ABSTRACT
Checkpoint inhibitors have improved the survival of patients with advanced tumors and show a manageable toxicity profile. However, auto-immune colitis remains a relevant side effect, and combinations of anti-PD1/PDL1 and anti-CTLA-4 increase its incidence and severity. Here, we report the case of a 50-year-old patient diagnosed with stage IV cervical cancer that relapsed following radical surgery, external radiation/brachytherapy and standard chemotherapy. She was subsequently treated with Nivolumab and Ipilimumab combination and developed grade 2 colitis presenting a dissociation between endoscopic and pathological findings. At cycle 10 the patient reported grade 3 diarrhea and abdominal discomfort, without blood or mucus in the stools. Immunotherapy was withheld and a colonoscopy was performed, showing normal mucosa in the entire colon. Puzzlingly, histologic evaluation of randomly sampled mucosal biopsy of the distal colon showed an intense intraepithelial lymphocyte infiltration with crypt loss and some regenerating crypts with a few apoptotic bodies set in a chronically inflamed lamina propria, consistent with the microscopic diagnosis of colitis. Treatment with methylprednisolone 2 mg/kg was initiated which led to a decrease in the number of stools to grade 1. Additional investigations to exclude other causes of diarrhea rendered negative results. The patient experienced a major partial response and, following the resolution of diarrhea, she was re-challenged again with immunotherapy, with the reappearance of grade 2 diarrhea, leading to permanent immunotherapy interruption. We conclude and propose that performing random colonic biopsies should be considered in cases of immune checkpoint-associated unexplained diarrhea, even when colonoscopy shows macroscopically normal colonic mucosa inflammatory lesions.
Introduction
Recurrent metastatic cervical cancer has a poor prognosis, with a 5-year survival rate of 17%.Citation1 Recently, immune-checkpoint inhibitors (ICPI) have emerged as an active treatment option for a variety of human malignant diseases,Citation2,Citation3 including metastatic cervical cancer.Citation4,Citation5 Several studies are currently being conducted in this tumor type with promising results.Citation6–Citation9
Immune-related adverse events (irAEs) have been extensively reported during treatment with immune-checkpoint inhibitorsCitation10,Citation11 and include, among others, dermatitis, thyroiditis, pneumonitis, colitis, myocarditis and hypophysitis.Citation12,Citation13 Although there is not a clear relation between the type of checkpoint inhibitor and the induction of irAEs, some toxicities, such as hypophysitis and colitis, are found with a higher prevalence among patients receiving anti-CTLA-4 mAb agents, either as single agents or in combination with anti PD1/PDL1 inhibitors.Citation14,Citation15
As described in the literature, diarrhea associated with anti-CTLA-4 therapy usually appears after 7–8 weeks from the first infusion, while anti-PD1 associated diarrhea usually onsets as late as 12–24 weeks following treatment initiation.Citation14,Citation15 These diarrheas have been linked to macroscopic and microscopic alterations similar to the ones found on immune-mediated colitis.Citation16 In general terms, drug-induced microscopic colitis (MC) can be grouped into two main categories, known as microscopic colitis: collagenous and lymphocytic colitis, both of which have a grossly normal endoscopic appearance and are differentiated by histology.Citation17 Both patterns are reported in patients receiving immune-checkpoint inhibitors. Interestingly, lymphocytic colitis has been related to other autoimmune disorders, such as type I diabetes, thyroid alterations or polyarthritis.Citation18 Importantly, in the context of microscopic colitis, the presence of diarrhea with the absence of macroscopic signs of inflammation should not discard a microscopic immune infiltration pattern. Microscopic colitis is not usually associated with endoscopic or radiological alterations.
Here we report the case of a patient diagnosed with metastatic cervical carcinoma treated with a combination of Ipilimumab and Nivolumab who presented different irAEs, including thyroiditis and pneumonitis. Interestingly, several weeks after withholding Ipilimumab and switching to Nivolumab monotherapy per protocol, while receiving Nivolumab, the patient presented grade 3 diarrhea with microscopic colitis, despite showing a normal-appearing colonic mucosa in the colonoscopy.
