ABSTRACT
Introduction
Despite some of the oncogenic driver mutations that have been associated with increased expression of programmed death-ligand 1 (PD-L1), the correlation between PD-L1 expression and ROS1 fusion in NSCLC cells, especially for those with Crizotinib resistance has not been fully addressed.
Materials and Methods
The expression of PD-L1 in 30 primary NSCLC tumors with/without ROS1-fusion protein was evaluated by immunohistochemical (IHC) analysis. To assess the correlation between ROS1 fusion and PD-L1 expression, we down-regulated ROS1 with RNA interference or specific inhibitor (Crizotinib) in ROS1-fusion positive NSCLC cell line HCC78; or up-regulate ROS1-fusion gene in an immortalized human bronchial epithelial cell line (HBE). Mouse xenograft models were also used to determine the effect of ROS1 expression on PD-L1 expression in vivo. Crizotinib-resistant cell line was generated for measuring the association between Crizotinib resistance and PD-L1 expression.
Results
ROS1-rearrangement in primary NSCLC tumor was significantly associated with up-regulated PD-L1 expression. PD-L1 expression was significantly up-regulated in bronchial epithelial cells after forced expression of ROS1 fusion and was eliminated when HCC78 xenograft mouse models were treated with Crizotinib. We found PD-L1 expression was modulated by MEK-ERK pathway signaling in both parental and Crizotinib-resistant NSCLC cells with ROS1 fusion.
Conclusions
The correlation between ROS1-fusion and PD-L1 overexpression suggested that PD-L1/PD-1 blockade could be the second-line treatment option for the Crizotinib-resistant NSCLC with ROS1 rearrangement.
Introduction
Blocking immune checkpoint signaling with antibodies against programmed cell death-1 (PD-1) or programmed cell death-ligand 1 (PD-L1) has been used to treat a wide spectrum of cancers, such as metastatic melanoma, renal cell carcinoma, NSCLC and other tumors.Citation1,Citation2 The PD-L1 expression in non-squamous subtype of NSCLC has been correlated with response to Nivolumab, an anti-programmed cell death-1 (PD-1) antibody,Citation3,Citation4 and PD-L1 expression remains the major tool for the selection of patients who might be beneficial from immune checkpoint inhibitor (ICI) treatment.Citation5,Citation6 Owning to the heterogeneity of PD-L1 expression levels, it is important to understand the mechanisms that regulate PD-L1 expression in tumor cells.
Accumulating evidences have been suggested that PD-L1 expression is regulated by oncogenic driver mutations in NSCLC.Citation7–Citation12 ROS1-rearrangements, a driver mutation in NSCLC, accelerate NSCLC cell proliferation through the activation of the receptor tyrosine kinase.Citation10–Citation12 The ROS1-fusion protein has also been found in many other cancers, including gastric cancer, ovarian cancer, cholangiocarcinoma, colorectal cancer and angiosarcoma.Citation13,Citation14 It has been observed that up-regulated PD-L1 expression in NSCLC is correlated with oncogenic RAS signaling, while the tyrosine kinase ROS1 is involved in RAS signaling.Citation12,Citation15 Most recently, high-level expression of PD-L1 has been associated with ROS1 rearrangement in NCSLC,Citation16,Citation17 which is in line with other studies that PD-L1 expression in NSCLC is driven by aberrant activation of oncogenes (such as EGFR and ALK).Citation18–Citation21 In this study, we found that PD-L1 expression was up-regulated by ROS1 rearrangement through the MEK-ERK pathway signaling in NSCLC with Crizotinib resistance. Our finding suggested that PD-L1/PD-1 blockade as a new strategy for treating Crizotinib-resistant NSCLC with ROS1 rearrangement.
Material & methods
Bio-specimens selection
Thirty NSCLC specimens were collected from NSCLC patients who had not been treated with chemotherapy or radiotherapy prior to surgery between December 2015 and December 2017 at West China Hospital. None of these tumors had EGFR mutation or ALK-rearrangement. ROS1 fusions were detected by Immunohistochemistry (IHC) and further confirmed by fluorescence in situ hybridization (FISH). Break-apart FISH approach was performed using BAC clones corresponding to the 5ʹ (RP11-835I21) and 3ʹ (RP11-1036C2) sequences flanking the ROS1 gene labeled by nick translation in green and red. Positive cases were defined as tumors harboring with more than 15% of cells with split signals. EGFR mutation status was detected by EGFR RGQ PCR Kit (QIAGEN, # 870121). ALK- rearrangement status was tested by IHC using D5F3 (CST, #3633). All samples were collected from patients with informed consent, and all related procedures were performed with the approval of the internal review and ethic boards of West China hospital (#2018-596).
