ABSTRACT
We previously reported that CIMAvax-EGF vaccine is safe, immunogenic and efficacious to treat advanced non-small-cell lung cancer (NSCLC) patients. A phase III trial was designed using an optimized immunization schedule. It included higher antigen dose and injections at multiple sites. Immune response and circulating biomarkers were studied in a subset of patients. EGF-specific antibody titers, IgG subclasses, peptide immunodominance and circulating biomarkers were assessed by ELISA. In vitro EGF-neutralization capacity of immune sera and EGF-IgG binding kinetics was evaluated by Western Blot and Surface Plasmon Resonance (SPR) technology, respectively. We show that CIMAvax-EGF elicited mainly IgG3/IgG4 antibodies at titers exceeding 1:4000 in 80% of vaccinated patients after 3 months of treatment. The EGF-specific humoral response was directed against the central region of the EGF molecule. For the first time, the kinetic constants of EGF-specific antibodies were measured evidencing affinity maturation of antibody repertoire up to month 12 of vaccination. Notably, the capacity of post-immune sera to inhibit EGFR phosphorylation significantly increased during the course of the immunization scheme and was related to clinical outcome (P = .013, log-rank test). Basal concentrations of EGF and TGFα in the serum were affected by EGF-based immunization. In conclusion, the CIMAvax-EGF vaccine induces an EGF-specific protective humoral response in a high percent of NSCLC vaccinated patients, the quantity and quality of which were associated with clinical benefit (clinical trial registration number: RPCEC00000161, http://registroclinico.sld.cu/).
Abbreviations
EGF: epidermal growth factor; EGFR: epidermal growth factor receptor; Ab: antibody; AR: amphiregulin; NSCLC: non-small-cell lung cancer; rhEGF: recombinant human epidermal growth factor; BSC: best supportive care; TGFα: tumor growth factor alpha; IL-8: interleukin 8; MAb: monoclonal antibody; SPR: surface plasmon resonance
Introduction
Lung cancer is one of the most prevalent tumors worldwide for decades with millions of new cases diagnosed every yearCitation1. Within lung neoplasms, non-small-cell lung cancer (NSCLC) is the dominant histology, representing approximately 85% of all the lung tumors.Citation2 Survival times obtained even after the best available chemotherapy are limitedCitation3 and, due to its toxicity, it is tolerable only in patients with good performance status. The finding that PD-1 blockade produces clinical responses in NSCLCCitation4 has yielded increased interest in cancer vaccines and their possible combination with checkpoint inhibitors.
Approximately 80% of patients with NSCLC overexpress the epidermal growth factor receptor (EGFR).Citation5 EGFR overexpression is associated with poor prognosis and resistance to chemotherapy, representing an attractive molecule for the development of therapies against this target.Citation6 EGF is one of the main ligands of the EGFR. Its binding induces receptor dimerization resulting in autophosphorylation and the transduction of mitogenic signals.Citation7,Citation8 EGFR overexpression and high levels of its ligands EGF and TGFα in clinical samples from cancer patients have been associated with survival disadvantages.Citation9,Citation10 The relation among the expression of EGFR ligands, the development of resistance to therapies directed against this receptorCitation11 and their value as prognostic markersCitation12,Citation13 render the targeting of these molecules an important research goal for antitumor therapy.
Thus, active immunotherapy against ligands of EGFR is an emerging concept that aims to generate antibodies specific for these molecules capable of blocking ligand/receptor binding and consequently intracellular signaling. The design of a therapeutic vaccine consisting of human recombinant EGF chemically conjugated to the P64k protein from Neisseria meningitides, emulsified in the oily adjuvant Montanide ISA 51VG (CIMAvax-EGF) was previously described by Gonzalez et al.Citation14 Clinical studies in patients with NSCLC have shown that the vaccine is immunogenic and well tolerated.Citation15,Citation16 It has been described that patients who exhibit a high Ab response against EGF able to inhibit its binding to EGFR, achieve an increased survival.Citation17 The search for more effective treatment schemes in terms of achieving a clinical benefit in a larger number of patients has been the focus of previous studies regarding this vaccine. Based on evidence obtained from a phase II study and aiming to improve vaccine immunogenicity, a phase III trial was designed using a higher antigen dose and injections at multiple sites.Citation18 In the present study, we characterize the humoral response generated in NSCLC patients using an optimized immunization schedule. Surrogate biomarkers of clinical benefit in immune-responsive NSCLC patients treated with CIMAvax-EGF were found.
Materials and methods
Study context
A total of 405 advanced unresectable NSCLC patients were enrolled in a randomized phase III clinical trial (National Public Registry of Clinical Trials: RPCEC00000161) with CIMAvax-EGF from June 2006 to January 2012.Citation19 Histologically confirmed stage IIIB or IV NSCLC patients with at least stable disease after first-line chemotherapy treatment were included. Other eligibility criteria were an Eastern Cooperative Oncology Group (ECOG) performance status index ranging from 0 to 2 and adequate organ function, as previously described.Citation18 The trial protocol, informed consent and investigator brochure were approved by the ethics boards from all participating institutions and by the National Regulatory Agency. The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practices guidelines. All patients provided a written informed consent.
Vaccine
CIMAvax-EGF consists of human recombinant EGF manufactured in yeast (hu-recEGF) chemically conjugated to the P64K Neisseria meningitides recombinant protein (reP64K), manufactured in Escherichia coli. Both hu-recEGF and P64K were supplied by the Center for Genetic Engineering and Biotechnology, Havana, Cuba. The rhEGF-rrP64K conjugate is stored at 4ºC. At the moment of immunization, the conjugate is emulsified in Montanide ISA-51 VG (NC0962946, Seppic).
Patient population and treatment
A set of 140 NSCLC patients (112 vaccinated and 28 controls) that received at least 4 vaccine doses, in the case of vaccinated patients, and with at least three follow-up sera samples in control subjects, were selected for different assays according to availability and quality of serum samples. The most important baseline features were balanced between treatment groups according to the chi-square test (P > .05) ().
Table 1. Patient characteristics at baseline.
At least 4 weeks after finishing the first-line chemotherapy, patients received a low dose of cyclophosphamide (200 mg/m2) and 3 d later the first immunization of CIMAvax as switch maintenance therapy. Each immunization consisted of intramuscular injection of 2.4 mg of CIMAvax-EGF, distributed in four separate anatomic sites (600 µg antigen/site). During the induction phase, four bi-weekly doses were administered followed by monthly immunizations until patient withdrawal, toxicity or performance status deterioration (maintenance phase). The immunization schedule is summarized in . Patients assigned to the control arm received best supportive care.