Case presentation
A 50-year-old woman without the previous medical history of gastrointestinal pathology was diagnosed with cervical carcinoma (T2N1M0) in April 2015. Radical surgery was performed in June 2015 and subsequently she received adjuvant radiotherapy using simultaneous integrated boost intensity-modulated radiotherapy (SIB-IMRT) followed by brachytherapy. Between August 17 and September 24, 2015, a total dose of 50.4 Gy (1.8 Gy/fraction) to the pelvic lymph node chains and 58.8 Gy (2.10 Gy/fraction) to the postsurgical high-risk area were administered. In addition, a total dose of 12 Gy (6 Gy/2fractions) was delivered using intracavitary high-dose-rate brachytherapy. Simultaneously, six cycles of cisplatin 40 mg/m2/week were administered. Radiation therapy ended in October 2015 and she started follow-up. In May 2016, a pelvic MRI and whole-body CT showed recurrence in the vulva and multiple lymph nodes. She was then treated with Paclitaxel 60 mg/m2 and Carboplatin AUC 1.5, showing clear disease progression at the first evaluation performed 4 weeks later.
In March 2017, she was enrolled in a phase I–II clinical trialCitation19 and she received Nivolumab 1 mg/kg plus Ipilimumab 3 mg/kg every 3 weeks for four doses, followed by Nivolumab 240 mg every 2 weeks until progression or unacceptable toxicity. After the first cycle (3 weeks), she developed grade 2 symptomatic thyroiditis, which required Methylprednisolone 2 mg/kg, Metamizole/acetaminophen for pain and beta-blockers to control hyperthyroidism-associated signs. After cycle 2 (9 weeks), she presented symptomatic grade2 pneumonitis. Methylprednisolone 2 mg/kg was started based on the clinical trial protocol. The pneumonitis resolved and Nivolumab was re-introduced 14 weeks after completed the steroid tapering.
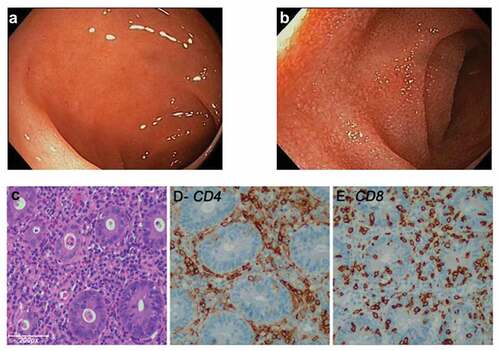
In the CT-SCAN performed 19 weeks after treatment onset, the patient showed a partial response (80% decrease) by RECIST 1.1 criteria, and she remained on treatment. Following cycle 10 (29 weeks), she presented grade-2 colitis due to grade-3 diarrhea (seven non-bloody stools per day over baseline), abdominal pain and a total weight loss of 4 kg during the diarrhea (31 weeks). Nivolumab was interrupted and infectious colitis was ruled out after bacteriological, virological and parasitological analyses of stools, including testing for Clostridium difficile toxin. Upper digestive tract endoscopy did not show any endoscopic abnormalities. However, a colonoscopy showed macroscopically normal-appearing mucosa in the whole length of the colon ( and ). Interestingly, severe lymphocytic colitis was observed in multiple colonic biopsies. Hematoxylin-eosin staining and immunohistochemical staining showed an increased intraepithelial infiltration of CD8+ lymphocytes (>10/100 enterocytes) with a severe CD4+ and CD8+ lymphocytic infiltration in the lamina propria (–).
Figure 1. (a and b) Endoscopic revealed grossly normal mucosa in patients treated with anti-PD-1 and anti-CTLA-4 combination mAbs. (c) Hematoxylin and Eosin (H&E)-stained section of the colonic biopsy shows diffuse colitis with crypt atrophy and loss of crypts with residual inflamed lamina propria. A severe lymphoplasmacytic infiltration of the lamina propria with increased intraepithelial lymphocytes and crypt epithelial cell apoptosis is seen. (d and e) A severe CD4+ and CD8+ lymphocytic infiltration is seen in lamina propria. (e) Increased intraepithelial CD8+ lymphocytes (>10/100 enterocytes) and lymphocytic cryptitis are observed.
