ABSTRACT
Adjuvant chemotherapy after surgery is the standard treatment modality for stage III and part of stage II or stage IV colorectal cancer (CRC) patients. However, the 5-year overall survival (OS) rate remains unsatisfactory. Thus, developing combination therapies is essential to improve the prognosis of patients with CRC. The present study aimed to determine the effect of a sequential combination of cytokine-induced killer cell (CIK) infusion and chemotherapy for patients with CRC. 122 patients with CRC treated with postoperative adjuvant chemotherapy were retrospectively included in this study. Among them, 62 patients received adjuvant chemotherapy only (control group), while the other 60 patients, with similar demographic and clinical characteristics, received adjuvant chemotherapy and sequential CIK cell immunotherapy (CIK group). Survival analysis showed significantly improved disease free survival (DFS) and OS rates in the CIK group compared with the control group (log-rank test, P = .0024; P = .008, respectively). Univariate and multivariate analyses indicated that sequential CIK cell treatment was an independent prognostic factor for patients’ DFS and OS. Subgroup analyses showed that sequential CIK cell treatment significantly improved the DFS and OS of patients with high-risk T4 stage and insufficient chemotherapy duration. In conclusion, these data indicate that sequential adjuvant CIK cell treatment combined with chemotherapy is an effective therapeutic strategy to prevent disease recurrence and prolong survival of patients with CRC, particularly for patients with high-risk T4 stage and insufficient chemotherapy duration.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer worldwide with the increased incidence in Asian and Eastern European countries.Citation1 About 50 percent of patients who undergo radical surgery alone relapse and die of metastatic disease.Citation2 Postoperative adjuvant chemotherapy is recommended for patients with high-risk stage II or more advanced disease to decrease the recurrence rate and prolong overall survival (OS) rate. However, approximately 35 percent of patients develop tumor recurrence with eventual distant metastases during the disease course, especially within the first 2 years after surgery,Citation3 and the 5-year OS rate of CRC remains unsatisfactory, reported as less than 70% after radical surgery and adjuvant chemotherapy.Citation3 Whether there are any effective treatments that prevent tumor recurrence and improve the long-term survival of patients with CRC is worth of further exploration.
Immune function of cancer patients is generally suppressed, which may be more serious after surgery and adjuvant chemotherapy.Citation4,Citation5 It is reported that surgery can hasten the metastatic development for some patients,Citation6 and chemotherapeutic treatments are directed against the tumor bulk but spare the putative cancer stem cells,Citation7 which may well prove to underlie certain forms of tumor dormancy after surgical resection or radio/chemotherapy and regenerate a tumor once therapy has been halted.Citation8,Citation9 Recently, cell-based immunotherapy, which is more effective in recovering the host immunity and killing residual chemo-resistant cancer cells, can be used to complement conventional management.Citation10,Citation11 Several studies have provided evidence to demonstrate the clinical activity of adoptive cell immunotherapies including tumor infiltrating lymphocytes, cytokine-induced killer (CIK) cells, NK cells, antigen-specific T lymphocytes, T cell receptor (TCR) T cells and chimeric antigen receptor (CAR) T cells.Citation12 Among them, CIK cells, which have several characters of rapid proliferation, strong MHC-unrestricted cytolytic activity against a broad range of tumor cells, and minimal toxicity,Citation13,Citation14 may be an alternative immunotherapeutic strategy for CRC patients.Citation12 Our and others previous clinical studies also observed the safety and efficacy of CIK cell treatment for several kinds of solid cancers,Citation15–Citation19 including CRC.Citation20,Citation21 However, to our knowledge, only one study had reported the role of CIK cell immunotherapy in the adjuvant therapeutic periods of CRC after radical surgery, and their results were limited by small sample size with only 21 patients receiving CIK cell treatment.Citation22 Therefore, more and larger sample size studies are needed to evaluate the therapeutic value of sequential adjuvant chemotherapy plus CIK cell treatment for patients with CRC.
According to National Comprehensive Cancer Network (NCCN) guidelines, neither any maintenance treatments are recommended to patients with CRC after surgery and adjuvant chemotherapy, nor are alternative treatments available for patients who are intolerance of chemotherapy. Thus, in the present study, we aimed to investigate whether sequential adjuvant chemotherapy plus CIK cell therapy could improve the clinical outcomes of patients with CRC after radical resection. Our data provide additional evidence on the clinical effectiveness of maintenance treatment using CIK cells for patients with CRC.
Results
Patient demographics and clinical characteristics
In total, 122 patients with CRC were retrospectively analyzed; 78 (63.9%) were men and 44 (36.1%) were women. The median age was 54 years (range, 30–81 years). The baseline characteristics of the patients in the CIK group and control group are shown in . There were no statistically significant differences in demographic or clinical characteristics between the two groups except that patients in the CIK group were more advanced stage disease than those in the control group (P = .021). The treatment strategies were similar between the two groups except the CIK cell treatment. Only a few of the patients accepted neoadjuvant chemotherapy or radiotherapy, which were also well matched between the two groups.