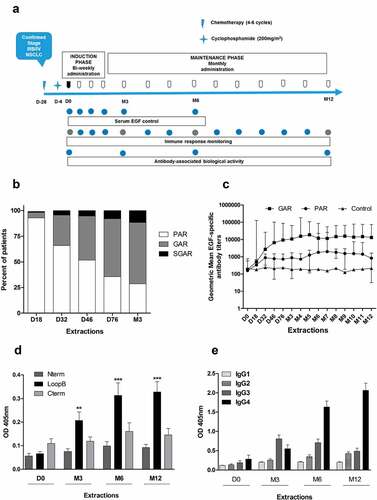
Figure 1. Induction of EGF-specific humoral immune response in NSCLC patients. (a) Vaccination and sampling schedules during CIMAvax-EGF immunotherapy. (b) Percent of vaccinated patients classified as poor antibody responders (PAR), good antibody responders (GAR) and super-good antibody responders (SGAR) during the induction phase of vaccination schedule. (c) EGF-specific antibody titers elicited in NSCLC patients from GAR (n = 85), PAR (n = 27) and control (n = 28) groups during 1 y of vaccination. Serum EGF IgG antibody titers were determined by ELISA at indicated time points and presented as the inverse of serum dilution. Significant differences were found among GAR, PAR and control curves according to Generalized Linear Model (P < .0001). D) IgG response to EGF-derived peptides from vaccinated patients classified as GAR (n = 40). Antibody levels against different regions of EGF molecule were determined by ELISA at indicated time points and presented as values of absorbance at 405 nm. Asterisks (*) represent significant differences according to Dunn’s test: **P < .01, ***P < .001. E) Levels of EGF-specific IgG subclasses from 40 vaccinated patients. Serum levels of EGF-specific IgG1, IgG2, IgG3 and IgG4 levels were determined by ELISA using subclass-specific antibodies and presented as values of absorbance at 405 nm. Asterisks (*) represent significant differences according to Dunn’s test: *P < .05, ***P < .001.
All recruited patients were considered assessable for toxicity according to the Common Toxicity Criteria from the National Cancer Institute version 3.0.
Sample collection and storage
Blood samples were collected before each immunization. Five milliliters of blood was spun for 10 min at 3000 rpm to isolate serum. Aliquots of the samples were stored at −80ºC until use.
Immune response measurements
ELISA, as previously described, determined anti-EGF Ab titers and IgG response to EGF-derived peptides.Citation17 Patients were classified as good antibody responders (GAR) if they elicited an antibody response four times higher than the baseline levels and a titer equal or higher than 1:4000. Patients with Ab titers below 1:4000 were classified as poor antibody responders (PAR). Additionally, patients who elicited antibody titers equal or higher than 1:64 000 were classified as super-good antibody responders (SGAR).
EGF-derived peptide immunodominance was defined as an optical density signal (405 nm) of at least two times the one obtained with the rest of the peptides used in the assay.
In order to characterize the anti-EGF IgG subclass, anti-human IgG1 (B6775, Sigma), IgG2 (B3398, Sigma) IgG3 (B3523, Sigma) and IgG4 (B3648, Sigma) subclass-specific secondary antibodies and alkaline phosphatase-conjugated streptavidin (189732, Sigma) were used in the ELISA assay previously described.Citation14
EGFR phosphorylation inhibition
The capacity of the anti-EGF antibodies to inhibit EGFR phosphorylation in the presence of EGF was measured by a previously described immunoblotting assay.Citation17 Briefly, A431 cells were serum starved for 24 h and then incubated with sera from control or vaccinated patients for 1 h at 37°C. Incubation with 1 mol/L tyrphostin AG1478 (tyrosine kinase inhibitor) was used as the positive control (100% of inhibition). Equal amounts of protein were resolved on SDS-PAGE, transferred onto polyvinylidene difluoride nitrocellulose membrane, and incubated with specific anti-phosphotyrosine antibody (2234L, Cell Signaling). After washing, the membranes were incubated with secondary anti-mouse (G-21040, Invitrogen) or anti-rabbit (G-21234, Invitrogen) antibodies conjugated with horseradish peroxidase. The signal was visualized by enhanced chemiluminescence according to the manufacturer’s instruction (Amersham Biosciences, UK) and band intensity was quantitated using a densitometer SI (Pharmacia Biotech) and ImageMaster 1D prime Software. To normalize the protein loading on the gel, the membranes were stripped and reprobed with anti-EGFR antibodies (2232S, Cell Signaling). We used ECL Plus Western blotting detection reagents (RPN2109, Amersham Biosciences) as detection system. The inhibition of phosphorylation occurred when values were higher than the mean of the percentages of inhibition reached in the control cohort (pre-immune sera) plus 2 SD.
EGF-specific antibodies’ affinity determination by surface plasmon resonance
Surface plasmon resonance (SPR) experiments were performed using a Biacore X unit (BR-1100-30, General Electric Health Care). Recombinant human EGF (hrEGF) was obtained at CIGB, Cuba.Citation20 hrEGF was immobilized in a CM5 chip (BR-1000-14, General Electric Health Care) by amine coupling chemistry at 25°C and a flowrate of 5 µL/min using HEPES 10 mmol/L, pH 7.4, 0.15 mol/L NaCl, 3 mmol/L EDTA, 0.005% Polysorbate 20 (HBS-EP, BR100188, General Electric Health Care) as running buffer.Citation21 For immobilization hrEGF was diluted in 10 mM sodium acetate buffer pH 4.0 to a final concentration of either 200 µg/mL to obtain high density surfaces (840 ± 35 RU, n = 5) or 50 µg/mL to obtain medium density surfaces (350 ± 20 RU, n = 6). The second flow channel was blocked with ethanolamine, to be used as a reference surface.
IgG fraction from patient’s serum samples was isolated using the Melon Gel kit (45206, Pierce) according to manufacturer’s instructions. Next, purified IgG buffer was changed to PBS using ultrafiltration devices (UFC205024, Millipore).
Quantification of anti-EGF antibodies was performed in conditions of total mass transfer limitation.Citation22 A calibration curve was generated by the application of MAb CB-EGF.1 diluted in HBS-EP buffer (0.03–7.5 µg/mL) at 5 µL/min. At least three different dilutions were assayed for each IgG patient’s sample with the same conditions used for the calibration curve. Antibody concentration was calculated from the interpolation of the binding slope of the interaction curve on the CB-EGF.1 calibration curve. The regeneration of the surface was carried out by applying 10 mM HCl for 1 min.