As severe diarrhea persisted despite full-dose loperamide and diet, methylprednisolone 2 mg/kg/day was started, leading to a decrease in the number of stools to grade 1 and resolution of the colitis. After careful consideration of the risks and benefits, and following thorough discussion with the patient, Nivolumab was restarted after tapering steroids. Grade 2 diarrhea recurred after 2 weeks and Nivolumab was permanently discontinued (35 weeks) (). The patient remains in partial response (80% decrease) after 153 weeks (3 years) of treatment initiation and is completely asymptomatic.
Figure 2. Treatment schema and AEs during the clinical trial. Week of cycle administration (upper line) and week at the moment of toxicity (lower line). EOT: end of treatment; FUP: follow up; G: grade; W:week; (*) Metilprednisolone.

Written informed consent was obtained from the patient for publication of this case report and accompanying images, in addition to the explicit permission from the Ethics Committee of Clínica Universidad de Navarra and the sponsor of the clinical trial (Bristol Myers Squibb).
Discussion
Auto-immune colitis is a well-described complication of treatment with immune-checkpoint inhibitors, and grades 3–4 episodes have been reported in up to 15.81%.Citation19–Citation21 Here, we report a case of a patient presenting with an adenocarcinoma of the cervix that relapsed following standard treatment with radical surgery, external radiation, brachytherapy and chemotherapy with carboplatin and paclitaxel. She started treatment combination with Nivolumab and Ipilimumab and developed grade 2 thyroiditis and pneumonitis, which required treatment with steroids (metilprednisolone 1 mg/kg weeks 3 to 6 and 9 to 13). After 19 weeks of treatment, a major partial response was observed, but on week 31 she progressed with diarrhea displaying a normal-appearing colonic mucosa, with no signs of inflammation, in the colonoscopy and a microscopic grade2 lymphocytic colitis. Lymphocytic colitis was observed on all random distal colonic biopsies. Treatment with steroids improved the diarrhea, which relapsed following rechallenge with Nivolumab, leading to permanent Nivolumab treatment discontinuation. The patient remains in a major partial response after 3 years.
Even though this patient progressed with lymphocytic colitis upon treatment with immune-checkpoint inhibitors, other possible causes of colitis should be considered as the role of previous surgery, external radiation, brachytherapy and chemotherapy. Nevertheless, severe and even fatal cases of acute and late-onset colitis associated with immune-checkpoint inhibitor treatment have been reported in the literature.Citation22–Citation24 The clinical course of the patient, with improvement after steroids and relapse upon rechallenge with Nivolumab, and the histological finding of lymphocytic colitis support the association between colitis and immune-checkpoint inhibitor treatment, but nevertheless the impact of previous therapy, either as a primary cause or as an associated risk factor, cannot be ruled out.
