On March 11, 2020, the WHO declared that the cluster of pneumonia cases caused by the novel coronavirus SARS-CoV-2 (COVID-19) from Wuhan, China was a global pandemic.Citation1 As of April 5th 2020, over to 1.2 million cases have been reported worldwide, with over 65,000 deaths (source: WHO website), knowing that both values are certainly underestimated.
Thus far, risk factors of COVID-19-related complications or fatal outcome are known to include advanced age (>70 years) and comorbidities such as preexisting respiratory, cardiac diseases, hypertension, and obesity.Citation2,Citation3 Patients living with cancer are at particularly high risk due to immunosuppression caused by both malignancy and anticancer treatments. Liang et al. were the first to describe a nation-wide analysis in China of COVID-19 cases in patients with a history of cancer.Citation4 Out of 1590 identified cases of COVID-19, 18 patients had a history of malignant disease, with lung cancer being the most frequent type of neoplasia. One quarter of these patients had received either chemotherapy or surgery during the month prior to diagnosis. The patients with cancer identified in this study were more likely to experience severe disease (with a higher risk of ICU-admission, invasive ventilation, and death), particularly those who received anti-cancer therapy in the month prior to COVID-19 diagnosis. Similarly, an Italian group reported that, among a COVID-19-positive population of 355 patients who succumbed to COVID-19 at the time of the publication, 20.3% had active cancer.Citation5
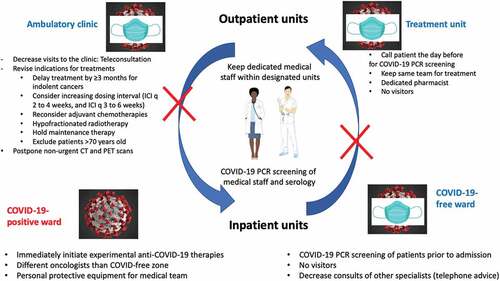
Amid the rapidly growing number of COVID-19 cases worldwide that have led to unprecedented stress on the health care system, cancer patients are also at a higher risk of not receiving planned treatment. To continue to provide care to oncological patients, prevention measures should immediately be implemented to protect cancer patients, health care professionals and maintain cancer units as COVID-free zones.
1. Outpatient management
As cancer patients are especially susceptible to COVID-19 infection, their visits to the hospital should be kept to a minimum and limited to treatment. In addition, to encourage strict confinement measures for patients, the following actions to decrease medical visits, invasive procedures and imaging should be considered.
First, telemedicine through web based-applications or via telephone represents a safe approach to assess patients regularly on a regular basis.Citation6 In our experience, up to 85% of regular follow-ups can be replaced by a telephone call.
Second, a careful balance between the risk of delaying a treatment versus the risk of COVID-19 infection should be addressed. For non-indolent cancers, a delay of 3 months potentially represents the best alternative.Citation7 However, if the treatment cannot be postponed or has already been initiated, alternative dosing should be considered. Indeed, for immune checkpoint inhibitors, strong pharmacological data demonstrated similar efficacy of anti-PD-1 Abs infused every 6 weeks compared to every 3 weeks.Citation8 In addition, the role of maintenance therapy should be reconsidered in patients with long-lasting stable disease or in remission. For radiotherapy, curative hypofractionated treatment should be recommended.Citation9 For each tumor site, more precise expert resources and guidance are available on the websites of the American Society of Clinical Oncology, European Society Medical Oncology and American Society of Hematology.
Unfortunately, in light of the unrelenting strain placed upon the health care system and the reassignment of medical teams to COVID-19-specific units, oncologists also need to base their clinical decisions on the impact of their treatments on the outcome. For example, treatments with limited impact on overall survival (such as adjuvant chemotherapy in node-negative non-small-cell lung carcinoma) or therapies that improve prognosis by only a few weeks or months should not be administered. In addition, patients over the age of 70 years are at extremely high risk of developing severe COVID-19,Citation3 suggesting that oncological treatments (for non-curative intent) should be carefully evaluated for their true utility.
Third, chemotherapy units must remain a COVID-free sanctuary. Prior to receiving treatment, telephone screening of patients should be mandatory. In the presence of symptoms, patients should be investigated thoroughly. Patients should receive their treatment without being accompanied by any family members to decrease the risk of contamination. Furthermore, to protect these units, medical staff assigned to treatment infusion and management of toxicities should be prohibited from working in other COVID-associated wards, whenever possible. ()
Fourth, as to follow-up exams, only important CT or PET scans that will change the clinical management/treatment should be performed. Efforts should focus on delaying non-urgent imaging and decreasing hospital visits.