Table 1. Baseline characteristics of patients with colorectal cancer
Phenotypic analysis of final CIK cells
After culturing and expansion, the final number of CIK cells was approximately 8.5 × 109–1.8 × 1010). The final cell products were assessed for viability by the trypan blue staining and the proportions of viable CIK cells exceeded 95%. The cells were also evaluated for possible contamination of bacterial, fungal, mycoplasma or endotoxins to make sure that they were safe to patients. The phenotype of CIK cells from 51 of 60 patients were determined by flow cytometry. The results suggested that there were obvious variations among different patients. The median percentage of CD3+, CD3+CD4+, CD3+CD8+, CD3−CD56+, and CD3+CD56+ population in the final CIK cells was 97.5% (range, 85.7–99.5%), 29.2% (range, 0.8–72.2%), 66.3% (range, 14.2–93.0%), 1.9% (range, 0.4–12.2%), and 16.4% (range, 6.9–38.1%), respectively (). Compared with peripheral blood mononuclear cells (PBMCs), we found that the percentage of CD3+CD56+ (P = .0001) and CD3+CD8+ (P = .0006) subsets significantly increased and CD3−CD56+ (P = .0025) subsets significantly decreased after in vitro expansion (Supplementary Figure S1); however, there was no significant difference in the percentage of CD3+CD4+ subsets after in vitro expansion (Supplementary Figure S1).
Figure 1. Phenotypic analysis of CIK cells after expansion. (a) The phenotype of autologous CIK cells after 14-d culture from 51 patients was evaluated using flow cytometry. The positive proportions of CD3+, CD3+CD4+, CD3+CD8+, CD3−CD56+, and CD3+CD56+ are shown. (b) The phenotypic evolution of CIK cells after culture for first four treatment cycles. The results were from 49 patients and are represented as mean ± SEM. NS, not significant. * p < .05
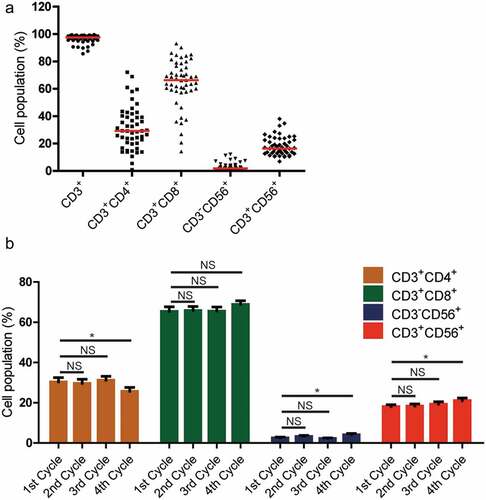
To identify whether there were any phenotypic evolution of patients with CRC after CIK cell infusions, the phenotype of CIK cells were also determined in the first four cycles of cell treatment. The results suggested that there were no statistically significant differences in CD3+CD4+, CD3−CD56+, and CD3+CD56+ population after the second or third treatment cycles. However, we found that the percentage of CD3+CD4+ subsets significantly decreased (; P = .0276), whereas the percentages of CD3−CD56+ or CD3+CD56+ subsets significantly increased (; P = .0343 and P = .0224, respectively) after the fourth treatment cycle. Besides, there was no significant difference in the percentage of CD3+CD8+ subsets during the four treatment cycles.
Side effects of CIK cell treatment
No significant induction of toxicity was observed in the patients who received CIK cell treatment. Across all processes of CIK cell immunotherapy in the CIK group, only 10 patients experienced adverse events, including 6 cases of self-limiting fever, 1 cases of transient hypertension, 1 cases of pruritus, and 2 cases of fatigue. All the adverse events were grade 1 or 2 and some of the patients recovered by symptomatic treatment. There were no immediate adverse reactions to the CIK cells treatment.
Survival analysis
The median follow-up period for all patients was 54.5 months (range, 6.5–129.5 months). By the end of follow-up, 19.7% (24/122) of the patients died. The 1-, 3-, and 5-year OS rates for the whole study population after postoperative adjuvant chemotherapy were 98.3%, 90.2%, and 80.3%, respectively; while the 1-, 3-, and 5-year DFS rate for these patients were 91.7%, 65.7%, and 58.8%, respectively.
We firstly assessed the difference of disease free survival (DFS) in the CIK and control groups. The 1-, 2-, 3-, 4-, and 5-year DFS rates were 98.3, 85.8, 80.2, 73.6, and 70.7%, respectively, in the CIK group, and 85.5, 61.0, 52.4, 48.3, and 48.3%, respectively, in the control group. There was a significant difference between the two groups (; log-rank test, P = .0024), with the CIK group showing a significantly improved DFS rate compared with the control group.
