ABSTRACT
The fms-related tyrosine kinase 3 (FLT3) ligand (FLT3LG) binds to FLT3 on dendritic cells to stimulate their differentiation and expansion, hence facilitating tumor antigen cross-presentation and anticancer immune responses. A recent study by Abrahamsson et al. demonstrates that, in patients receiving a hepatic arterial infusion of oxaliplatin for the treatment of colorectal cancer metastases, an increase in circulating FLT3LG predicts long-term survival of those individuals whose metastases have been rendered resectable. Thus, FLT3LG constitutes a potential biomarker of immune activation by immunogenic cell death-inducing chemotherapeutics such as oxaliplatin.
Abbreviations: DC, dendritic cell; FLT3, fms-related tyrosine kinase 3; FLT3LG, FLT3 ligand; ICI, immune checkpoint inhibitor; OXA, oxaliplatin
Editorial
Immunogenic cell death (ICD) refers to a type of cellular demise (usually by apoptosis), that, rather than being tolerogenic, elicits an adaptive immune response against antigens of the dying entity. ICD has been described in cancer cells following cytotoxic or cytolytic interventions such as chemotherapy, radiotherapy or oncolytic virotherapy.Citation1 The mechanisms of ICD involve the release and surface exposure of danger-associated molecular patterns (DAMPs) that attract antigen-capturing immune sentinels such as dendritic cells (DCs). Activation of recruited DCs within the tumor bed ignites their migration to the secondary lymphoid organs (or alternatively their movement to tertiary lymphoid structures within the tumor) where they cross-present tumor antigens to T lymphocytes, thus priming an antitumor response. Not only tumor-specific CD8+ T cells can eliminate residual cancer cells spared by the treatment, but they also generate an immune memory compartment that can protect from tumor recurrence.Citation1–Citation3
A series of DAMPs and cytokines has been characterized upon chemotherapy as hallmarks of ICD. They include calreticulin (CALR) exposure on the outer layer of the plasma membrane, the release of the nucleotide ATP and of the proteins high–mobility group box 1 (HMGB1) and annexin A1 (ANXA1) into the tumor microenvironment, as well as the production of type I interferons (IFN) and of chemokines like C-X-C motif chemokine ligand 10 (CXCL10).Citation1–Citation4 In vitro screening for these hallmarks, in vivo vaccination experiments, as well as comparisons of anticancer drug effects in immunocompetent versus immunodeficient hosts, led to the identification of several pharmaceutical agents capable of inducing ICD.Citation5–Citation7 Such ICD inducers include the platinum salt oxaliplatin (OXA), anthracyclines such as doxorubicin (DOX) or daunorubicin (DAU), as well as the alkylating agent cyclophosphamide.Citation4 Of note, certain chemotherapies that do not produce the whole spectrum of ICD hallmarks can complement each other to stimulate bona fide ICD. For instance, cisplatin (CDDP) fails to elicit ICD as it is unable to trigger the endoplasmic reticulum stress module responsible for CALR translocation to the plasma membrane. However, a combination of CDDP with the ER stress inducers thapsigargin or tunicamycin facilitated CRT exposure at the cancer cell surface, resulting in the induction of full-blown ICD and superior therapeutic efficacy.Citation8 Thus, preclinical studies have proven the that ICD-inducing chemotherapeutics are de facto immunotherapeutics and that the induction of anticancer immune responses is indispensable for the long-term efficacy of any kind of antineoplastic treatment.
Cumulative evidence supports the translation of the concept of ICD to the clinics. First, variations of the expression level of ICD-related DAMPs and receptors, as well as loss-of-function mutations affecting the corresponding signaling cascades, have prognostic and predictive impact.Citation9,Citation10 For example, formyl peptide receptor 1 (FPR1) is expressed on DCs and detects the DAMP ANXA1. Upon OXA-based chemotherapy, colorectal cancer (CRC) patients that are homozygous for a loss-of-function allele of FPR1 exhibit shorter progression-free and overall survival than subjects harboring one or two copies of the wild-type allele. Similarly, breast cancer patients receiving an anthracycline-based adjuvant chemotherapy have a poor prognosis when they bear one or two copies of the FPR1 loss-of-function allele.