Medium density surfaces were used to characterize antibody binding kinetics. The application of MAb CB.EGF-1 at different flowrates yielded superimposable curves indicating the absence of mass transport limitation on these surfaces. At least three different dilutions of each IgG patient’s sample were applied at 30 µL/min, and association phase was registered for 5 min and antibody dissociation was measured for 3 min. Binding curves were adjusted to a bivalent analyte model using BIAevaluation software V4.1 (General Electric Health Care).
Quantification of circulating EGFR ligands and inflammatory factors
Circulating levels of EGF, TGFα, AR, VEGF, IL-6 and IL-8 were determined in sera from NSCLC patients before treatment and at least 6 months after the administration of CIMAvax-EGF. The concentration of the circulating factors was measured using commercial ELISA kits (Quantikine, R&D Systems Inc, USA). The plates were read in a Thermo Fisher microplate reader (Thermo Scientific, USA). Serum samples were diluted as recommended by the manufacturers.
Statistical analysis
Chi-square test, used for testing relationships between categorical variables, was applied for evaluating distribution differences in demographic and tumor characteristics between control and vaccinated groups. Comparison of levels of anti-EGF Abs between the two treatment groups was performed using the Generalized Linear Model. This method is an extension of generalized linear models, as a means of testing hypotheses regarding the influence of binary factors (Treatment) on other variables collected within subjects across time (levels of anti-EGF Abs). For the analyses of the immunological data, non-parametric Wilcoxon signed-rank test was used for matched-pairs taking into account that the samples were not normally distributed. Dunn’s multiple comparison test was utilized, as post hoc analysis, after finding means differences by ANOVA. It was used to test differences in antibody response inside vaccinated patients. Spearman rank correlation is a non-parametric test that is applied to measure the degree of association between two variables. In our case, it was employed to estimate the correlation between the immunological variables. Cox Regression confirmed the correlation between survival and measured variables. Survival data were analyzed using the Kaplan–Meier method and the log-rank test. Statistical analyses were done with SPSS program (version 17.0).
Results
Elicited anti-EGF antibody titers
Antibody response against EGF was evaluated in 140 patients (vaccinated, n = 112, control, n = 28). A preexisting immune response to EGF was detected in both groups, with a geometric mean of Ab titers of 1:184. Levels of antibody titers before treatment did not correlate with the humoral response elicited after vaccine administration, according to Spearman coefficient (r = 0.25; P > .05).
After three administrations of vaccine (D46), 50% of immunized patients were classified as good antibody responders (GAR) and the other 50% were classified as poor antibody responders (PAR). After induction phase (M3), 72% of patients elicited Ab titers higher than 1:4000 (). In addition, 10% of patients were classified as super-good antibody responders (SGAR) because they developed Ab titers higher than 1:64 000. None of the control patients were classified as GAR.
In patients classified as GAR, there was a gradual increase in Ab titers during the first 2 months of treatment followed by a plateau starting around d 76 (P < .001) (). Significant differences were obtained between the kinetics of anti-EGF Ab titers of vaccinated (GAR vs PAR) and control patients (P < .0001).
Moreover, no associations between baseline characteristics of patients and immune response were observed (chi-square test, P > .05).
Antibody response against different regions of EGF molecule
To check the reactivity to the EGF regions, serum from 40 vaccinated patients classified as GAR were tested against three peptides corresponding to N-terminal, central (Loop B) and C-terminal (Loop C) regions of the EGF molecule (). An equally distributed IgG Ab response to all peptides was detected before immunizations (P > .05). Remarkably, after four doses of vaccine, the EGF-specific Ab response was significantly directed against the main region involved in the binding of EGF to EGFR (Loop B) in 46% of evaluated patients (P < .01). The remaining patients had an equally distributed Ab response against all regions of the EGF molecule.
IgG subclasses elicited during treatment with CIMAvax-EGF
A previous studyCitation14 described the induction of a predominant IgG3 response measured in the highest anti-EGF Ab titer reached after CIMAvax-EGF administration. In the present study, we assessed the kinetics of anti-EGF IgG subclasses elicited in 40 vaccinated patients classified as GAR (). After CIMAvax-EGF administration, an increase in IgG2, IgG3 and IgG4 levels was observed. After 3 months of treatment, the humoral response against EGF was predominantly IgG3 (P < .05). However, after 6 months, IgG4 was the predominant subclass (P < .001) and was maintained for the rest of the evaluated period (P < .001). These results suggest a change in T helper response pattern from Th1 at month 3 to Th2 at month 6.
In vitro biological activity of EGF-specific antibodies
The mechanism of action of CIMAvax-EGF is based on the ability of the elicited antibodies to prevent the binding of EGF to EGFR, thus inhibiting the signaling cascade associated with tumor cell proliferation. To evaluate the ability of the antibodies to inhibit EGF-mediated activation of EGFR, samples from 24 patients also classified as GAR, but with similar EGF-specific Ab titers were selected.
Elicited Abs were capable to inhibit EGFR phosphorylation upon treatment of lung cancer cells with EGF (). A gradual increase of EGFR-phosphorylation inhibitory capacity was observed during the first year of treatment (P < .01). Remarkably, after the induction phase (3 months), percentages of inhibition over 50% were observed in 10 out 24 patients.
Figure 2. In vitro biological activity of EGF-specific antibodies elicited in vaccinated NSCLC patients. (a) Inhibition percent of EGFR activation in H292 lung cancer cells by sera from immunized NSCLC patients (n = 24). Starved H292 cells were incubated with NSCLC patient immune sera (1:100) and activated with rhEGF. The levels of phosphorylated (pEGFR) and EGFR from H292 lysates were determined by Western blot using specific antibodies. Pre-immune serum was used to set 100% EGFR activation signaling in each evaluated patient. Asterisks (*) represent significant differences according to Wilcoxon signed-rank test: **P < .01, ***P < .001. (b) Relationship between the anti-EGF Ab titers and inhibition of EGFR activation. Asterisks (*) indicate a significant correlation according to Spearman’s correlation coefficient: *P < .05, ***P < .001. (c) Immunoblots showing the EGFR phosphorylation levels obtained using the sera of one representative patient.