Figure 2. Kaplan–Meier estimates of disease-free survival (DFS) (a) and overall survival (OS) (b) of patients with CRC by treatment group. Significantly improved DFS and OS were observed in the CIK group (n = 60) versus the control group (n = 62)
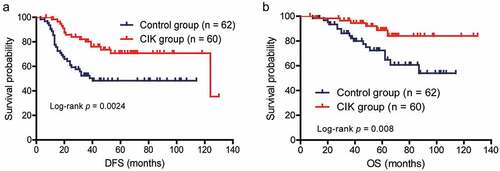
To assess the survival benefit resulting from CIK cell treatment, the difference of OS in the two groups was also evaluated. The 1-, 2-, 3-, 4-, and 5-year OS rates were 98.3, 98.3, 96.5, 92.0, and 88.7%, respectively, in the CIK group, and 98.4, 93.4, 84.2, 75.0, and 72.4%, respectively, in the control group. Similarly, Kaplan-Meier curves showed that the patients who received CIK cell treatment also exhibited a better OS than the control group (; log-rank test, P = .008).
Univariate and multivariate analysis
Univariate and multivariate Cox proportional hazards regression analysis were used to evaluate the impact of CIK cell treatment on the prognosis of patients with CRC. Early T stage, adequate duration of chemotherapy, and CIK cell treatment were significantly associated with better DFS in the univariate analysis (). Further multivariate survival analysis indicated that early T stage and CIK cell treatment were independent prognostic factor for improved DFS (). However, only CIK cell treatment was associated with improved OS regardless of in the univariate or multivariate survival analysis ().
Table 2. Univariate and multivariate analysis of disease-free survival in patients with CRC
Table 3. Univariate and multivariate analysis of overall survival in patients with CRC
Subgroup analysis
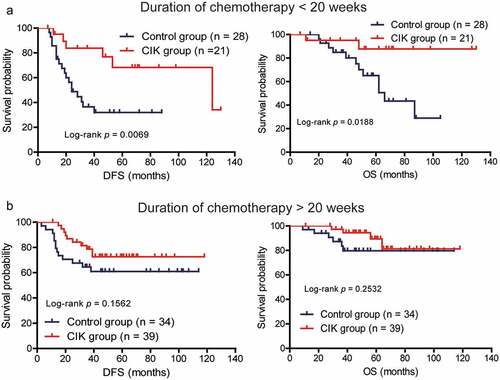
The T stage and chemotherapy duration have been associated with the prognosis of patients; therefore, we assessed which subgroup of patients with CRC, classified according to these clinical parameters, could benefit most from CIK cell immunotherapy. In the low-risk stage (T1, T2, T3) group, CIK cell treatment did not significantly affect the DFS and OS of patients (; P = .2944 for DFS; P = .3498 for OS). In the high-risk stage (T4) group, CIK cell immunotherapy significantly prolonged the DFS and OS in comparison with the control group (; P = .0014 for DFS; P = .0038 for OS). Moreover, for patients receiving less than 20 weeks of chemotherapy, the DFS and OS of patients with CRC were significantly improved in the CIK group compared to the control group (; P = .0069 for DFS; P = .0188 for OS). By contrast, patients who received more than 20 weeks of chemotherapy might derive some survival benefit from CIK cell immunotherapy; however, this benefit was not statistically significant (; P = .1563 for DFS; P = .2532 for OS). Moreover, the correlation between the mean number of the total infused CIK cells and patients’ prognosis was also investigated. Based on the median of mean number of total infused CIK cells, patients were divided into high-dose CIK cells group and low-dose CIK cells group. We found that patients in the high-dose CIK cells group exhibited significantly better DFS than patients in the low-dose CIK cells group, but the OS did not significantly differ between the two groups (Supplementary Figure S2).