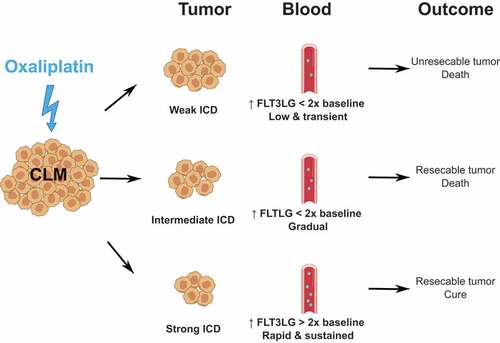
Figure 1. Serum FLT3LG as a prognostic biomarker of ICD-induced systemic anticancer immunity in colorectal cancer
Second, ICD-inducing chemotherapies have demonstrated a positive interaction with immunotherapies. T cell activation consecutive to ICD sensitizes to immunotherapy with checkpoint blockade or interleukin (IL)-2.Citation11–Citation14 A clinical trial compared two consolidation treatments in acute myeloid leukemia. These regimens consisted of dual immunotherapy with IL-2 + histamine dihydrochloride after cytarabine-based chemotherapy comprising or not the ICD inducer DAU. Interestingly, higher levels of circulating effector memory CD8+ T cells at the beginning of immunotherapy, together with extended survival, were observed in subjects that received the anthracycline DAU.Citation11 Moreover, a Phase II trial enrolling patients with triple-negative breast cancer revealed an improved objective response rate in a cohort co-infused with the ICD inducer DOX + the immune checkpoint inhibitor (ICI) nivolumab (anti-PD-1) over individuals treated with the same ICI + the non ICD chemotherapeutic CDDP.Citation12
Finally, A.H. Ree’s group recently reported on the prognostic value of circulating levels of the hematopoietic cytokine fms-related tyrosine kinase 3 (FLT3) ligand (FLT3LG) in CRC after OXA-based chemotherapy.Citation15,Citation16 FLT3LG is produced by stromal cells in the bone marrow and by lymphocytes in the tumor microenvironment.Citation17,Citation18 FLT3LG regulates the differentiation and expansion of conventional and plasmacytoid DCs from most FLT3+ bone marrow progenitors, including both lymphoid and myeloid common progenitors. Moreover, mature DCs express FLT3 and proliferate in the presence of FLT3LG.Citation19,Citation20 In the clinic, elevation of serum FLT3 has been described as a marker of recovery from chemotherapy-induced myelosuppression.Citation21,Citation22 In locally advanced CRC, Kalanxhi E. et al observed higher levels of serum FLT3LG at baseline and post-neoadjuvant therapy with OXA in patients with signs of a histologic tumor response.Citation15 Moreover, elevated levels of circulating FLT3LG post-neoadjuvant treatment strongly correlated with better progression-free survival, mostly due to a reduced risk of metastatic events. In their latest study, Abrahamsson H. et al measured circulating FLT3LG first at baseline and then along the course of 1st-line chemotherapy with OXA in individuals with CRC liver metastases.Citation16 Treatment consisted of 1 to 3 sequences, each sequence consisting of one hepatic arterial infusion of OXA (complemented with oral capecitabine) every 2 weeks over an 8-week period. Liver metastases that responded to treatment underwent surgical resection. Interestingly, the mean serum level of FLT3LG rose following the 1st sequence of OXA in all patients. This increase of circulating FLT3LG was similar between the two groups of patients stratified according to resectability of CRC liver metastases. Of note, eligibility for surgical removal was associated with wild-type RAS status, rather than the more aggressive RAS mutation. Remarkably, within the resectable category (n = 33/55; 60%), patients that remained alive at the end of the 8 to 12-year follow-up (n = 9/33; 27.7%) demonstrated an immediate and prolonged 2-fold increase of circulating FLT3LG (from 76.5 pg/ml at baseline to 159 pg/ml post-1st sequence of OXA on average). In contrast, mean FLT3LG levels were lower by 40 pg/ml in resected individuals that ultimately succumbed to the disease (n = 24/33; 72.3%).Citation16 Further investigations should confirm FLT3LG as a prognosis biomarker in CRC. Collectively, accretion of serum FLT3LG reflected the establishment of a systemic anticancer immune response consecutive to OXA-mediated ICD and to supportive DC expansion.