Endoscopy and microscopic examination poignantly rendered dissociated results in this case. This suggests that infiltrating immune cells are capable of functionally damaging the intestinal inner lining, causing diarrhea without obvious ulcers or even milder signs of inflammation. We interpret this dissociation as a result of different timing of cellular changes and the translation to pathological impact in the tissue architecture. The microstructural changes likely precede the macroscopic alterations. It remains to be seen what would have been the natural course of the lesions if left untreated. Perhaps, macroscopic lesions denoting inflammation would have appeared at a later point. Also, there is a possibility that previous treatment of the thyroiditis and pneumonitis with methylprednisolone 18 weeks before the diarrhea contributed to decrease the inflammation, masking the presence of macroscopical lesions. Despite some histopathological similarities between microscopic colitis with and without prior exposure to ICPIs, differences have been demonstrated in clinical presentations, disease courses and treatments between non-ICPI-induced MC and ICPI-induced MC. ICPI-induced MC has greater symptom severity and a more aggressive disease course that requires more potent immunosuppressive treatment regimens.Citation25
This case highlights the potential for the development of immune-related toxicity in patients treated with immunotherapy combinations,Citation10,Citation11 including multiple irAEs affecting several organs in a single patient. An important unresolved question is which factors render certain patients more susceptible to irAEs. Probably, several factors underlie this increased risk, including genetic predisposition, microbiomeCitation22-Citation24 and perhaps subclinical pretreatment inflammation of the target organ. Conversely, the correlation of irAE and clinical benefit is weak at bestCitation26 and cannot be used as a predictor, even if in this case our patient experienced a major and sustained response. Another relevant observation confirmed by this case is that treatment cessation and steroid treatment do not seem to spoil clinical benefit.Citation26,Citation27
The most important message for clinical practice from this case is that autoimmune colitis would have remained undiagnosed unless random biopsies would have been obtained from apparently healthy mucosa. Therefore, random obtention of colonic biopsies should be considered in cases of unexplained diarrhea in patients treated with immune checkpoint-inhibitors, even if an experienced endoscopist fails to identify alterations on a macroscopically normal-appearing mucosal tissue, and especially in patients that have received previous steroids, that may have contributed to mask macroscopic inflammation.
Abbreviations
Availability of data and materials
Not applicable
Authors’ contributions
All authors contributed to their order in writing the manuscript. All authors read and approved the final manuscript.
Consent for publication
Informed consent by the patient.
Competing interests
M consultant for BMS, Roche, Seattle Genetics, Tusk, Genmab, Alligator, Merck-Serono, Bioncotech, Molecular Partners; receives Grants from Alligator, Roche, BMS. JL Research funding, travel grants and speaker honoraria from BMS.
Ethics approval and consent to participate
Not applicable.
Acknowledgments
We are grateful for Scientific discussion and excellent endoscopic image by Dr. Subtil. Excellent work by personnel at the Unidad Central de Ensayos Clinicos of CUN (UCEC) is acknowledged.
Additional information
Funding
References
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–5. doi:10.1002/ijc.29210.
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi:10.1126/science.aar4060.
- Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7(2):95–106. doi:10.1038/nrc2051.
- Borcoman E, Le Tourneau C. Pembrolizumab in cervical cancer: latest evidence and clinical usefulness. Ther Adv Med Oncol. 2017;9(6):431–439. doi:10.1177/1758834017708742.
- Frenel JS, Le Tourneau C, O’Neil B, Ott PA, Piha-Paul SA, Gomez-Roca C, van Brummelen EMJ, Rugo HS, Thomas S, Saraf S, Rangwala R, Varga A. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the Phase Ib KEYNOTE-028 trial. J Clin Oncol. 2017;35(36):4035–4041. doi:10.1200/JCO.2017.74.5471.
- Scarpignato C, Varga G, Dobronyi I, Papp M. Bombesin-induced pancreatic secretion and growth in rats: effect of proglumide, spantide and ranitidine. Int J Tissue React. 1990;12:299–307.
- Shephard RJ. The need for sport-specific tests. Can J Sport Sci. 1990;15:163–164.
- Le Roux PD, Jardine DS, Kanev PM, Loeser JD. Pediatric intracranial pressure monitoring in hypoxic and nonhypoxic brain injury. Childs Nerv Syst. 1991;7(1):34–39. doi:10.1007/BF00263831.
- Galluzzi L, Chan TA, Kroemer G, Wolchok JD, Lopez-Soto A. The hallmarks of successful anticancer immunotherapy. Sci Transl Med. 2018;10(459):459. doi:10.1126/scitranslmed.aat7807.
- Guillermo De Velasco, Youjin Je, Dominick Bosse, Mark M. Awad, Patrick A. Ott, Raphael B. Moreira, Fabio Schutz, Joaquim Bellmunt, Guru P. Sonpavde, F. Stephen Hodi, et al. comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res. 2018;6(4):498–499. doi:10.1158/2326-6066.CIR-18-0078.