Cell culture and reagents
HCC78 was purchased from the DSMZ. H3122 was kindly provided by Prof. Yong Peng (Sichuan University). PC-9 was purchased from the European Collection of Authenticated Cell Cultures (ECACC). Other cell lines including A549, H1299, HBE, HCC827 and H1975 cells were purchased from the American Type Culture Collection (ATCC). PC-9 and A549 were cultured in DMEM medium with 10% fetal bovine serum (FBS). Other cells were cultured in RPMI-1640 medium in the presence with 10% FBS fetal bovine serum. To establish the Crizotinib-resistant cells, HCC78 cells were maintained in complete medium with Crizotinib from a starting concentration of 100 nM to a final concentration of 1 μM over 6 months. The Crizotinib-resistant cells were maintained in complete medium with 0.5 μM Crizotinib. All cells were maintained under a humidified atmosphere of 5% CO2 at 37°C. Crizotinib (Sigma, #PZ0191), U0126 (Selleck, #S1102 chem), PD0325901 (Selleck, #S1036chem) and Cisplatin (Selleck, # S1166) were dissolved in dimethyl sulfoxide (DMSO) and stored at −20° or −80°C.
Immunohistochemistry
Surgical specimens were fixed overnight for 24 h in 10% buffered formalin. The paraffin-embedded tissue was sectioned at 5 μm thick slices. Each sections slide was incubated for 2 h with human PD-L1 antibody (Proteintech, #66248-1-Ig) at 1:500 dilutions. All stains were quantified in 10 tumor-containing fields. The percentage of PD-L1 positive tumor cell surface staining was scored as 0 (<5%), 1 (≥5–20%), 2 (≥20–50%) or 3 (≥50%). All immunohistochemical images were evaluated by two experienced pathologists who were unaware of the identity of the specimens. The average of the two determinations was used in subsequent analyses.
Plasmid transfection
The plasmids expressing SLC34A2-ROS1 and FIG-ROS1 fusion proteins as well as the negative control plasmid were generously provided by Prof. Druker’s laboratory (Knight Cancer Institute). HBE cells were plated in 6-well plates with 60% confluence. We used 2 µg of plasmid DNA, 4 µl P3000 reagent and 6µ Lipofectamine 3000 reagent (Invitrogen, #L3000001) for transfection. The culture medium was changed 12 h after transfection.
RNA interference
Cells were plated in 6-well plates with 70% confluence. We used 7 µl siRNA (20 µM) and 7 µl Lipofectamine 3000 reagent for the transfection, and the culture medium was changed 24 h after transfection. The ROS1 mRNA-specific siRNAs were obtained from Shanghai GenePharma Co., including ROS1-1 sense (5ʹ-CAGAGUAGUAGCUGCAAAUTT-3ʹ); ROS1-1 antisense (5ʹ-AUUUGCAGCUACUACUCUGTT-3ʹ); ROS1-2 sense (5ʹ-GGAUCUGGCAGCUAGAAAUTT-3ʹ); and ROS1-2 antisense (5ʹ-AUUUCUAGCUGCCAGAUCCTT-3ʹ).
Antibodies for immunoblot and flow cytometry
Cells were washed once with ice-cold PBS and lysed in 100 µl RIPA lysis buffer (Solarbio, #R0010) with PMSF and Cocktail. Lysate was extracted with SDS-PAGE loading buffer (Solarbio, #P1040) for 5 min at 100°C. Proteins were transferred to Immobilon-FL 0.45 µm PVDF membranes (Millipore) and subjected to immunoblot analysis with following antibodies:PD-L1 (1:1000, CST, #13684 S), phospho-ROS1 (1:1000, CST, #3078 S), ROS1 (1:1000, CST, #3266 S), p-ERK (1:1000, CST, #9106 S), ERK (1:1000, CST, #4695 S), p-STAT3(1:1000, CST, #9145 S), STAT3 (1:1000, CST, #9139 S), p-AKT (1:1000, CST, #4060 S) and AKT (1:1000, CST, #4685), β-actin (1:5000, ZSGB-BIO, #TA-09). Bio-Rad ChemiDocTM MP imaging system or LI-COR Odyssey Platform was used for western blot detection with either HRP-conjugated or IR dye secondary antibodies, respectively. Flow cytometry antibodies for PD-L1 were purchased from BD Biosciences, conjugated with PE-CY7 (BD, #558017) or APC (BD, #563741).