Some preclinical investigations have comforted the beneficial role of systemic FLT3LG in sensitizing cancer to immunotherapeutic approaches.Citation23,Citation24 Nowadays, ICIs targeting the immune checkpoints PD-1, PD-L1 or CTLA-4 represent the most transversal cancer treatment. Nevertheless, only a fraction of patients do benefit from ICI monotherapies due to primary or acquired cancer resistance. Such resistance mechanisms include DC dysfunction or reduced DC recruitment, as well as decreased T cell priming and/or infiltration.Citation25 These mechanisms of resistance can be overcome by therapeutic agents that induce cancer ICD or facilitate DC recruitment/activation.Citation23,Citation24,Citation26–Citation36 The main DC subsets involved in cancer immunity are Ly6ChiCD11b+ monocyte-derived DCs and Clec9A+ conventional DCs.Citation23,Citation24,Citation26,Citation35,Citation37 M. Merad and colleagues showed limited efficacy of anti-PD-L1 therapy in a murine model of melanoma (B16).Citation23 However, when anti-PD-L1 was combined with repeated infusions of FLT3LG, together with intratumoral administrations of the toll-like receptor 3 agonist poly I:C, tumor growth control was achieved. Mechanistically, systemic FLT3LG triggers the differentiation of CD103+ conventional DCs from bone marrow progenitors, followed by DC expansion and accumulation in the tumor bed. These events favor DC activation upon the capture of tumor antigens and initiate their migration to the draining lymph nodes, where mature DCs efficiently prime CD8+ T cells. Remarkably, the tumor enrichment of DCs not only led to an expansion of effector T lymphocytes but also facilitated their access to the neoplastic core. Interestingly, this tritherapy of anti-PD-L1 + FLT3LG + poly I:C could synergize with BRAF inhibitors for efficient treatment of BRAF mutated melanoma.Citation23 Furthermore, FLT3LG + poly I:C can be advantageously combined with ICD-inducing radiotherapy to treat murine A20 lymphomas. This triple combination also sensitized the neoplasm to anti-PD-1 ICI, leading to complete and durable remission in most animals.Citation23 In a clinical trial, the combination of FLT3LG + poly I:C + radiotherapy was evaluated in 11 patients with indolent B cell lymphoma. The treatment promoted the peripheral expansion of CD8+ T cells, particularly of the naive and exhausted effector memory subsets. At censoring, the best overall responses recorded consisted of one complete and one partial responses, 6 stable and 2 progressive diseases. One more patient with a partial response remained under monitoring. In one subject with a systemic partial response, untreated (abscopal) lesions exhibited an increased infiltration by myeloid cells (mainly DCs and monocytes), as well as by lymphoid cells (notably exhausted CD8+ T cells, as well as naive and memory B cells).Citation24
In sum, there is overwhelming preclinical and clinical evidence that FLT3LG may be useful for stimulating the anticancer effects of DCs. Whether FLT3LG can overcome stress-induced (glucocorticoid-mediated) DC defects that negatively affect tumor immunosurveillanceCitation38 remains to be determined, it appears that, at least in specific circumstances, circulating FLT3LG levels may constitute a proxy for the assessment of incipient anticancer immune responses ignited by immunogenic chemotherapy. Future studies will have to evaluate whether the assessment of fluctuations in plasma FLT3LG concentrations, alone or in combination with other immune parameters, will yield a clinically useful biomarker for predicting the outcome of anticancer treatments.
