of overweight were estimated. Statistical analyses were carried out with STATA SE version 14.1 (StataCorp, College Station, TX, USA).
Abbreviations
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Ethics approval and consent to participate
The study was approved by the Ethics and Research Committee of Hospital Universitario de la Princesa. All patients signed a written informed consent before being included in this study.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to the Spanish and European official law of data protection (LOPD) but are available from the corresponding author on reasonable request.
Competing interests
N Romero-Laorden has received grants support from Roche, Janssen, Astellas, Pfizer and Pharmamar, and travel grants from Pfizer and Janssen. R Colomer is consultant/member of advisory board of Lilly, MSD, Roche, Servier and Novartis; received research funding from BMS, MSD, Roche, Pfizer, Janssen, MSD and Novartis. No potential conflicts of interest were disclosed by the other authors.
Author contributions
J.R. contributed to the conception and design of the study, data acquisition, statistical analysis, interpretation of the data and writing of the manuscript. J.M.S.T. and N.R.L. contributed to the acquisition and interpretation of the data and revision of the manuscript. P.G. and A.L. contributed to the statistical analysis and interpretation of the data. A.B., V.P.B., A.M.R.L., R.A., A.L., M.A., R.M., P.C., A.G., J.M.S.L.M., J.A. and A.A. contributed to the acquisition of the data. R.C. contributed to the conception and design of the study, statistical analysis, interpretation of the data and writing of the manuscript. All authors reviewed and approved the final version of the manuscript.
Acknowledgments
The authors express their gratitude to the patients and their families for participation in this study.
Additional information
Funding
References
- Hanfei Xu MS, Cupples LA, Stokes A, Liu C-T. Association of obesity with mortality over 24 years of weight history findings from the framingham heart study. JAMA Netw Open. 2018;1(7):e184587. doi:10.1001/jamanetworkopen.2018.4587.
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293(15):1861–7. doi:10.1001/jama.293.15.1861.
- Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index cat egories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi:10.1001/jama.2012.113905.
- Prospective studies collaboration. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–1096. doi:10.1016/S0140-6736(09)60318-4.
- Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer-viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794–798. doi:10.1056/NEJMsr1606602.
- Klevorn LE, Teague RM. Adapting cancer immunotherapy model for the real world. Trends Immunol. 2016;37(6):354–363. doi:10.1016/j.it.2016.03.010.
- Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass índex and risk of 22 specific cancers: a population-based cohort study of 5.24 million UD adults. Lancet. 2014;384(9945):755–765. doi:10.1016/S0140-6736(14)60892-8.
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proponed mechanisms. Nat Rev Cancer. 2004;4(8):579–591. doi:10.1038/nrc1408.
- Parr CL, Batty GD, Lam TH, Barzi F, Fang X, Ho SC, Jee SH, Ansary-Moghaddam A, Jamrozik K, Ueshima H, et al. Body-mass índex and cancer mortality in the Asia-Pacific cohort studies collaboration: pooled analyses of 424,519 participants. Lancet Oncol. 2010;11(8):741–752. doi:10.1016/S1470-2045(10)70141-8.
- Tao W, Lagergren J. Clinical management of obese patients with cancer. Nat Rev Clin Oncol. 2013;10(9):519–533. doi:10.1038/nrclinonc.2013.120.
- Quante M, Dietrich A, Elkhal A, Tullius SG. Obesity-related immune responses and their impact on surgical outcomes. Int J Obes (Lond). 2015;39(6):877–883. doi:10.1038/ijo.2015.21.
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003 Apr 24;348(17):1625–1638. doi:10.1056/NEJMoa021423.
- Dahlberg SE, Schiller JH, Bonomi PB, Sandler AB, Brahmer JR, Ramalingam SS, Johnson DH. Body mass index and its association with clinical outcomes for advanced non-small-cell lung cancer patients enrolled on Eastern Cooperative Oncology Group clinical trials. J Thorac Oncol. 2013;8(9):1121–1127. doi:10.1097/JTO.0b013e31829cf942.
- Yang R, Cheung MC, Pedroso FE, Byrne MM, Koniaris LG, Zimmers TA. Obesity and weight loss at presentation of lung cancer are associated with opposite effects on survival. J Surg. Res. 2011;170(1):e75–83. doi:10.1016/j.jss.2011.04.061.