Quantitative real time-PCR
Total RNA was extracted from cells using TRIzol reagent (Invitrogen, #10296010). Reverse transcription was done using the PrimeScript™ RT Master Mix (Takara, #RR036A) using 1 µg of total RNA, followed by real-time PCR analysis with TB Green Premix Ex Taq (Takara, #RR420A). The PCR primers (forward and reverse, respectively) included those for PD-L1 (5ʹ-TGGCATTTGCTGAACGCATTT-3ʹ and 5ʹ-TGCAGCCAGGTCTAATTGTTTT-3ʹ) and 18 s-rRNA (5ʹ- GATGGGCGGCGGAAAATAG-3ʹ and 5ʹ-GCGTGGATTCTGCATAATGGT-3ʹ). The amount of PD-L1 mRNA was normalized by that of 18 s-rRNA.
Mouse xenograft models
Human HCC78 NSCLC cell line was used to establish a subcutaneous xenograft mouse model in 6-week-old nude mice (BALB/c nude; GemPharmatech, Nanjing, China). A total of 1 × 107 HCC78 cells were resuspended in 80 µl Matrigel and were inoculated subcutaneously to the left dorsal flanks of the nude mice. Twelve nude mice were randomly divided into control and Crizotinib treatment groups (six mice per group). Tumors with a diameter of 4–6 mm were treated by oral gavage with vehicle or Crizotinib (25 mg/kg) once per day for 14 consecutive d. Tumor size was measured by electronic caliper and recorded every 3 d. Tumor volumes were calculated by taking length to be the longest diameter across the tumor and width to be the corresponding perpendicular diameter, using the following formula: 0.5× (length×width2) mm2. The study was approved by the Animal Care Committee of West China Hospital (#2018217A).
Statistical analysis
Pairwise comparison for RT-PCR results was performed using T-test. Other logistic regression analyses were conducted using non-parametric Mann–Whitney U test. Analysis of variance in conjugation with Bonferroni’s correction was used to compare multiple groups of data. The statistical testing results were determined by the SPSS 22.0 or GraphPad Prism 6 software (GraphPad, San Diego, CA) and R Project for Statistical Computing (Augasse, Austria). p < .05 was considered statistically significant and denoted as follows: *p < .05, **p < .01, ***p < .001.
Results
PD-L1 expression is correlated with ROS1 fusion in NSCLC
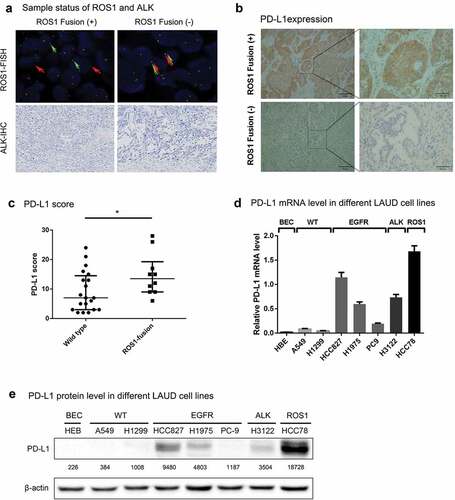
To determine the correlation between ROS1 fusion and PD-L1 expression, we measured PD-L1 protein levels in 30 NSCLC tumors without EGFR mutation and ALK-rearrangement, including 10 ROS1-fusion positive and 20 ROS1-fusion negative cases ( and Supplementary Table 1). We found that PD-L1 expression was significantly upregulated in tumors with ROS1 fusion, compared with those without ROS1 rearrangement ().
To further characterize the association between ROS1 fusion and PD-L1 expression, we measured PD-L1 expression in a series of NSCLC cell lines with/without ROS fusion. HCC78 is known as an NSCLC cell line with SLC34A2-ROS1 rearrangement but without EGFR mutation and ALK fusion. We confirmed the SLC34A2-ROS1 fusion in HCC78 cell line using short tandem repeat (STR) experiment (Supplementary Figure 1a). Since PD-L1 expression was correlated EGFR mutation and ALK fusion,Citation18,Citation22 for the comparison, NSCLC cell lines with EGFR mutation (HCC827, H1975 and PC9) and NSCLC cell line with ALK fusion (H3122) were selected in this analysis. As control, an immortalized human bronchial epithelial cell line (HBE) and NSCLC cell lines (A549 and H1299) without EGFR mutation, ALK fusion and ROS1 fusion were also included in this study. As expected, NSCLC cell lines with EGFR mutation and ALK fusion were correlated with a higher level of PD-L1 expression (). We found that PD-L1 expression was significantly up-regulated in HCC78 cells with SLC34A2-ROS1 fusion than those without EGFR mutation, ALK fusion and ROS1 fusion (). Since HCC78 cell is negative for EGFR mutation and ALK fusion, ROS1 fusion might be an independent factor that modulates PD-L1 expression in NSCLC cells.