Author disclosure
JGP and GK share ownership of patents for the use of caloric restriction mimetics to improve cancer chemo-immunotherapy. GK has been holding research contracts with Bayer Healthcare, Genentech, Glaxo Smyth Kline, Institut Mérieux, Lytix Pharma, PharmaMar, Sotio and Vasculox. GK is member of the Board of Directors of the Bristol Myers Squibb Foundation France. GK is a scientific co-founder of everImmune, Samsara Therapeutics, and Therafast Bio. The other author declares no conflict of interest.
Acknowledgments
JP is supported by the Seerave Foundation and the Société Française d’Hépatologie (AFEF). GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Association “Le Cancer du Sein, Parlons-en!”; Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Gustave Roussy Odyssea, the European Union Horizon 2020 Project Oncobiome; Fondation Carrefour; High-end Foreign Expert Program in China (GDW20171100085), Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology (ANR-18-IDEX-0001); the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and the SIRIC Cancer Research and Personalized Medicine (CARPEM).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–5.
- Serrano-Del Valle A, Anel A, Naval J, Marzo I. Immunogenic cell death and Immunotherapy of Multiple Myeloma. Front Cell Dev Biol. 2019;7:50. doi:10.3389/fcell.2019.00050.
- Garg AD, Agostinis P. Cell death and immunity in cancer: from danger signals to mimicry of pathogen defense responses. Immunol Rev. 2017;280:126–148. doi:10.1111/imr.12574.
- Vanmeerbeek I, Sprooten J, De Ruysscher D, Tejpar S, Vandenberghe P, Fucikova J, Spisek R, Zitvogel L, Kroemer G, Galluzzi L. et al. Trial watch: chemotherapy-induced immunogenic cell death in immuno-oncology. Oncoimmunology. 2020;9(1):1703449. doi:10.1080/2162402X.2019.1703449.
- Kepp O, Sauvat A, Leduc M, Forveille S, Liu P, Zhao L, Bezu L, Xie W, Zitvogel L, Kroemer G. et al. A fluorescent biosensor-based platform for the discovery of immunogenic cancer cell death inducers. Oncoimmunology. 2019;8(8):1606665. doi:10.1080/2162402X.2019.1606665.
- Humeau J, Levesque S, Kroemer G, Pol JG. Gold Standard Assessment of Immunogenic Cell death in Oncological Mouse Models. Methods Mol Biol. 2019;1884:297–315.
- Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, Apetoh L, Aranda F, Barnaba V, Bloy N. et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology. 2014;3(9):e955691. doi:10.4161/21624011.2014.955691.
- Martins I, Kepp O, Schlemmer F, Adjemian S, Tailler M, Shen S, Michaud M, Menger L, Gdoura A, Tajeddine N. et al. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene. 2011;30(10):1147–1158. doi:10.1038/onc.2010.500.
- Fucikova J, Moserova I, Urbanova L, Bezu L, Kepp O, Cremer I, Salek C, Strnad P, Kroemer G, Galluzzi L. et al. Prognostic and predictive value of DAMPs and DAMP-Associated processes in Cancer. Front Immunol. 2015;6:402. doi:10.3389/fimmu.2015.00402.
- Vacchelli E, Ma Y, Baracco EE, Sistigu A, Enot DP, Pietrocola F, Yang H, Adjemian S, Chaba K, Semeraro M. et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science. 2015;350:972–978. doi:10.1126/science.aad0779.
- Aurelius J, Mollgard L, Kiffin R, Ewald Sander F, Nilsson S, Thoren FB, Hellstrand K, Martner A. Anthracycline-based consolidation may determine outcome of post-consolidation immunotherapy in AML. Leuk Lymphoma. 2019;60(11):2771–2778. doi:10.1080/10428194.2019.1599110.