Figure 3. Subgroup analysis to estimate the survival benefits from sequential CIK cell immunotherapy according to the T stage. (a) Sequential CIK cell immunotherapy slightly prolonged the disease-free survival (DFS) and overall survival (OS) of patients with T1-3 stage disease. (b) Sequential CIK cell immunotherapy significantly improved the DFS and OS of patients with T4 stage disease
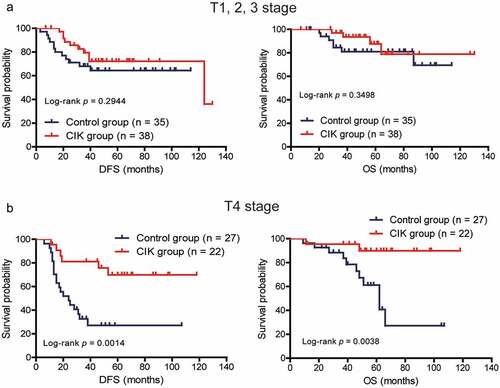
Figure 4. Subgroup analysis to estimate the survival benefits from sequential CIK cell immunotherapy according to the duration of chemotherapy. (a) Sequential CIK cell immunotherapy significantly prolonged the disease-free survival (DFS) and overall survival (OS) of patients receiving less than 20 weeks of chemotherapy. (b) Sequential CIK cell immunotherapy slightly prolonged the DFS and OS of patients receiving more than 20 weeks of chemotherapy
Discussion
Adjuvant chemotherapy after surgery is the standard treatment modality for stage III and part of stage II or stage IV colorectal cancer patients. However, 35% patients experience tumor recurrence after surgery and adjuvant chemotherapy, and most relapses occurred within 2 years after surgery.Citation3 Besides, patients who are intolerable of chemotherapy and discontinue as a consequence of treatment-related adverse event may have increased relapse risk.Citation23,Citation24 Thereafter, such postoperative CRC patients after adjuvant chemotherapy require available maintenance or alternative treatments to prevent tumor recurrence and improve their prognosis. CIK cell immunotherapy has shown potential efficacy in controlling tumor growth and prolonging patients’ survival. Several clinical studies have confirmed survival benefit and safety of CIK cell immunotherapy for patients with cancers.Citation14,Citation25 Recently, the role of CIK cell treatment in patients with CRC was also reported.Citation22,Citation26–Citation28 However, to date, there is only one study on the therapeutic effects of adjuvant CIK cell treatment in patients with CRC after surgery, and their results were limited by small sample size and short follow-up time.Citation22 Thus, in the present study, we sought to further validate the efficacy of sequential CIK cell treatment combined with adjuvant chemotherapy in patients with CRC after curative resection through a retrospective analysis of a relatively larger sample size of 122 cases.
The results presented here indicate that, relative to the control group that received only postoperative adjuvant chemotherapy, patients with CRC who received sequential adjuvant chemotherapy plus CIK cell treatment exhibited improved DFS and OS rates. Furthermore, multivariate survival analysis suggested that the CIK cell treatment was an independent prognostic factor for DFS and OS, indicating that CIK treatment is an effective intervention that prevents disease recurrence and prolongs the survival of patients with CRC after postoperative adjuvant chemotherapy. Previously, Zhu et al. reported the improvement of DFS, but not OS, in twenty-one patients with CRC received adjuvant CIK cell treatment.Citation22 The difference in effect of CIK cell treatment on OS of patients with CRC in our and Zhu’s studies might be a consequence of the sample size, different patients’ condition, and chemotherapeutic regimes. Nonetheless, these results collectively provide strong evidence in support of the efficacy of sequential adjuvant chemotherapy plus CIK cell–based treatment for improved outcomes of patients with CRC.
Preclinical studies have shown that CIK cells possess cytotoxic activities against different solid tumors,Citation25 including colorectal cancer.Citation29 Mechanism analysis found that CIK cell cytotoxicity is mediated by releasing perforin and granzyme granules and dependent on several activating receptors such as NKG2D, NKp30, NKp44, NKp46 and DNAM-1.Citation25 Thus, the clinical benefit of CIK cells may be due to the direct tumor killing activity in a MHC-independent way,Citation30 which is different from chemotherapeutic cytotoxic drugs. Besides, CIK cells can enhance the efficacy of chemotherapy in patients by eliminating potential or residual tumor cells including even drug-resistant tumor cells and putative cancer stem cells.Citation31,Citation32 Furthermore, CIK cell treatment can improve the immunological status of patients with CRC.Citation33 Our results also suggested that the percentages of CD3+CD56+ subsets, representing the main anti-tumor immuno-effector cells,Citation34,Citation35 significantly increased after the fourth treatment cycle in patients with CRC. Thus, these results together with our findings indicated that sequential adjuvant chemotherapy plus CIK cell treatment have synergistic anti-tumor effects and might be an optimized modality to gain improved therapeutic efficacy in patients with CRC.
Whether or not to receive adjuvant chemotherapy, as well as the duration of chemotherapy, for patients with CRC after surgery is depended on the recurrence risk. Patients with T4 category have by far the worst survival rate regardless of stage II or III disease,Citation36 and adequate duration of adjuvant chemotherapy is recommended to these patients.Citation37,Citation38 Consistent with previous observations,Citation36–Citation39 our study also found that the 5-year OS rate of patients with T4 category or shorter chemotherapy duration was approximately 60%, whereas the 5-year OS rate of patients with T1-3 category or longer chemotherapy duration was approximately 80%. In the subgroup analyses, CIK cell immunotherapy was found to be significantly associated with an improved DFS and OS in patients with T4 category or shorter chemotherapy duration, but this association was absent in patients with T1-3 category or longer chemotherapy duration. The reason for this discrepancy may be due to patients with the low-risk stage already possessing better prognosis,Citation36 and hence might derive some benefit from adjuvant CIK cell treatment, but the benefit would not be statistically significant. Conversely, patients in the high-risk stage (T4 category) exhibited worst OS rates, and CIK cell treatment could significantly improve the prognosis of this subset of patients. Moreover, patients in the CIK group had more advanced disease, but exhibited significantly improved prognosis than that in the control group, which indicated the therapeutic effect of CIK cells for the high-risk patients. Thus, our findings provide evidence to support the recommendation of sequential CIK cell treatment for patients who are with high-risk T4 stage and insufficient chemotherapy duration.