ROS1 fusion drives PD-L1 expression
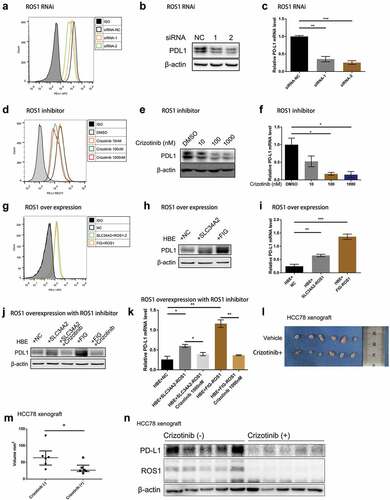
To assess whether ROS1 fusion is a driver of PD-L1 expression, we depleted ROS1-fusion protein in HCC78 cells by a ROS1-targeted siRNA (Supplementary Figure 2a) and found that both protein () and mRNA levels of PD-L1 were down-regulated after knockdown ROS1 (). Furthermore, we treated HCC78 cells with Crizotinib, a potent ROS1 inhibitor, and measured PD-L1 expression using flow cytometry, western blot and qPCR analyses. We found that Crizotinib blocked ROS1 phosphorylation (Supplementary Figure 2b) and significantly reduced PD-L1 expression in HCC78 cells ().
Furthermore, both mRNA and protein expression of PD-L1 was significantly increased after transfected SLC34A2-ROS1 and FIG-ROS1 fusion into HBE cells (, Supplementary Figure 2c). When SLC34A2-ROS1 or FIG-ROS1 transfected HBE cells were treated with Crizotinib, the PD-L1 expression was down-regulated ().
Targeting ROS1 fusion leads to the elimination of PD-L1 expression in vivo
We next sought to determine the effect of targeting ROS1 fusion on PD-L1 expression in vivo, using a ROS1-rearrangement HCC78 xenograft mouse model. Twelve nude mice were randomly divided into two groups with/without Crizotinib treatment and were subcutaneously inoculated with 1 × 107 HCC78 cells. Tumors with a diameter of 4–6 mm were treated by oral gavage with vehicle or Crizotinib (25 mg/kg) once per day for 14 consecutive d. We found that the elimination of ROS1 expression in HCC78 xenograft model by Crizotinib inhibited tumor growth, and was correlated with down-regulated expression of PD-L1 (-n), which was in line with our in vitro results that ROS1 fusion modulated PD-L1 expression.
ROS1 fusion modulates PD-L1 expression through MEK-ERK pathway signaling
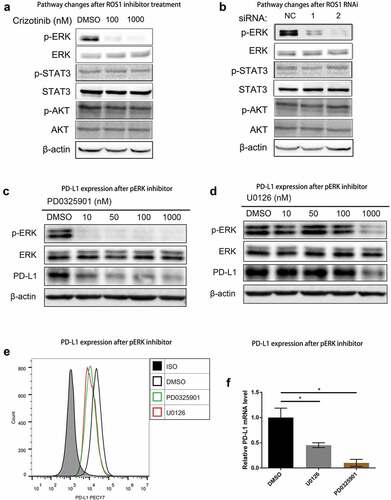
To characterize the signaling pathways that are involved in the modulation of PD-L1 expression by ROS1 fusion, we measured downstream signaling of ROS1 in response to Crizotinib treatment, including MEK-ERK, JAK-STAT and PI3K-AKT.Citation13,Citation23 Increasing concentrations of Crizotinib were significantly correlated with downregulation of ERK phosphorylation in HCC78 cells, whereas the phosphorylation of STAT3 and AKT was barely affected by Crizotinib treatment (). Similarly, when ROS1-fusion protein in HCC78 cells was depleted by ROS1 siRNAs, only ERK phosphorylation was deactivated ().
To further assess the correlation between ERK activation and ROS1 fusion-induced PD-L1 expression, we measured the effects of MEK-ERK specific inhibitor (PD0325901 and U0126) on PD-L1 expression in HCC78 cells. Both ERK phosphorylation and PD-L1 expression were down-regulated after treating HCC78 cells with PD0325901 or U0126 (), suggesting that MEK-ERK signaling facilitated PD-L1 expression in ROS1-fusion positive cells in NSCLC, rather than JAK-STAT or PI3 K-AKT pathways.