- Voorwerk L, Slagter M, Horlings HM, Sikorska K, van de Vijver KK, de Maaker M, Nederlof I, Kluin RJC, Warren S, Ong S. et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25(6):920–928. doi:10.1038/s41591-019-0432-4.
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R. et al. Overall survival with Durvalumab after Chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–2350. doi:10.1056/NEJMoa1809697.
- Pol JG, Caudana P, Paillet J, Piaggio E, Kroemer G. Effects of interleukin-2 in immunostimulation and immunosuppression. J Exp Med. 2020;217.
- Kalanxhi E, Meltzer S, Schou JV, Larsen FO, Dueland S, Flatmark K, Jensen BV, Hole KH, Seierstad T, Redalen KR. et al. Systemic immune response induced by oxaliplatin-based neoadjuvant therapy favours survival without metastatic progression in high-risk rectal cancer. Br J Cancer. 2018;118(10):1322–1328. doi:10.1038/s41416-018-0085-y.
- Abrahamsson H, Jensen BV, Berven LL, Nielsen DL, Saltyte Benth J, Johansen JS, Larsen FO, Johansen JS, Ree AH. Antitumour immunity invoked by hepatic arterial infusion of first-line oxaliplatin predicts durable colorectal cancer control after liver metastasis ablation: 8-12 years of follow-up. Int J Cancer. 2020;146:2019–2026. doi:10.1002/ijc.32847.
- Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, Nelson AE, Loo K, Kumar R, Rosenblum MD. et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat Med. 2018;24:1178–1191. doi:10.1038/s41591-018-0085-8.
- Lisovsky M, Braun SE, Ge Y, Takahira H, Lu L, Savchenko VG, Lyman SD, Broxmeyer HE. Flt3-ligand production by human bone marrow stromal cells. Leukemia. 1996;10:1012–1018.
- Durai V, Bagadia P, Briseno CG, Theisen DJ, Iwata A, JTt D. et al. Altered compensatory cytokine signaling underlies the discrepancy between Flt3(-/-) and Flt3l(-/-) mice. J Exp Med. 2018;215:1417–1435. doi:10.1084/jem.20171784.
- Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J Exp Med. 2003;198(2):305–313. doi:10.1084/jem.20030323.
- Greystoke A, O’Connor JP, Linton K, Taylor MB, Cummings J, Ward T, Maders F, Hughes A, Ranson M, Illidge TM. et al. Assessment of circulating biomarkers for potential pharmacodynamic utility in patients with lymphoma. Br J Cancer. 2011;104(4):719–725. doi:10.1038/sj.bjc.6606082.
- Wodnar-Filipowicz A, Lyman SD, Gratwohl A, Tichelli A, Speck B, Nissen C. Flt3 ligand level reflects hematopoietic progenitor cell function in aplastic anemia and chemotherapy-induced bone marrow aplasia. Blood. 1996;88(12):4493–4499. doi:10.1182/blood.V88.12.4493.bloodjournal88124493.
- Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, Casanova-Acebes M, Khudoynazarova M, Agudo J, Tung N. et al. Expansion and Activation of CD103 + Dendritic Cell Progenitors at the Tumor site enhances Tumor responses to Therapeutic PD-L1 and BRAF inhibition. Immunity. 2016;44(4):924–938. doi:10.1016/j.immuni.2016.03.012.
- Hammerich L, Marron TU, Upadhyay R, Svensson-Arvelund J, Dhainaut M, Hussein S, Zhan Y, Ostrowski D, Yellin M, Marsh H. et al. Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat Med. 2019;25(5):814–824. doi:10.1038/s41591-019-0410-x.
- Liu D, Jenkins RW, Sullivan RJ. Mechanisms of resistance to Immune Checkpoint Blockade. Am J Clin Dermatol. 2019;20:41–54. doi:10.1007/s40257-018-0389-y.