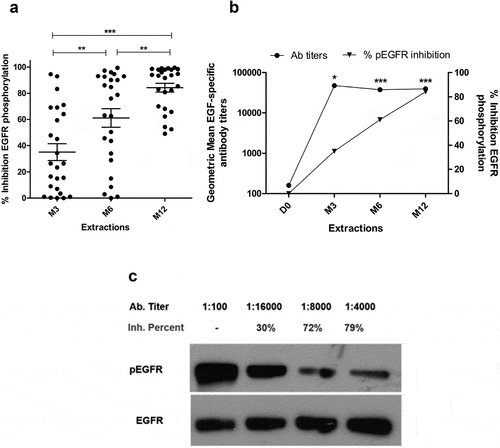
Moreover, a high correlation between anti-EGF Ab titers and their capacity to inhibit EGFR phosphorylation was observed over time, according to Spearman correlation coefficient (r = 0.62; P < .001) (). Nevertheless, some sera with similar EGF-specific Ab titers showed differences in their inhibitory capacity, as exemplified in one representative patient, in which increasing percentages of EGF-EGFR inhibition were obtained with lower antibody titers ().
Serial Biacore analysis of relative antibody dissociation rates
In addition to epitope specificity, the neutralization capacity of an antibody response depends on the strength of the interaction with the antigen.Citation23 To characterize the patient’s serum samples in terms of antigen-binding affinity and its relation with the inhibitory capacity, we investigated the affinity maturation of the specific antibody response in post-vaccination polyclonal sera from NSCLC patients selected according to their in vitro biological activity.
For quantitation of specific antibodies, purified IgG from serial serum samples of 18 vaccinated patients were injected over a surface of immobilized hrEGF in conditions of total mass transfer limitation. Under these conditions, the initial slope of the binding curve depends on antibody concentration but not on the kinetics of the interaction.Citation24 Concentration of anti-EGF antibodies was then determined from the binding response measured during the early binding phase of the SPR sensorgram. For this purpose, a calibration curve of different concentrations of the MAb CB.EGF1 was used. Preexisting antibodies were detected at D0 with an average concentration of 23.7 µg/mL (1.6x10−7 M) followed by an increase upon CIMAvax-EGF administration that becomes statistically significant after induction phase (P < .001). This concentration remains rather constant around 218 µg/mL (1.5x10−6 M) after month 3 (). Anti-EGF antibody concentration determined by SPR correlated with the ELISA antibody titers in all tested samples (P < .05).
Figure 3. Affinity maturation of EGF-specific immune response elicited in NSCLC vaccinated with CIMAvax-EGF. (a) Concentration of EGF-specific antibodies in immunized patient’s sera (n = 20). Asterisks represent significant differences according to Wilcoxon signed-rank test: **P < .01, ***P < .001. (b) Kinetic constants for the first binding (koff1 and kon1) of EGF-specific antibodies elicited in vaccinated patients. Asterisks represent significant differences according to the Wilcoxon signed-rank test: **P < .01. Only significant differences are represented. Concentration and kinetic constants of elicited EGF-specific antibodies were determined by SPR technology. (c) Correlation between in vitro biological activity of anti-EGF antibodies and their affinities. Asterisks (*) indicate a significant correlation P < .05 according to Spearman’s correlation coefficient.
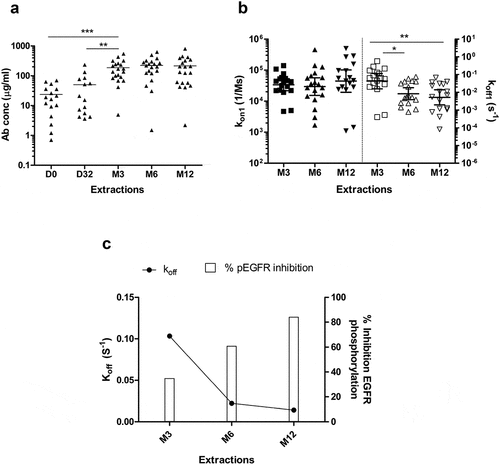
To compare the strength of binding, IgG samples were injected over a surface with hrEGF immobilized at a density that allowed a sensitive detection of reactive antibodies, not only the high-affinity repertoire. Such medium density surface also allowed bivalent antibody binding as verified with MAb CBEGF.1 (data not shown). Thus, interaction curves were fitted to a bivalent model. Kinetic constants for the first binding (koff1 and kon1) determined by fitting to a bivalent model has been demonstrated to be in close agreement with the constants obtained for the Fab fragment, thus reflecting the intrinsic affinity of the antibody.Citation25 Statistical residuals were evenly distributed with an average Chi2 of 1.83 ± 1.43 (n = 42), evidencing a homogeneous fit to this interaction model. Results showed no significant differences in kon1 from month 3 to month 12 (). Interestingly, a sustained decrease in koff1 values was observed during the evaluated period with a significant decrease between month 3 (4.2x10−2 s−1, 95% CI, 1.6 × 10−2-1.2x10−1) and M6 (8.3x10−3 s−1, 95% CI, 3.8 × 10−3-1.8x10−2) and month 3 and month 12 (5.2x10−3 s−1, 95% CL, 1,9x10−3-1.4x10−2) (P < .01). These results indicated the existence of affinity maturation due to a 30-fold increase on the average stability of EGF: antibody complexes ().
The contribution of the antibody affinity to in vitro EGF-neutralization capacity of vaccine-elicited antibodies was evaluated by correlating the Koff1 of hrEGF:antibody interaction with the percentage of EGFR-activation inhibition (). A significant negative correlation was observed between those parameters at month 12 (P = .032), where lower dissociation constants correlated with higher EGFR-phosphorylation inhibition.
Altogether, these data indicate that the optimized CIMAvax-EGF vaccination schedule generated antibodies with increasing affinity against the EGF molecule that correspondingly leads to a higher neutralization capacity.
Association between immune response and patient’s clinical outcome
In order to identify the subset of vaccinated patients who might benefit from treatment with CIMAvax-EGF, we correlated different features of the humoral response with patient survival.
Confirming our previous results,Citation17 a significant survival advantage was observed in patients who developed Ab titers higher than 1:4000 after induction phase (GAR, n = 68; median: 15.9 months) compared to patients classified as poor antibody responders (PAR) (n = 27; median: 10.07 months, P = .028). Interestingly, patients who elicited Ab titers higher than 1:64 000 (SGAR) did not show a statistically significant survival advantage (n = 17; median: 26.9 months, P = .324) ().
Figure 4. Immune response and survival outcome of NSCLC patients immunized with CIMAvax-EGF. (a) Overall survival of vaccinated patients according to EGF-specific antibody titers 3 months after first immunization. (b) Clinical impact of in vitro biological activity. Differences in survival times were assessed by the log-rank test.