2. Inpatient management
Unavoidably, some patients will need to be admitted to the hospital for therapeutic complications or inpatient treatments. It is imperative that the hemato-oncology wards remain COVID-free, and that all patients undergo a thorough screening process before they are admitted in these units. Medical personnel including orderlies, nurses, pharmacists and physicians, should be exclusively assigned to these units and should not be in contact with COVID-19 positive patients. Indications for autologous and allogeneic stem cell transplantations should be reconsidered and discussed during virtual tumor board meetings. Only urgent transplants with curative intent should be offered to patients.
For patients that are already hospitalized, a systematic screening (that is not limited to suspected cases of COVID-19 but includes all patients) should confirm the absence of COVID-19. Cancer patients who test positive for COVID-19 must be treated in a dedicated COVID-19 unit that is kept separate, with distinct personnel engaged in the use of personal protective equipment and strict infection prevention measures. It will be important to treat COVID-19 patients as soon as they are positive (even before they develop any symptoms of the infection) using under medical supervision agents that target viral enzymes (such as favipiravir) or host cells (such as hydroxychloroquine plus azithromycin) to reduce viral replication. Although these treatments have not been evaluated in Phase III clinical trials, at this point it appears a moral obligation to attempt experimental treatments rather than to passively await the results of such trials.
Finally, even the asymptomatic medical staff should be systematically tested for the presence of oropharyngeal COVID-19 on a weekly basis.
3. Clinical trials
To protect medical personnel and provide support to COVID-19-specific units, most ongoing oncology clinical trials have been temporarily stopped.Citation10 The COVID-19 pandemic has driven unprecedented research efforts in finding treatments and vaccines, and has galvanized the medical and research community to cooperate and to share data on high-risk individuals (and hence to create a COVID-19 cancer registry) and clinical observations (such as the impact of immunotherapy on COVID-19) in order to improve patient management.
4. Unmet research needs
It will be important to find out i) novel diagnostics to stratify COVID-19 patients not only based on viral load and virulence, but also according to their status of inflammation or immunosuppression, or in contrast their immune activation (humoral and cellular immune responses), ii) which type of cancer therapy favors (or attenuates) the susceptibility to severe COVID-19, in particular the effects of PD-1/PD-L1-targeting immunotherapy on COVID-19, iii) whether experimental or clinically approved anti-COVID-19 medications interfere with the efficacy of anticancer immunotherapies.
In summary, the current COVID-19 outbreak requires a reorganization of cancer care and oncological wards at multiple levels, imposing a series of difficult choices to optimize a health care system that is under stress. Several European and Canadian COVID-19 cancer consortia are following the Chinese efforts including https://ccc19.org and ONCOVID trial (NCT04341207) at Gustave Roussy and across multiple cancer centers. In this pandemic setting, innovative approaches to maintain safe follow-ups and treatments are needed.
References
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3. doi:10.1038/s41586-020-2012-7.
- Guan W, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, Liu L, Shan H, Lei C-L, Hui DSC, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;NEJMoa2002032. doi:10.1056/NEJMoa2002032.
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3.
- Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi:10.1016/S1470-2045(20)30096-6.
- Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020. doi:10.1001/jama.2020.4683.
- Spicer J, Chamberlain C, Papa S. Provision of cancer care during the COVID-19 pandemic. Nat Rev Clin Oncol. 2020. doi:10.1038/s41571-020-0370-6.
- Kutikov A, Weinberg DS, Edelman MJ, Horwitz EM, Uzzo RG, Fisher RI. A war on two fronts: cancer care in the time of COVID-19. Ann Intern Med. 2020. doi:10.7326/M20-1133.
- Lala M, Li M, Sinha V, de Alwis D, Chartash E, Jain L. A six-weekly (Q6W) dosing schedule for pembrolizumab based on an exposure-response (E-R) evaluation using modeling and simulation. JCO. 2018;36(15_suppl):3062. doi:10.1200/JCO.2018.36.15_suppl.3062.
- You B, Ravaud A, Canivet A, Ganem G, Giraud P, Guimbaud R, Kaluzinski L, Krakowski I, Mayeur D, Grellety T, et al. The official French guidelines to protect patients with cancer against SARS-CoV-2 infection. Lancet Oncol. 2020;S1470204520302047. doi:10.1016/S1470-2045(20)30204-7.
- American Association for Cancer Research. Clinical research slows as COVID-19 surges. Cancer Discov. 2020. doi:10.1158/2159-8290.CD-NB2020-021.