- Levesque S, Le Naour J, Pietrocola F, Paillet J, Kremer M, Castoldi F, Baracco EE, Wang Y, Vacchelli E, Stoll G. et al. A synergistic triad of chemotherapy, immune checkpoint inhibitors, and caloric restriction mimetics eradicates tumors in mice. Oncoimmunology. 2019;8(11):e1657375. doi:10.1080/2162402X.2019.1657375.
- Gujar S, Pol JG, Kroemer G. Heating it up: oncolytic viruses make tumors ‘hot’ and suitable for checkpoint blockade immunotherapies. Oncoimmunology. 2018;7:e1442169. doi:10.1080/2162402X.2018.1442169.
- Liu P, Zhao L, Kepp O, Kroemer G. Crizotinib – a tyrosine kinase inhibitor that stimulates immunogenic cell death. Oncoimmunology. 2019;20(7):1596652. doi:10.1080/2162402X.2019.1596652.
- Liu P, Zhao L, Pol J, Levesque S, Petrazzuolo A, Pfirschke C, Engblom C, Rickelt S, Yamazaki T, Iribarren K. et al. Crizotinib-induced immunogenic cell death in non-small cell lung cancer. Nat Commun. 2019;10(1):1486. doi:10.1038/s41467-019-09415-3.
- Manukian G, Bar-Ad V, Lu B, Argiris A, Johnson JM. Combining Radiation and Immune Checkpoint Blockade in the treatment of Head and Neck Squamous Cell Carcinoma. Front Oncol. 2019;9:122. doi:10.3389/fonc.2019.00122.
- Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, Yamazaki T, Poirier-Colame V, Newton A, Redouane Y. et al. Immunogenic Chemotherapy sensitizes Tumors to Checkpoint Blockade Therapy. Immunity. 2016;44(2):343–354. doi:10.1016/j.immuni.2015.11.024.
- Sivanandam V, LaRocca CJ, Chen NG, Fong Y, Warner SG, Viruses O. Immune Checkpoint Inhibition: the best of both Worlds. Mol Ther Oncolytics. 2019;13:93–106. doi:10.1016/j.omto.2019.04.003.
- Xie W, Mondragon L, Mauseth B, Wang Y, Pol J, Levesque S, Zhou H, Yamazaki T, Eksteen JJ, Zitvogel L. et al. Tumor lysis with LTX-401 creates anticancer immunity. Oncoimmunology. 2019;8(7):1594555. doi:10.1080/2162402X.2019.1594555.
- Yamazaki T, Pitt JM, Vetizou M, Marabelle A, Flores C, Rekdal Ø, Kroemer G, Zitvogel L. The oncolytic peptide LTX-315 overcomes resistance of cancers to immunotherapy with CTLA4 checkpoint blockade. Cell Death Differ. 2016;23(6):1004–1015. doi:10.1038/cdd.2016.35.
- Cauwels A, Van Lint S, Paul F, Garcin G, De Koker S, Van Parys A, Wueest T, Gerlo S, Van der Heyden J, Bordat Y. et al. Delivering Type I Interferon to Dendritic Cells empowers Tumor Eradication and Immune combination treatments. Cancer Res. 2018;78(2):463–474. doi:10.1158/0008-5472.CAN-17-1980.
- Hwang HS, Shin H, Han J, Na K. Combination of photodynamic therapy (PDT) and anti-tumor immunity in cancer therapy. J Pharm Investig. 2018;48(2):143–151. doi:10.1007/s40005-017-0377-x.
- Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani J, Hannani D, Duret H, Steegh K. et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38(4):729–741. doi:10.1016/j.immuni.2013.03.003.
- Yang H, Xia L, Chen J, Zhang S, Martin V, Li Q. et al. Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced antitumor immunity. Nat Med. 2019;25:1428–1441. doi:10.1038/s41591-019-0566-4.