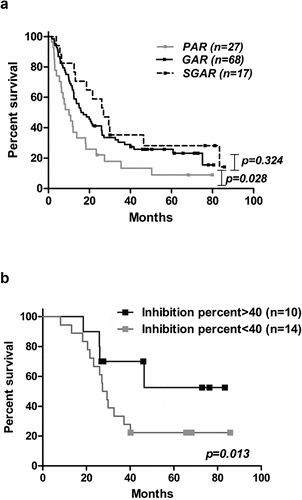
Moreover, inside the GAR subset, the quality of the anti-EGF Ab response correlated with patient survival. As early as 3 months after treatment, we observed that patients with a higher percentage (>40%) of EGFR phosphorylation inhibition had longer survival times (n = 10; median SV: not reached) in comparison to lower percentage (<40%) of EGR phosphorylation (n = 14; median SV: 27.4 months, P = .013) ().
Serum concentration of EGFR ligands and NSCLC-associated proteins
Few studies have indicated the influence of EGFR-targeted therapies on the EGFR ligand and other tumor growth factor levels.Citation26,Citation27 In this sense, we evaluated the circulating levels of EGF, TGFα, AR, IL-6, IL-8 and VEGF in a set of vaccinated and control NSCLC patients.
The reduction in EGF serum concentration has been used as an indicator of the in vivo effect of the antibodies generated after immunization of NSCLC patients with CIMAvax-EGF. In the present study, we evaluated how the induced antibody response was capable of reducing basal EGF serum levels of NSCLC patients during the first 6 months of treatment. High levels of EGF were detected in serum at baseline (mean 1057 pg/mL, range: 0–2980 pg/mL) as it was previously reported.Citation17 Vaccinated patients (n = 40) displayed a significant decrease in EGF levels after 1 month of treatment (P < .01) (). More than 50% of the evaluated patients decreased at least two times their EGF levels after two administrations of CIMAvax-EGF. EGF decreased to undetectable levels at month 6 in 80% of evaluated patients. On the contrary, no variation in the EGF levels was observed in serum from control patients (P > .05) ().
Figure 5. Serum levels of EGF and different NSCLC-associated proteins in patients vaccinated with CIMAvax-EGF. Circulating EGF levels in immunized (a), n = 40) and control (b), n = 15) patients during 6 months. (c) Serum levels of TGFα at baseline and after 6 months of treatment with CIMAvax-EGF (n = 40). Serum levels were determined by a commercial ELISA kit at indicated time points. Asterisks (*) represent significant differences according to Wilcoxon signed-rank test: **P < .01, ***P < .001.
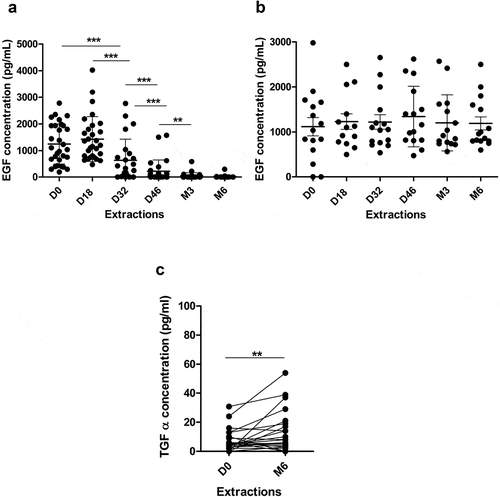
On the other hand, 6 months after the first immunization, while the EGF levels were undetectable in 80% of patients, basal TGFα levels increased in vaccinated patients (P < .01) (). In contrast, basal concentration of AR, VEGF, IL-8 and IL-6 was not affected by CIMAvax-EGF immunotherapy. These circulating factors did not show significant variations in the follow-up of control NSCLC patients (data not shown).
Discussion
Vaccine scheme optimization is a complex and challenging process that involves multiple factors such as doses, intervals, routes and administration sites. In the context of cancer vaccines, these factors must be carefully selected in order to increase the number of patients that elicit a protective immune response.
Based on preclinical resultsCitation28 and the increased immunogenicity observed in a previous clinical study using CIMAvax-EGF,Citation29 a phase III clinical trial was performed. Advanced NSCLC patients were vaccinated after first-line chemotherapy using a scheme optimized for the vaccine dose and number of immunization sites. The vaccine was well tolerated. The most frequent adverse events were injection site reactions, fever, headache, vomiting, chills and nausea.Citation19
The existence of a natural response specific to EGF was found in all evaluated patients. This response was increased 10-folds during the induction phase of the vaccine. A significant difference between the Ab titers of vaccinated and controls patients was observed in a shorter time period as compared to previous studies.Citation15,Citation17 The changes in the dose and in the number of immunization sites performed in this study led to higher frequency of good antibody responders (GAR) after 6 months of treatment, compared with previous clinical trials. The proportion of patients who developed Ab titers higher than 1:64 000 was also improved. These findings confirm what was found in the preclinical setting. In this case, low dose fractionated in multiple anatomical sites at priming accelerated the induction and enhanced the long-term maximal antibody response.Citation28 Previous studies have demonstrated that the distribution of vaccine in different immunization sites impacts on vaccine efficacy.Citation30,Citation31 These results suggest that the spatial distribution of the immunogen would increase the total number of presenting cells that are exposed to the antigen, thereby increasing the number of activated specific effector cells.