Some survival benefits have been observed in patients with CRC; however, the results should interpret carefully and more studies are required. The present study has a few shortcomings. First, selection bias was the most important bias for this study, because all the patients were from our hospital. Second, the frequency of follow-up in the chemotherapy alone group was lower than in the CIK group. Therefore, a well-designed prospective study should be performed to confirm these results further. However, our study demonstrated that sequential CIK cell treatment combined with adjuvant chemotherapy is a safe and potential therapeutic modality for patients with CRC.
In conclusion, this single-center retrospective study revealed that sequential CIK cell treatment combined with adjuvant chemotherapy could improve the DFS and OS for patients with CRC. Moreover, patients who are with high-risk T4 stage and insufficient chemotherapy duration could benefit more from CIK cell immunotherapy. External validation and prospective randomized studies are warranted to further confirm the present findings and to further define optimal combinational treatment modality for patients with CRC.
Patients and methods
Patients
Between January 16, 2007 and October 30, 2016, the medical records of patients with colon or rectal cancer from a computerized database in the Sun Yat-sen University Cancer Center were retrospective reviewed. Patients were eligible if they have undergone complete resection of histologically proven high-risk stage II or stage III CRC or they have had their resectable stage IV disease underwent radical resection. Patients who relapsed within 1 month after surgery were defined as non-curative resection and were excluded from this study. Patients were also excluded from the study based on the following criteria: a concurrent malignancy other than CRC, prior immunotherapy, with unresectable distant metastasis at diagnosis, the occurrence of serious adverse events during chemotherapy, without receiving surgery or postoperative adjuvant chemotherapy, or receiving CIK cell immunotherapy after recurrence. A total of 122 patients with CRC met the described criteria and were enrolled in this study. Among them, 60 patients received sequential CIK cell treatment (CIK group), while the other 62 patients diagnosed at the same or near day but without CIK cell treatment were used as the control group for comparisons. The treatment decision regarding whether to receive adjuvant CIK cell therapy was made basing on the patients’ preference after complete communications and understandings of each possible accessible therapeutic option by our multidisciplinary team of doctors, as described in previous studies.Citation40
The study fulfilled the Helsinki declaration and Guidelines for Good Clinical Practice and was approved by the Institutional Review Board of the Sun Yat-sen University Cancer Center. Written informed consent from each patient was sought before receiving CIK cell treatment.
Treatment procedures
All patients underwent completion resection. Following surgery, all patients in the control and CIK groups received adjuvant chemotherapy with FOLFOX (bolus and infused fluorouracil with oxaliplatin), CAPOX (oxaliplatin and capecitabine), or single-agent capecitabine regimen. For patients receiving FOLFOX, treatment was given every 2 weeks with the intention of delivering twelve cycles to patients assigned 24 weeks of therapy. For patients receiving CAPOX or single-agent capecitabine, treatment was given every 3 weeks with an intention of delivering eight cycles to patients assigned 24 weeks of therapy. The duration of chemotherapy included neoadjuvant and adjuvant treatment stage (Supplementary Table S1). Dose reductions or treatment delays were calculated according to the treatment- related adverse events, which were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events version 4.0.
For patients treated with sequential adjuvant chemotherapy plus CIK cell immunotherapy, the CIK cells transfusions were started 4 weeks after last chemotherapy. In general, patients would receive at least 4 cycles of CIK cell infusion with 1-week intervals between each cycle, and then another 4 cycles of treatment would be given at an interval of two weeks. The detailed CIK cell treatment protocol is shown in Supplementary Figure S3. The patients were eligible for CIK cell maintenance treatment at an interval of 1- to 3-month if they had stable disease (Supplementary Table S2).
CIK cell preparation
Autologous CIK cells were prepared using a standard method as described in our previous studies.Citation16,Citation18 Briefly, peripheral blood mononuclear cells were separated using Ficoll-Hypaque density centrifugation, rinsed twice using saline solution, and then suspended in X-VIVO 15 serum-free medium (Lonza, Visp, Switzerland). After culturing for 1 h in the atmosphere with 5% CO2 at 37°C, the non-adherent cells were removed by aspiration and the cell density was adjusted to 2 × 106 cells/ml using X-VIVO 15 medium supplemented with 1,000 U/mL recombinant human IFN-γ (ShangClone, Shanghai, China) for the first 24 h. Subsequently, 1,000 U/mL IL-2 (Beijing Sihuan Pharm, Beijing, China), 100 ng/mL mouse anti-human CD3 monoclonal antibody (R&D Systems, MN, USA), and 100 U/mL IL-1α (Life Technologies, CA, USA) were added to the medium. Fresh medium containing IL-2 was added periodically according to the cell growth and the CIK cells were harvested at 14 d. Before infusion, a fraction of the cultured CIK cells was collected to evaluate the number, viability, phenotype analysis, and possible contamination; the majority of the harvested CIK cells were administered intravenously (iv) into the patients within 30 minutes. Before and after transfusion, vital signs such as breath, pulse, blood pressure, and temperature were monitored and recorded.