PD-L1 expression is upregulated in Crizotinib-resistant NSCLC Cells
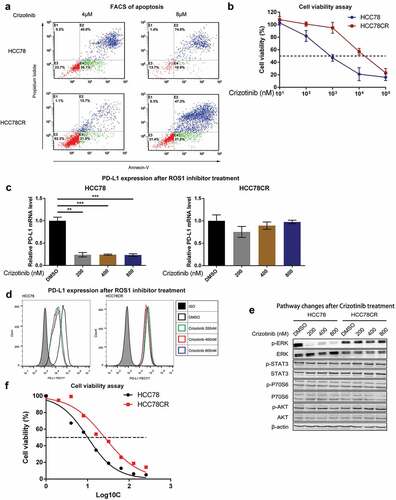
Since both ROS1 and PD-L1 expressions in Crizotinib-sensitive HCC78 cells were downregulated by ROS1 inhibitor, we further determined the correlation between ROS1-fusion and PDL-1 expression in Crizotinib-resistant NSCLC cells. We established a Crizotinib-resistant HCC78 (HCC78CR) NSCLC cell line using the method as Song et al. described previouslyCitation24 (Supplementary Figure 3). Comparing with parental HCC78 cells, HCC78CR cells were resistant to Crizotinib with over eightfold higher IC50, and significantly less apoptosis (p < .001, Fisher’s exact test) (). Though PD-L1 expression in HCC78CR cells was not affected by Crizotinib (), the overexpression of PD-L1 in Crizotinib-resistant HCC78CR cells was also specifically correlated with the activation ERK pathway signaling ().
Since platinum-based therapy is widely used as the second-line treatment for patients who have received prior Crizotinib,Citation25 we performed cisplatin IC50 test on Crizotinib-resistant and sensitive strains. Crizotinib-resistant strains (HCC78CR) showed higher IC50 levels than sensitive strains (HCC78), with cisplatin IC50 25.86 uM and 10.08 uM, respectively (). These results suggest that Crizotinib-resistant NSCLC cells with ROS1-rearrangement are also resistant to traditional chemotherapy, and PD-L1/PD-1 blockade may be a new strategy to overcome Crizotinib resistance in ROS1-rearranged NSCLC.
Discussion
In this study, we demonstrated that ROS1 rearrangement plays a critical role in modulating PD-L1 expression through activation of ERK signaling, which contributes to immune escape of Crizotinib-resistance NSCLC cells with ROS1-rearrangement.
PD-L1 expression has been approved as a biomarker for PD-1/PD-L1 inhibitor therapy by the US Food and Drug Administration for patients with advanced NSCLC treated with pembrolizumab.Citation26 Several oncogenic alterations, such as mutations in MET, KRAS and ALK, have been associated with upregulated expression of PD-L1 in NSCLC.Citation7,Citation22,Citation27 However, discordant correlation between these common oncogenic alterations and PD-L1 expression was also observed in a subset of NSCLC patients,Citation5,Citation16 suggesting additional oncogenic alterations is needed to defined subpopulation of patients who might be beneficial from PD-1/PD-L1 inhibitors. Here, we reported that ROS1-fusion protein was correlated with up-regulated PD-L1 expression in primary NSCLC without EGFR and ALK alterations. Forced expression of SLC34A2-ROS1 or FIG-ROS1 in human bronchial epithelial cells dramatically increased the expression of PD-L1; while attenuated PD-L1 expression was observed in ROS1-fusion positive NSCLC cell line HCC78 after depletion of ROS1-fusion protein by RNA interference or ROS1 inhibitor. Our finding suggested that ROS1 fusion is not only an oncogenic driver of NSCLC cell proliferation and survivalCitation28 but is also involved in remodeling the immune microenvironment. This finding is consistent with a recent report that high PD-L1 expression was significantly associated with ROS1 rearrangement in lung adenocarcinoma cohort.Citation16
Analyzing downstream signaling of ROS1 in response to Crizotinib (ROS1 inhibitor), we found depletion of PD-L1 expression was associated with the downregulation of MEK-ERK pathway signaling when HCC78 cells were treated with Crizotinib or ROS1 siRNAs. Our finding is in line with previous reported that PD-L1 expression is regulated through PI3K-AKT and RAS signaling in various solid tumors, as well as STAT3 or MEK-ERK pathway in some hematologic neoplasms.Citation15,Citation29,Citation30 Collectively, our results highlighted the molecular basis of ROS1 rearrangement in regulating PD-L1 expression in NSCLC.