Moreover, during the evaluation of the antibodies specific for different regions of the EGF molecule, we observed an increase in the response against the central region of EGF (Loop B) over the vaccination period, whereas the humoral response against the rest of the molecule did not change during treatment. These results are similar to those obtained using a suboptimal treatment scheme, and highlight the reduced impact of changes in the immunization schedule in the immunodominance of the different epitopes of the EGF molecule. This behavior is in line with some experimental evidences showing that the immunodominant nature of an antigenic determinant is not affected by its concentration, position within the sequence of a given protein or the route of administration.Citation32
Although it is well known that the administration of CIMAvax-EGF using different immunization schemes was able to induce an IgG response,Citation29 there is not a detailed report about the subclass distribution during patient’s immunization. In agreement with previous data,Citation14 an IgG3 Ab response was generated after 3 months of vaccination, which is associated with the immune response against proteins and the secretion of Th1 cytokines.Citation33 However, in the present study, after 6 months of treatment, we observed a change to IgG4, which remained the predominant subclass for the rest of the evaluated period. As it is very well known, the nature of the antigen influences IgG subclass production, with protein antigens canonically stimulating IgG1 and IgG3.Citation34 Since the human EGF is a 6-kDa protein with 53 amino acid residues,Citation35 it might be anticipated the induction of the above-mentioned subclasses. However, the strong effect on IgG3 response instead of IgG1 is unclear and suggests additional influence of other factors like the adjuvant or the carrier protein. Interesting, as the number of immunizations increased, there was a gradual shift in the anti-EGF response from a predominant IgG3 at 3 months to IgG4 after 6 months. This distribution pattern of the IgG subclass indicates that both Th1 (IgG3) and Th2 (IgG4) T-cell responses are induced. Although there are few studies on the characterization of IgG subclass responses using cancer antigens as vaccines,Citation36,Citation37 the generation of IgG4 Ab with potent neutralization activityCitation38 has been associated with prolonged administration of high antigen doses.Citation39,Citation40 In this sense, Ullenhag et al. showed a shift from IgG1 to IgG4 after few months of vaccination of colorectal carcinoma patients with a recombinant carcinoembryonic antigen (CEA) vaccine.Citation36 Recently, advanced cancer patients who were long-term treated with a VEGF-based vaccine showed a gradual switch in the anti-VEGF IgG response from IgG1 to IgG4.Citation41
Antibody affinity maturation is an antigen-dependent processCitation24. To our knowledge, no previous studies have addressed affinity maturation of antibody response generated by cancer vaccines. This characterization could provide a quantitative analysis regarding the contribution of affinity maturation to the clinical outcome of treated patients. The EGF-specific antibody response developed in NSCLC patients during CIMAvax-EGF administration was characterized by an early and significant increment in antibody concentration and by discrete but continuous decrease in the dissociation rates. These results evidence that long-term maturation of antibody response elicited by CIMAvax-EGF vaccine increases the average affinity of antibody population due to lower dissociation rates of EGF-antibody complexes. Considering the high heterogeneity on affinity maturation among individuals found in human response against protein antigens, larger sample number would be also necessary to identify the relationship between the affinity of anti-EGF antibodies and the clinical outcome of patients. Interestingly, according to a theoretical model, the increase in the affinity seems to be related to IgG4 switchCitation42 which is in line with the aforementioned switch in IgG subclass.
The generation of EGF-specific antibodies with a high neutralizing capacity during immunization with CIMAvax-EGF has been the main objective of the different immunization schedules used in NSCLC patients. In the present study, the inhibitory activity of the elicited antibodies was, not only superior to that previously reported,Citation17 but also increased in the course of the immunization schedule. However, that increase was not associated with a significant change in antibody titers. These results indicate that this biological activity is dependent not only on the quantity of specific antibodies present in a given period of time, but also is influenced by the quality of the interaction or affinity, between these antibodies and the circulating EGF.
Several studies have demonstrated the role of the ligands of EGFR (EGF, TGFα) in tumor biology.Citation43 However, the different therapies directed against EGFR (monoclonal antibodies, tyrosine kinase inhibitors) currently used in the clinic, do not target its ligands. Additionally, resistance to such therapies has been linked to an increased secretion of these molecules,Citation43,Citation44 and therefore their concentrations in the serum of patients are currently monitored as biomarkers of clinical response to treatment.Citation45,Citation46 In the case of CIMAvax-EGF, the reduction in serum concentration of EGF has been used as an indicator of the effect of the antibodies generated by immunization. In the present study, the initial EGF concentration values after first-line chemotherapy in NSCLC patients were high and similar to those found in previous studies.Citation15,Citation17 Notably, this baseline EGF is a predictive biomarker of clinical response as it was recently reported.Citation19 Significant reduction of these pre-treatment EGF levels was achieved during the first month of vaccination (d 32), in contrast with our previous results, where this occurred after 6 months of treatment.Citation15,Citation17,Citation47 This behavior can be associated with changes in the immunization schedule and their effects on the neutralizing capacity of the generated anti-EGF antibodies, as addressed above. In addition, it was observed that TGFα levels increased after 6 months of vaccination, in agreement with other results obtained by blocking EGFR using different treatments.Citation26,Citation27 The usefulness of TGFα concentration as an indicator of an effective blockade of EGFR will be addressed in future trials with CIMAvax-EGF.
The selection of subgroups of patients who could benefit from therapies, as well as the search for immunological biomarkers associated with an effective antitumor response, have been the focus of several clinical studies. The association of the magnitude of the immune response and survival of cancer patients was observed in previous studies with CIMAvax-EGFCitation16,Citation17 and other therapeutic vaccines.Citation48,Citation49 In the present study, immunized patients classified as good responders after 4 months of treatment had a six-month survival benefit compared with poor responders. However, within the immune responders, no advantage in survival was observed in patients who developed Ab titers higher than 1:64 000. This finding suggests that not only the magnitude of antibody response in terms of titers but also the intrinsic characteristics of the immunoglobulins, such as their affinities, may be related to better EGF neutralization.
Moreover, biological activity, expressed as percentage of inhibition of EGFR phosphorylation after 2 months of treatment, showed a direct correlation with patient´s survival. Previously, antibody functionality measured as inhibition of the binding of EGF to EGFR was linked with longer survival times.Citation17 Both studies show that not only magnitude of anti-EGF antibody response but also their functionalities are related to clinical benefit.
In summary, for the first time, the kinetics of specific antibody subclass switch and the EGF-specific antibody affinity maturation were characterized in patients treated with CIMAvax. Regarding clinical surrogacy, the titers and quality (measured as EGFR phosphorylation inhibition) of the elicited EGF-antibodies have been described in this study and provide information about vital features to consider for the design of future clinical trials with CIMAvax-EGF. The usefulness of the current treatment schedule to elicit an immune response capable of producing clinical benefit in a high number of advanced NSCLC patients was also validated.
Disclosure of Potential Conflicts of Interest
BG, KPF, CEV, PCR, ZG, AG, TC and ZM are employees of the Center of Molecular Immunology, which sponsored all the clinical studies and produced CIMAvax-EGF. The rest of the authors have no conflict of interest. No consulting fees or payments for speeches or appearances have been received by any of the authors.
Author’s contributions
Conception and design: X. Popa and B. García
Clinical investigators (recruited and treated patients): E. Neninger
Immunological assessments: X. Popa, K. P. Fuentes, Z. Gonzalez and A. Gonzalez
SPR measurements and analysis: K. Alvarez and V. Huerta
Trial conduction and supervision: P.C. Rodriguez, T. Crombet and E. Neninger
Writing and or/revision of the manuscript: X. Popa, B. García, T. Crombet and Z. Mazorra
Analysis and interpretation of data: X. Popa, B. Garcia, C. E. Viada and Z. Mazorra
Acknowledgments
This study was conducted by the Center of Molecular Immunology in Havana, Cuba. We are especially grateful to the participating patients and their families, as well as the staffs of all institutions involved in this study.