Phenotypic analysis of CIK cells
Before and after expansion, the phenotypes of CIK cells were characterized using flow cytometry (FC500, Beckman Coulter, CA, USA). The following mouse-anti-human monoclonal antibodies were used: anti-CD3-Phycoerythrin (PE)-cyanine (Cy) 5 (Clone: HIT3a), anti-CD4-PE-Cy7 (Clone: SK3), anti-CD8-PE (Clone: HIT8a), and anti-CD56-fluorescein isothiocyanate (FITC) (Clone: B159) (all from BD Bioscience, NJ, USA). After washing twice, the cells were analyzed using a Cytomics™ FC500 Flow Cytometer (Beckman Coulter, USA), and data analysis was performed using CXP analysis software (Beckman Coulter).
Follow-up
All the patients were followed-up regularly after discharge, including clinic or telephone contact once every 3–6 months during the first 2 year, every 6–12 months for the next 3 years, and every year thereafter. Postoperative follow-up included clinical, laboratory and instrument examinations. Routine blood examination, carcinoembryonic antigen (CEA) levels, colonoscopy, and chest/abdominal/pelvis computed tomography were followed up every 6–12 months. The diagnosis of recurrence was made on the basis of imaging and, if necessary, cytologic analysis or biopsy. An elevated carcinoembryonic antigen level as a solitary finding was not accepted as evidence of relapse. DFS was defined from the date of completion surgery to the date of first recurrence (local or distant) or date of last follow-up (Supplementary Table S3). Patients who died before experiencing a disease recurrence were considered censored at their date of death. The diagnosis of recurrence was made on the basis of imaging, and only an elevated carcinoembryonic antigen level was not accepted as evidence of relapse. OS was defined from the time of completion surgery until death or the end of follow-up (Supplementary Table S3). If recurrence or metastases were suspected during follow-up, remedial treatment included surgery or systemic chemotherapy was recommended by our multidisciplinary team. Otherwise, supportive treatment was provided for patients who were intolerant of any systemic and local treatment.
Statistical analysis
Differences in demographic and clinical variables of the two groups were tested using the Pearson χ2 test or the Fisher’s exact test where appropriate. Student’s t-test was used to compare the difference of CIK cell phenotype among cycles. DFS and OS curves were calculated using the Kaplan–Meier method and compared using the log-rank test. Hazard ratios with 95% confidence intervals (CIs) were calculated using the Cox proportional hazards model. A difference of 0.05 was considered significant in all analyses. SPSS software (Statistical Package for the Social Science, version 17.0, IBM Corp. Armonk, NY, USA) and GraphPad Prism 5 (Version 5.01, GraphPad Software, Inc.) were used for all the statistical evaluations.
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest.
Supplemental Material
Download ()Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–9. doi:10.3322/caac.21262.
- Obrand DI, Gordon PH. Incidence and patterns of recurrence following curative resection for colorectal carcinoma. Dis Colon Rectum. 1997;40(1):15–24. doi:10.1007/BF02055676.
- Sargent D, Sobrero A, Grothey A, O’Connell MJ, Buyse M, Andre T, Zheng Y, Green E, Labianca R, O’Callaghan C, et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009;27(6):872–877. doi:10.1200/JCO.2008.19.5362.
- Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330(6005):827–830. doi:10.1126/science.1195300.
- Kiessling R, Wasserman K, Horiguchi S, Kono K, Sjoberg J, Pisa P, Petersson M. Tumor-induced immune dysfunction. Cancer Immunol Immunother. 1999;48(7):353–362. doi:10.1007/s002620050586.
- Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Gukas ID. The effects of surgery on tumor growth: a century of investigations. Ann Oncol. 2008;19(11):1821–1828. doi:10.1093/annonc/mdn386.
- Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12(2):133–143. doi:10.1038/nrc3184.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013.
- Clarke MF. Clinical and Therapeutic Implications of Cancer Stem Cells. N Engl J Med. 2019;380(23):2237–2245. doi:10.1056/NEJMra1804280.
- Zhang D, Tang DG, Rycaj K. Cancer stem cells: regulation programs, immunological properties and immunotherapy. Semin Cancer Biol. 2018;52:94–106. doi:10.1016/j.semcancer.2018.05.001.