Although ROS1- or ALK-rearranged non-small cell lung cancer (NSCLC) is sensitive to Crizotinib, a subset of ROS1 fusion-positive patients may acquire drug resistance following Crizotinib treatment.Citation14,Citation24 EGFR pathway activation and secondary mutations in ROS1, such as G2032R and L2155S, have been recognized as the mechanisms of the Crizotinib resistance.Citation24,Citation31 Although efforts have been made to develop more potent inhibitors to overcome Crizotinib resistance in ROS1-rearranged NSCLC, these clinical trials on unselected populations have been discontinued due to the less significant results.Citation32 While the National Comprehensive Cancer Network (NCCN) guideline of NSCLC recommended that Crizotinib-resistance patients with positive ROS1-rearrangement might be treated with PD-1/PD-L1 inhibitor,Citation32 the genetic basis of this approach has not been addressed.
American Society of Clinical Oncology Clinical Practice Guideline recommends that platinum-based therapy in the second line with or without bevacizumab for patients who have received prior Crizotinib.Citation25 However, the response rate is not very encouraging.Citation33 It has been reported that NSCLC with EMT-positive tumors were less sensitive to chemotherapy or radiotherapy.Citation34 Since PD1/PD-L1 blockade therapy for NSCLC gives superior treatment responses than chemotherapy for those PD-L1 positive patients,Citation34–Citation38 effort has been made to define the NSCLC patient with Crizotinib resistance who might be beneficial from PD-L1 inhibitors. For instance, it has been suggested that EGFR/PD-L1 positive NSCLC with EGFR-TKI resistance response to PD-L1 inhibitors.Citation39
In our study, we found that IC50 of cisplatin in Crizotinib-resistant line of HCC78CR cells was also significantly higher than parental line HCC78. The highly expressed PD-L1 in HCC78CR cells with relatively lower response to cisplatin indicating that immunotherapy may be a promising second-line therapeutic strategy to overcome Crizotinib resistance, especially for the NSCLC patients with ROS1 fusion. Our results warrant further in vivo studies and clinical trials to evaluate the efficacy of immunotherapies for the NSCLC with ROS1 rearrangement.
Disclosure of potential conflicts of interest
The authors declare no potential conflicts of interest.
Author Contributions
L.L. and Y.P. conceived the project. Z.L., K.Z. and S.W. performed all experiments. X.W. performed pathological analysis. C.L., J.Z., Q.G., Z.Y. and Y.Y. assisted with some experiments. L.L. and Y.P. supervised the study. Z.L., K.Z., Q.C. and L.L. wrote the manuscript. All authors discussed the results and revised the manuscript.
Competing interests
The authors declare no conflict of interest.
Supplemental Material
Download ()Acknowledgments
We thank Prof. Druker (Knight Cancer Institute) for his generosity of offering us the plasmids expressing SLC34A2-ROS1 and FIG-ROS1 fusion proteins as well as the negative control plasmid. We thank Dr. Weiya Wang (Department of pathology, West China Hospital) for assistance on tissue acquisition.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-l1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–9. doi:10.1056/NEJMoa1200694.
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi:10.1056/NEJMoa1200690.
- Borghaei H, Paz-Ares L, Horn L, Spigel D, Steins M, Ready N, Chow L, Vokes E, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi:10.1056/NEJMoa1507643.
- Brahmer J, Reckamp K, Baas P, Crinò L, Eberhardt W, Poddubskaya E, Antonia S, Pluzanski A, Vokes E, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi:10.1056/NEJMoa1504627.
- Rangachari D, VanderLaan PA, Shea M, Le X, Huberman MS, Kobayashi SS, Costa DB. Correlation between classic driver oncogene mutations in egfr, alk, or ros1 and 22c3-PD-l1 >/=50% expression in lung adenocarcinoma. J Thorac Oncol. 2017;12(5):878–883. doi:10.1016/j.jtho.2016.12.026.
- Shien K, Papadimitrakopoulou VA, Wistuba II. Predictive biomarkers of response to PD-1/PD-l1 immune checkpoint inhibitors in non-small cell lung cancer. Lung Cancer. 2016;99:79–87. doi:10.1016/j.lungcan.2016.06.016.
- Saigi M, Alburquerque-Bejar JJ, Mc Leer-Florin A, Pereira C, Pros E, Romero OA, Baixeras N, Esteve-Codina A, Nadal E, Brambilla E, et al. Met-oncogenic and jak2-inactivating alterations are independent factors that affect regulation of PD-l1 expression in lung cancer. Clin Cancer Res. 2018;24(18):4579–4587. doi:10.1158/1078-0432.Ccr-18-0267.
- Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, Matsumoto K, Takayama K, Takamori S, Kage M, et al. Association of PD-l1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25(10):1935–1940. doi:10.1093/annonc/mdu242.