Additional information
Funding
References
- Global Burden of Disease Cancer C. Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019. doi:10.1001/jamaoncol.2019.2996.
- Jemal A. Global burden of cancer: opportunities for prevention. Lancet. 2012;380:1797–11. doi:10.1016/S0140-6736(12)61688-2.
- Gridelli C, Maione P, Rossi A, Guerriero C, Ferrara C, Del Gaizo F, Colantuoni G, Nicolella D, Napolitano L. Chemotherapy of advanced NSCLC in special patient population. Ann Oncol. 2006;17(Suppl 5):v72–78. doi:10.1093/annonc/mdj955.
- Langer CJ. Emerging immunotherapies in the treatment of non-small cell lung cancer (NSCLC): the role of immune checkpoint inhibitors. Am J Clin Oncol. 2015;38:422–430. doi:10.1097/COC.0000000000000059.
- Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi:10.1016/1040-8428(94)00144-I.
- Ryan PD, Chabner BA. On receptor inhibitors and chemotherapy. Clin Cancer Res. 2000;6:4607–4609.
- Prigent SA, Lemoine NR. The type 1 (EGFR-related) family of growth factor receptors and their ligands. Prog Growth Factor Res. 1992;4:1–24. doi:10.1016/0955-2235(92)90002-Y.
- Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A. The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr Relat Cancer. 2001;8:11–31. doi:10.1677/erc.0.0080011.
- Tominaga T, Tsuchiya T, Mochinaga K, Arai J, Yamasaki N, Matsumoto K, Miyazaki T, Nagasaki T, Nanashima A, Tsukamoto K, et al. Epidermal growth factor signals regulate dihydropyrimidine dehydrogenase expression in EGFR-mutated non-small-cell lung cancer. BMC Cancer. 2016;16:354. doi:10.1186/s12885-016-2392-0.
- Yonemura Y, Takamura H, Ninomiya I, Fushida S, Tsugawa K, Kaji M, Nakai Y, Ohoyama S, Yamaguchi A, Miyazaki I. Interrelationship between transforming growth factor-alpha and epidermal growth factor receptor in advanced gastric cancer. Oncology. 1992;49:157–161. doi:10.1159/000227031.
- Wheeler DL, Huang S, Kruser TJ, Nechrebecki MM, Armstrong EA, Benavente S, Gondi V, Hsu KT, Harari PM. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944–3956. doi:10.1038/onc.2008.19.
- Ishikawa N, Daigo Y, Takano A, Taniwaki M, Kato T, Hayama S, Murakami H, Takeshima Y, Inai K, Nishimura H, et al. Increases of amphiregulin and transforming growth factor-alpha in serum as predictors of poor response to gefitinib among patients with advanced non-small cell lung cancers. Cancer Res. 2005;65:9176–9184. doi:10.1158/0008-5472.CAN-05-1556.
- Fontanini G, De Laurentiis M, Vignati S, Chine S, Lucchi M, Silvestri V, Mussi A, De Placido S, Tortora G, Bianco AR, et al. Evaluation of epidermal growth factor-related growth factors and receptors and of neoangiogenesis in completely resected stage I-IIIA non-small-cell lung cancer: amphiregulin and microvessel count are independent prognostic indicators of survival. Clin Cancer Res. 1998;4:241–249.
- Gonzalez G, Crombet T, Torres F, Catala M, Alfonso L, Osorio M, Neninger E, Garcia B, Mulet A, Perez R, et al. Epidermal growth factor-based cancer vaccine for non-small-cell lung cancer therapy. Ann Oncol. 2003;14:461–466. doi:10.1093/annonc/mdg102.
- Ramos TC, Vinageras EN, Ferrer MC, Verdecia BG, Rupale IL, Perez LM, Marinello GG, Rodriguez RP, Davila AL. Treatment of NSCLC patients with an EGF-based cancer vaccine: report of a phase I trial. Cancer Biol Ther. 2006;5:145–149. doi:10.4161/cbt.5.2.2334.
- Neninger E, Verdecia BG, Crombet T, Viada C, Pereda S, Leonard I, Mazorra Z, Fleites G, Gonzalez M, Wilkinson B, et al. Combining an EGF-based cancer vaccine with chemotherapy in advanced nonsmall cell lung cancer. J Immunother. 2009;32:92–99. doi:10.1097/CJI.0b013e31818fe167.
- Garcia B, Neninger E, de la Torre A, Leonard I, Martinez R, Viada C, Gonzalez G, Mazorra Z, Lage A, Crombet T. Effective inhibition of the epidermal growth factor/epidermal growth factor receptor binding by anti-epidermal growth factor antibodies is related to better survival in advanced non-small-cell lung cancer patients treated with the epidermal growth factor cancer vaccine. Clin Cancer Res. 2008;14:840–846. doi:10.1158/1078-0432.CCR-07-1050.
- Rodriguez PC, Neninger E, Garcia B, Popa X, Viada C, Luaces P, Gonzalez G, Lage A, Montero E, Crombet T. Safety, immunogenicity and preliminary efficacy of multiple-site vaccination with an Epidermal Growth Factor (EGF) based cancer vaccine in advanced non small cell lung cancer (NSCLC) patients. J Immune Based Ther Vaccines. 2011;9:7. doi:10.1186/1476-8518-9-7.
- Rodriguez PC, Popa X, Martinez O, Mendoza S, Santiesteban E, Crespo T, Amador RM, Fleytas R, Acosta SC, Otero Y, et al. A phase III clinical trial of the epidermal growth factor vaccine CIMAvax-EGF as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res. 2016;22:3782–3790. doi:10.1158/1078-0432.CCR-15-0855.
- Santana H, Garcia G, Vega M, Beldarrain A, Paez R. Stability studies of a freeze-dried recombinant human epidermal growth factor formulation for wound healing. PDA J Pharm Sci Technol. 2015;69:399–416. doi:10.5731/pdajpst.2015.01052.
- Hahnefeld C, Drewianka S, Herberg FW. Determination of kinetic data using surface plasmon resonance biosensors. Methods Mol Med. 2004;94:299–320. doi:10.1385/1-59259-679-7:299.
- Pol E, Karlsson R, Roos H, Jansson A, Xu B, Larsson A, Jarhede T, Franklin G, Fuentes A, Persson S. Biosensor-based characterization of serum antibodies during development of an anti-IgE immunotherapeutic against allergy and asthma. J Mol Recognit. 2007;20:22–31. doi:10.1002/jmr.804.