- Miyamoto S, Kochin V, Kanaseki T, Hongo A, Tokita S, Kikuchi Y, Takaya A, Hirohashi Y, Tsukahara T, Terui T, et al. The antigen ASB4 on cancer stem cells serves as a target for CTL immunotherapy of colorectal cancer. Cancer Immunol Res. 2018;6(3):358–369. doi:10.1158/2326-6066.CIR-17-0518.
- Fan J, Shang D, Han B, Song J, Chen H, Yang JM. Adoptive cell transfer: is it a promising immunotherapy for colorectal cancer? Theranostics. 2018;8(20):5784–5800. doi:10.7150/thno.29035.
- Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med. 1991;174(1):139–149. doi:10.1084/jem.174.1.139.
- Schmeel LC, Schmeel FC, Coch C, Schmidt-Wolf IG. Cytokine-induced killer (CIK) cells in cancer immunotherapy: report of the international registry on CIK cells (IRCC). J Cancer Res Clin Oncol. 2015;141(5):839–849. doi:10.1007/s00432-014-1864-3.
- Zhou Y, Chen CL, Jiang SW, Feng Y, Yuan L, Chen P, Zhang L, Huang S, Li J, Xia J-C, et al. Retrospective analysis of the efficacy of adjuvant CIK cell therapy in epithelial ovarian cancer patients who received postoperative chemotherapy. Oncoimmunology. 2019;8(2):e1528411. doi:10.1080/2162402X.2018.1528411.
- Pan QZ, Tang Y, Wang QJ, Li YQ, Zhang L, Li XD, Zhao -J-J, Weng D-S, Liu Q, Huang L-X, et al. Adjuvant cellular immunotherapy in patients with resected primary non-small cell lung cancer. Oncoimmunology. 2015;4(9):e1038017. doi:10.1080/2162402X.2015.1038017.
- Pan K, Li YQ, Wang W, Xu L, Zhang YJ, Zheng HX, Zhao -J-J, Qiu H-J, Weng D-S, Li -J-J, et al. The efficacy of cytokine-induced killer cell infusion as an adjuvant therapy for postoperative hepatocellular carcinoma patients. Ann Surg Oncol. 2013;20(13):4305–4311. doi:10.1245/s10434-013-3144-x.
- Pan K, Guan XX, Li YQ, Zhao JJ, Li JJ, Qiu HJ, Weng D-S, Wang Q-J, Liu Q, Huang L-X, et al. Clinical activity of adjuvant cytokine-induced killer cell immunotherapy in patients with post-mastectomy triple-negative breast cancer. Clin Cancer Res. 2014;20(11):3003–3011. doi:10.1158/1078-0432.CCR-14-0082.
- Li JJ, Gu MF, Pan K, Liu LZ, Zhang H, Shen WX, Xia J-C. Autologous cytokine-induced killer cell transfusion in combination with gemcitabine plus cisplatin regimen chemotherapy for metastatic nasopharyngeal carcinoma. J Immunother. 2012;35(2):189–195. doi:10.1097/CJI.0b013e318241d9de.
- Zhou X, Mo X, Qiu J, Zhao J, Wang S, Zhou C, Su Y, Lin Z, Ma H. Chemotherapy combined with dendritic cell vaccine and cytokine-induced killer cells in the treatment of colorectal carcinoma: a meta-analysis. Cancer Manag Res. 2018;10:5363–5372. doi:10.2147/CMAR.S173201.
- Liu Y, Zheng Z, Zhang Q, Zhou X, Feng Y, Yan A. FOLFOX regimen plus dendritic cells-cytokine-induced killer cells immunotherapy for the treatment of colorectal cancer: a meta-analysis. Onco Targets Ther. 2017;10:2621–2633. doi:10.2147/OTT.S138011.
- Zhu Y, Zhang H, Li Y, Bai J, Liu L, Liu Y, Qu Y, Qu X. Efficacy of postoperative adjuvant transfusion of cytokine-induced killer cells combined with chemotherapy in patients with colorectal cancer. Cancer Immunol Immunother. 2013;62(10):1629–1635. doi:10.1007/s00262-013-1465-z.
- Iveson TJ, Kerr RS, Saunders MP, Cassidy J, Hollander NH, Tabernero J, Haydon A, Glimelius B, Harkin A, Allan K, et al. 3 versus 6 months of adjuvant oxaliplatin-fluoropyrimidine combination therapy for colorectal cancer (SCOT): an international, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2018;19(4):562–578. doi:10.1016/S1470-2045(18)30093-7.
- Grothey A, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, Souglakos J, Shi Q, Kerr R, Labianca R, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378(13):1177–1188. doi:10.1056/NEJMoa1713709.
- Mata-Molanes JJ, Sureda Gonzalez M, Valenzuela Jimenez B, Martinez Navarro EM, Brugarolas Masllorens A. Cancer immunotherapy with cytokine-induced killer cells. Target Oncol. 2017;12(3):289–299. doi:10.1007/s11523-017-0489-2.