- Lastwika KJ, Wilson W, Li QK, Norris J, Xu H, Ghazarian SR, Kitagawa H, Kawabata S, Taube JM, Yao S, et al. Control of PD-l1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res. 2016;76(2):227–238. doi:10.1158/0008-5472.Can-14-3362.
- Takeuchi K, Soda M, Togashi Y, Suzuki R, Sakata S, Hatano S, Asaka R, Hamanaka W, Ninomiya H, Uehara H, et al. Ret, ros1 and alk fusions in lung cancer. Nat Med. 2012;18(3):378–381. doi:10.1038/nm.2658.
- Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, Massion PP, Siwak-Tapp C, Gonzalez A, Fang R, et al. Ros1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863–870. doi:10.1200/JCO.2011.35.6345.
- Davies KD, Doebele RC. Molecular pathways: ros1 fusion proteins in cancer. Clin Cancer Res. 2013;19(15):4040–4045. doi:10.1158/1078-0432.CCR-12-2851.
- Jun HJ, Johnson H, Bronson RT, de Feraudy S, White F, Charest A. The oncogenic lung cancer fusion kinase cd74-ros activates a novel invasiveness pathway through e-syt1 phosphorylation. Cancer Res. 2012;72(15):3764–3774. doi:10.1158/0008-5472.CAN-11-3990.
- Shaw AT, Hsu PP, Awad MM, Engelman JA. Tyrosine kinase gene rearrangements in epithelial malignancies. Nat Rev Cancer. 2013;13(11):772–787. doi:10.1038/nrc3612.
- Coelho M, de Carné Trécesson S, Rana S, Zecchin D, Moore C, Molina-Arcas M, East P, Spencer-Dene B, Nye E, Barnouin K, et al. Oncogenic ras signaling promotes tumor immunoresistance by stabilizing pd-l1 mrna. Immunity. 2017;47(6):1083–1099.e1086. doi:10.1016/j.immuni.2017.11.016.
- Lee J, Park CK, Yoon HK, Sa YJ, Woo IS, Kim HR, Kim SY, Kim TJ. Pd-l1 expression in ros1-rearranged non-small cell lung cancer: A study using simultaneous genotypic screening of egfr, alk, and ros1. Thoracic Cancer. 2019;10(1):103–110. doi:10.1111/1759-7714.12917.
- Ogawa H, Tanaka Y, Tachihara M, Uehara K, Shimizu N, Doi T, Hokka D, Maniwa Y. Ros1-rearranged high-PD-l1-expressing lung adenocarcinoma manifesting as mediastinal tumor: A case report. Oncol Lett. 2019;17(1):488–491. doi:10.3892/ol.2018.9622.
- Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp EM, Pugh TJ, et al. Activation of the PD-1 pathway contributes to immune escape in egfr-driven lung tumors. Cancer Discov. 2013;3(12):1355–1363. doi:10.1158/2159-8290.CD-13-0310.
- Hong S, Chen N, Fang W, Zhan J, Liu Q, Kang S, He X, Liu L, Zhou T, Huang J, et al. Upregulation of PD-l1 by EML4-ALK fusion protein mediates the immune escape in ALK positive NSCLC: implication for optional anti-PD-1/PD-l1 immune therapy for ALK-TKIs sensitive and resistant nsclc patients. Oncoimmunology. 2016;5(3):e1094598. doi:10.1080/2162402x.2015.1094598.
- Koh J, Jang JY, Keam B, Kim S, Kim MY, Go H, Kim TM, Kim DW, Kim CW, Jeon YK, et al. Eml4-alk enhances programmed cell death-ligand 1 expression in pulmonary adenocarcinoma via hypoxia-inducible factor (HIF)-1alpha and stat3. Oncoimmunology. 2016;5(3):e1108514. doi:10.1080/2162402x.2015.1108514.
- Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, Zhang Y, He X, Zhou T, Qin T, et al. Upregulation of PD-l1 by egfr activation mediates the immune escape in egfr-driven nsclc: implication for optional immune targeted therapy for NSCLC patients with egfr mutation. J Thorac Oncol. 2015;10(6):910–923. doi:10.1097/jto.0000000000000500.
- Ota K, Azuma K, Kawahara A, Hattori S, Iwama E, Tanizaki J, Harada T, Matsumoto K, Takayama K, Takamori S, et al. Induction of PD-l1 expression by the eml4-alk oncoprotein and downstream signaling pathways in non-small cell lung cancer. Clin Cancer Res. 2015;21(17):4014–4021. doi:10.1158/1078-0432.CCR-15-0016.