- De Velasco G, Je Y, Bosse D, Awad MM, Ott PA, Moreira RB, Schutz F, Bellmunt J, Sonpavde GP, Hodi FS, Choueiri TK Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res. 2017;5(4):312–318. doi:10.1158/2326-6066.CIR-16-0237.
- Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS One. 2016;11(7):e0160221. doi:10.1371/journal.pone.0160221.
- Vanpouille-Box C, Lhuillier C, Bezu L, Aranda F, Yamazaki T, Kepp O, Fucikova J, Spisek R, Demaria S, Formenti SC, et al. Trial watch: immune checkpoint blockers for cancer therapy. Oncoimmunology. 2017;6(11):e1373237. doi:10.1080/2162402X.2017.1373237.
- Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol. 2017;8:49. doi:10.3389/fphar.2017.00049.
- Wang DY, Mooradian MJ, Kim D, Shah NJ, Fenton SE, Conry RM, Mehta R, Silk AW, Zhou A, Compton ML, et al. Clinical characterization of colitis arising from anti-PD-1 based therapy. Oncoimmunology. 2019;8(1):e1524695. doi:10.1080/2162402X.2018.1524695.
- Garcia-Varona A, Odze RD, Makrauer F. Lymphocytic colitis secondary to ipilimumab treatment. Inflamm Bowel Dis. 2013;19(2):E15–6. doi:10.1002/ibd.22846.
- Pisani LF, Tontini GE, Vecchi M, Pastorelli L. Microscopic colitis: what do we know about pathogenesis? Inflamm Bowel Dis. 2016;22(2):450–458. doi:10.1097/MIB.0000000000000628.
- Parfitt JR, Driman DK. Pathological effects of drugs on the gastrointestinal tract: a review. Hum Pathol. 2007;38(4):527–536. doi:10.1016/j.humpath.2007.01.014.
- Naumann RW, Hollebecque A, Meyer T, Devlin MJ, Oaknin A, Kerger J, López-Picazo JM, Machiels J-P, Delord J-P, Evans TRJ, et al. Safety and efficacy of nivolumab monotherapy in recurrent or metastatic cervical, vaginal, or vulvar carcinoma: results from the phase I/II checkmate 358 trial. J Clin Oncol. 2019;37(31):2825–2834. doi:10.1200/JCO.19.00739.
- Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, et al.. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;JCO2017776385.
- Abdel-Rahman O, ElHalawani H, Fouad M. Risk of gastrointestinal complications in cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Immunotherapy. 2015;7(11):1213–1227. doi:10.2217/imt.15.87.
- Kountouras J, Zavos C. Recent advances in the management of radiation colitis. World J Gastroenterol. 2008;14(48):7289–7301. doi:10.3748/wjg.14.7289.
- Kennedy GD, Heise CP. Radiation colitis and proctitis. Clin Colon Rectal Surg. 2007;20(1):64–72. doi:10.1055/s-2007-970202.
- Andreyev J. Gastrointestinal complications of pelvic radiotherapy: are they of any importance? Gut. 2005;54(8):1051–1054. doi:10.1136/gut.2004.062596.
- Choi K, Abu-Sbeih H, Samdani R, Nogueras Gonzalez G, Raju GS, Richards DM, Can immune checkpoint inhibitors induce microscopic colitis or a brand new entity? Inflamm Bowel Dis. 2019;25(2):385–393. doi:10.1093/ibd/izy240.
- Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, Carvajal RD, Dickson MA, D'Angelo SP, Woo KM, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193–3198. doi:10.1200/JCO.2015.60.8448.
- Aston WJ, Hope DE, Cook AM, Boon L, Dick I, Nowak AK, Lake RA, Lesterhuis WJ. Dexamethasone differentially depletes tumour and peripheral blood lymphocytes and can impact the efficacy of chemotherapy/checkpoint blockade combination treatment. Oncoimmunology. 2019;8(11):e1641390. doi:10.1080/2162402X.2019.1641390.