- Lam VK, Bentzen SM, Mohindra P, Nichols EM, Bhooshan N, Vyfhuis M, Scilla KA, Feigenberg SJ, Edelman MJ, Feliciano JL, et al. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2017;104:52–57. doi:10.1016/j.lungcan.2016.11.017.
- Albiges L, Hakimi AA, Xie W, McKay RR, Simantov R, Lin X, Lee J-L, Rini BI, Srinivas S, Bjarnason GA et al. Body mass index and metastatic renal cell carcinoma: clinical and biological correlations. J Clin Oncol. 2016 Oct 20;34(30):3655–3663. doi:10.1200/JCO.2016.66.7311.
- Zhang X, Liu Y, Shao H, Zheng C. Obesity paradox in lung cancer prognosis: evolving biological insights and clinical implications. J Thorac Oncol. 2017 Oct;12(10):1478–1488. doi:10.1016/j.jtho.2017.07.022.
- Aparicio T, Ducreux M, Faroux R, Barbier E, Manfredi S, Lecomte T, Etienne P-L, Bedenne L, Bennouna J, Phelip J-M, et al. Overweight is associated to a better prognosis in metastatic colorectal cancer: A pooled analysis of FFCD trials. Eur J Cancer. 2018 Jul;98:1–9. doi:10.1016/j.ejca.2018.03.031.
- Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, Mirsoian A, Minnar CM, Stoffel KM, Sturgill IR, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019 Jan;25(1):141–151. doi:10.1038/s41591-018-0221-5.
- Richtig G, Hoeller C, Wolf M, Wolf I, Rainer BM, Schulter G, Richtig M, Grübler MR, Gappmayer A, Haidn T, et al. Body mass index may predict the response to ipilimumab in metastatic melanoma: an observational multi-centre study. PLoS One. 2018;13(10):e0204729. doi:10.1371/journal.pone.0204729.
- Mcquade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, Park JJ, Haydu LE, Spencer C, Wongchenko M, et al. Association of body-mass índex and outcomes in patients metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19(3):310–322. doi:10.1016/S1470-2045(18)30078-0.
- Murphy WJ, Longo DL. The surprisingly positive association between obesity and cancer immunotherapy efficacy. JAMA. 2019 Mar 18;321(13):1247. doi:10.1001/jama.2019.0463.
- Rogado J, Sánchez-Torres JM, Romero-Laorden N, Ballesteros AI, Pacheco-Barcia V, Ramos-Leví A, Arranz R, Lorenzo A, Gullón P, Donnay O, et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur J Cancer. 2019 Jan 22;109:21–27. doi:10.1016/j.ejca.2018.10.014.
- Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, Armand P, Fanale M, Ratanatharathorn V, Kuruvilla J, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016 Sep;17(9):1283–1294. doi:10.1016/S1470-2045(16)30167-X.
- Nghiem P, Bhatia S, Lipson EJ, Sharfman WH, Kudchadkar RR, Brohl AS, Friedlander PA, Daud A, Kluger HM, Reddy SA et al. Durable tumor regression and overall survival in patients with advanced merkel cell carcinoma receiving pembrolizumab as first-line therapy. J Clin Oncol. 2019 Mar 20;37(9):693–702. doi:10.1200/JCO.18.01896.
- WHO. Health topics. Obesity. [accessed 2020 Apr 12]. https://www.who.int/topics/obesity/en
- NIH. Classification of overweight and obesity by BMI, waist circumference, and associated disease risks. [accessed 2020 Apr 12]. https://www.nhlbi.nih.gov/
- Turner DC, Kondic AG, Anderson KM, Robinson AG, Garon EB, Riess JW, Jain L, Mayawala K, Kang J, Ebbinghaus SW et al. Pembrolizumab exposure-response assessments challenged by association of cancer cachexia and catabolic clearance. Clin Cancer Res. 2018 Dec 1;24(23):5841–5849. doi:10.1158/1078-0432.CCR-18-0415.
- Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017 Mar;18(3):e143e52. doi:10.1016/S1470-2045(17)30074-8.