- Zhao H, Wang Y, Yu J, Wei F, Cao S, Zhang X, Dong N, Li H, Ren X. Autologous cytokine-induced killer cells improves overall survival of metastatic colorectal cancer patients: results from a phase II clinical trial. Clin Colorectal Cancer. 2016;15(3):228–235. doi:10.1016/j.clcc.2016.02.005.
- Xie Y, Huang L, Chen L, Lin X, Chen L, Zheng Q. Effect of dendritic cell-cytokine-induced killer cells in patients with advanced colorectal cancer combined with first-line treatment. World J Surg Oncol. 2017;15(1):209. doi:10.1186/s12957-017-1278-1.
- Zhang J, Zhu L, Zhang Q, He X, Yin Y, Gu Y, Guo R, Lu K, Liu L, Liu P, et al. Effects of cytokine-induced killer cell treatment in colorectal cancer patients: a retrospective study. Biomed Pharmacother. 2014;68(6):715–720. doi:10.1016/j.biopha.2014.07.010.
- Kim JS, Kim YG, Park EJ, Kim B, Lee HK, Hong JT, Kim Y, Han S-B. Cell-based immunotherapy for colorectal cancer with cytokine-induced killer cells. Immune Netw. 2016;16(2):99–108. doi:10.4110/in.2016.16.2.99.
- Verneris MR, Karami M, Baker J, Jayaswal A, Negrin RS. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood. 2004;103(8):3065–3072. doi:10.1182/blood-2003-06-2125.
- Schmidt-Wolf IG, Lefterova P, Johnston V, Scheffold C, Csipai M, Mehta BA, Tsuruo T, Huhn D, Negrin RS. Sensitivity of multidrug-resistant tumor cell lines to immunologic effector cells. Cell Immunol. 1996;169(1):85–90. doi:10.1006/cimm.1996.0094.
- Gammaitoni L, Giraudo L, Macagno M, Leuci V, Mesiano G, Rotolo R, Sassi F, Sanlorenzo M, Zaccagna A, Pisacane A, et al. Cytokine-induced killer cells kill chemo-surviving melanoma cancer stem cells. Clin Cancer Res. 2017;23(9):2277–2288. doi:10.1158/1078-0432.CCR-16-1524.
- Lin T, Song C, Chuo DY, Zhang H, Zhao J. Clinical effects of autologous dendritic cells combined with cytokine-induced killer cells followed by chemotherapy in treating patients with advanced colorectal cancer: a prospective study. Tumour Biol. 2016;37(4):4367–4372. doi:10.1007/s13277-015-3957-2.
- Schmidt-Wolf IG, Lefterova P, Mehta BA, Fernandez LP, Huhn D, Blume KG, Weissman IL, Negrin RS. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp Hematol. 1993;21:1673–1679.
- Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153:1687–1696.
- Snaebjornsson P, Coupe VM, Jonasson L, Meijer GA, van Grieken NC, Jonasson JG. pT4 stage II and III colon cancers carry the worst prognosis in a nationwide survival analysis. Shepherd’s local peritoneal involvement revisited. Int J Cancer. 2014;135(2):467–478. doi:10.1002/ijc.28676.
- Andre T, Vernerey D, Mineur L, Bennouna J, Desrame J, Faroux R, Fratte S, Hug de Larauze M, Paget-Bailly S, Chibaudel B, et al. Three versus 6 months of oxaliplatin-based adjuvant chemotherapy for patients with stage III colon cancer: disease-free survival results from a randomized, open-label, International Duration Evaluation of Adjuvant (IDEA) France, phase III trial. J Clin Oncol. 2018;36(15):1469–1477. doi:10.1200/JCO.2017.76.0355.
- Lieu C, Kennedy EB, Bergsland E, Berlin J, George TJ, Gill S, Gold PJ, Hantel A, Jones L, Mahmoud N, et al. Duration of oxaliplatin-containing adjuvant therapy for stage III colon cancer: ASCO clinical practice guideline. J Clin Oncol. 2019;37(16):1436–1447. doi:10.1200/JCO.19.00281.
- Kumar A, Kennecke HF, Renouf DJ, Lim HJ, Gill S, Woods R, Speers C, Cheung WY. Adjuvant chemotherapy use and outcomes of patients with high-risk versus low-risk stage II colon cancer. Cancer. 2015;121(4):527–534. doi:10.1002/cncr.29072.
- Qiao G, Wang X, Zhou L, Zhou X, Song Y, Wang S, Zhao L, Morse MA, Hobeika A, Song J, et al. Autologous dendritic cell-cytokine induced killer cell immunotherapy combined with S-1 plus cisplatin in patients with advanced gastric cancer: a prospective study. Clin Cancer Res. 2019;25(5):1494–1504. doi:10.1158/1078-0432.CCR-18-2360.