- Davies KD, Le AT, Theodoro MF, Skokan MC, Aisner DL, Berge EM, Terracciano LM, Cappuzzo F, Incarbone M, Roncalli M, et al. Identifying and targeting ros1 gene fusions in non-small cell lung cancer. Clin Cancer Res. 2012;18(17):4570–4579. doi:10.1158/1078-0432.ccr-12-0550.
- Song A, Kim T, Kim D, Kim S, Keam B, Lee S, Heo D. Molecular changes associated with acquired resistance to crizotinib in ros1-rearranged non-small cell lung cancer. Clin Cancer Res. 2015;21(10):2379–2387. doi:10.1158/1078-0432.CCR-14-1350.
- Hanna N, Johnson D, Temin S, Baker S Jr., Brahmer J, Ellis PM, Giaccone G, Hesketh PJ, Jaiyesimi I, Leighl NB, et al. Systemic therapy for stage iv non-small-cell lung cancer: american society of clinical oncology clinical practice guideline update. J Clin Oncol. 2017;35(30):3484–3515. doi:10.1200/jco.2017.74.6065.
- Shukuya T, Carbone DP. Predictive markers for the efficacy of anti-PD-1/PD-l1 antibodies in lung cancer. J Thorac Oncol. 2016;11(7):976–988. doi:10.1016/j.jtho.2016.02.015.
- Chen N, Fang W, Lin Z, Peng P, Wang J, Zhan J, Hong S, Huang J, Liu L, Sheng J, et al. Kras mutation-induced upregulation of PD-l1 mediates immune escape in human lung adenocarcinoma. Cancer Immunol Immuno. 2017;66(9):1175–1187. doi:10.1007/s00262-017-2005-z.
- Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131(6):1190–1203. doi:10.1016/j.cell.2007.11.025.
- Jiang X, Zhou J, Giobbie-Hurder A, Wargo J, Hodi FS. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-l1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res. 2013;19(3):598–609. doi:10.1158/1078-0432.CCR-12-2731.
- Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M, Ruggeri BA, et al. Oncogenic kinase npm/alk induces through stat3 expression of immunosuppressive protein cd274 (PD-l1, b7-h1). Proc Natl Acad Sci U S A. 2008;105(52):20852–20857. doi:10.1073/pnas.0810958105.
- Awad MM, Katayama R, McTigue M, Liu W, Deng YL, Brooun A, Friboulet L, Huang D, Falk MD, Timofeevski S, et al. Acquired resistance to crizotinib from a mutation in cd74-ros1. N Engl J Med. 2013;368(25):2395–2401. doi:10.1056/NEJMoa1215530.
- Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, D’Amico TA, DeCamp MM, Dilling TJ, Dobelbower M, et al. Non-small cell lung cancer, version 5.2017, nccn clinical practice guidelines in oncology. J National Compr Cancer Network. 2017;15(4):504–535.
- Zhou J, Hu Q, Zhang X, Zheng J, Xie B, Xu Z, Zhang W. Sensitivity to chemotherapeutics of NSCLC cells with acquired resistance to EGFR-TKIs is mediated by t790m mutation or epithelial-mesenchymal transition. Oncol Rep. 2018;39(4):1783–1792. doi:10.3892/or.2018.6242.
- Jakobsen KR, Demuth C, Sorensen BS, Nielsen AL. The role of epithelial to mesenchymal transition in resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Transl Lung Cancer Res. 2016;5(2):172–182. doi:10.21037/tlcr.2016.04.07.
- Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr., Srimuninnimit V, Laktionov KK, Bondarenko I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-l1-expressing, locally advanced or metastatic non-small-cell lung cancer (keynote-042): A randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi:10.1016/s0140-6736(18)32409-7.
- Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: A randomised, phase 2 cohort of the open-label keynote-021 study. Lancet Oncol. 2016;17(11):1497–1508. doi:10.1016/s1470-2045(16)30498-3.
- Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, Park K, Alexandru A, Lupinacci L, de la Mora Jimenez E, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020–2031. doi:10.1056/NEJMoa1910231.
- Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Pembrolizumab versus chemotherapy for PD-l1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa1606774.
- Haratani K, Hayashi H, Tanaka T, Kaneda H, Togashi Y, Sakai K, Hayashi K, Tomida S, Chiba Y, Yonesaka K, et al. Tumor immune microenvironment and nivolumab efficacy in egfr mutation-positive non-small-cell lung cancer based on t790m status after disease progression during egfr-tki treatment. Ann Oncol. 2017;28(7):1532–1539. doi:10.1093/annonc/mdx183.