- Klasse PJ. How to assess the binding strength of antibodies elicited by vaccination against HIV and other viruses. Expert Rev Vaccines. 2016;15:295–311. doi:10.1586/14760584.2016.1128831.
- Poulsen TR, Jensen A, Haurum JS, Andersen PS. Limits for antibody affinity maturation and repertoire diversification in hypervaccinated humans. J Immunol. 2011;187:4229–4235. doi:10.4049/jimmunol.1000928.
- Yasmeen A, Ringe R, Derking R, Cupo A, Julien JP, Burton DR, Ward AB, Wilson IA, Sanders RW, Moore JP, et al. Differential binding of neutralizing and non-neutralizing antibodies to native-like soluble HIV-1 Env trimers, uncleaved Env proteins, and monomeric subunits. Retrovirology. 2014;11:41. doi:10.1186/1742-4690-11-41.
- Mutsaers AJ, Francia G, Man S, Lee CR, Ebos JM, Wu Y, Witte L, Berry S, Moore M, Kerbel RS. Dose-dependent increases in circulating TGF-alpha and other EGFR ligands act as pharmacodynamic markers for optimal biological dosing of cetuximab and are tumor independent. Clin Cancer Res. 2009;15:2397–2405. doi:10.1158/1078-0432.CCR-08-1627.
- Loupakis F, Cremolini C, Fioravanti A, Orlandi P, Salvatore L, Masi G, Schirripa M, Di Desidero T, Antoniotti C, Canu B, et al. EGFR ligands as pharmacodynamic biomarkers in metastatic colorectal cancer patients treated with cetuximab and irinotecan. Target Oncol. 2014;9:205–214. doi:10.1007/s11523-013-0284-7.
- Rodriguez PC, Gonzalez I, Gonzalez A, Avellanet J, Lopez A, Perez R, Lage A, Montero E. Priming and boosting determinants on the antibody response to an Epidermal Growth Factor-based cancer vaccine. Vaccine. 2008;26:4647–4654. doi:10.1016/j.vaccine.2008.07.003.
- Saavedra D, Crombet T. CIMAvax-EGF: a new therapeutic vaccine for advanced non-small cell lung cancer patients. Front Immunol. 2017;8:269. doi:10.3389/fimmu.2017.00269.
- Mould RC, AWK A, van Vloten JP, Susta L, Mutsaers AJ, Petrik JJ, Wood GA, Wootton SK, Karimi K, Bridle BW. Enhancing immune responses to cancer vaccines using multi-site injections. Sci Rep. 2017;7:8322. doi:10.1038/s41598-017-08665-9.
- Jaffee EM, Thomas MC, Huang AY, Hauda KM, Levitsky HI, Pardoll DM. Enhanced immune priming with spatial distribution of paracrine cytokine vaccines. J Immunother Emphasis Tumor Immunol. 1996;19:176–183. doi:10.1097/00002371-199605000-00002.
- Kim A, Sadegh-Nasseri S. Determinants of immunodominance for CD4 T cells. Curr Opin Immunol. 2015;34:9–15. doi:10.1016/j.coi.2014.12.005.
- Kyogoku N, Ikeda H, Tsuchikawa T, Abiko T, Fujiwara A, Maki T, Yamamura Y, Ichinokawa M, Tanaka K, Imai N, et al. Time-dependent transition of the immunoglobulin G subclass and immunoglobulin E response in cancer patients vaccinated with cholesteryl pullulan-melanoma antigen gene-A4 nanogel. Oncol Lett. 2016;12:4493–4504. doi:10.3892/ol.2016.5253.
- Valenzuela NM, Schaub S. The biology of IgG subclasses and their clinical relevance to transplantation. Transplantation. 2018;102:S7–S13. doi:10.1097/TP.0000000000001816.
- Carpenter G, Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi:10.1146/annurev.bi.48.070179.001205.
- Ullenhag GJ, Frodin JE, Strigard K, Mellstedt H, Magnusson CG. Induction of IgG subclass responses in colorectal carcinoma patients vaccinated with recombinant carcinoembryonic antigen. Cancer Res. 2002;62:1364–1369.
- Oji A, Noda T, Fujihara Y, Miyata H, Kim YJ, Muto M, Nozawa K, Matsumura T, Isotani A, Ikawa M. CRISPR/Cas9 mediated genome editing in ES cells and its application for chimeric analysis in mice. Sci Rep. 2016;6:31666. doi:10.1038/srep31666.
- Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy. 2009;39:469–477. doi:10.1111/j.1365-2222.2009.03207.x.
- Senti G, Von Moos S, Tay F, Graf N, Sonderegger TJP, Kundig TM. Epicutaneous allergen-specific immunotherapy ameliorates grass pollen-induced rhinoconjunctivitis: A double-blind, placebo-controlled dose escalation study. J Allergy Clin Immunol. 2012;129:128–135. doi:10.1016/j.jaci.2011.08.036.
- Deisenhammer F, Reindl M, Berger T. Immunoglobulin subclasses in patients with neutralizing and nonneutralizing antibodies against IFN-beta1b. J Interferon Cytokine Res. 2001;21:167–171. doi:10.1089/107999001750133195.
- Morera Y, Sanchez J, Bequet-Romero M, Selman-Housein KH, de la Torre A, Hernandez-Bernal F, Martin Y, Garabito A, Pinero J, Bermudez C, et al. Specific humoral and cellular immune responses in cancer patients undergoing chronic immunization with a VEGF-based therapeutic vaccine. Vaccine. 2017;35:3582–3590. doi:10.1016/j.vaccine.2017.05.020.
- Collins AM, Jackson KJ. A temporal model of human IgE and IgG antibody function. Front Immunol. 2013;4:235. doi:10.3389/fimmu.2013.00235.
- Esparis-Ogando A, Montero JC, Arribas J, Ocana A, Pandiella A. Targeting the EGF/HER ligand-receptor system in cancer. Curr Pharm Des. 2016;22:5887–5898. doi:10.2174/1381612822666160715132233.
- Taniguchi H, Takeuchi S, Fukuda K, Nakagawa T, Arai S, Nanjo S, Yamada T, Yamaguchi H, Mukae H, Yano S. Amphiregulin triggered epidermal growth factor receptor activation confers in vivo crizotinib-resistance of EML4-ALK lung cancer and circumvention by epidermal growth factor receptor inhibitors. Cancer Sci. 2017;108:53–60. doi:10.1111/cas.13111.
- Pentheroudakis G, Kotoula V, De Roock W, Kouvatseas G, Papakostas P, Makatsoris T, Papamichael D, Xanthakis I, Sgouros J, Televantou D, et al. Biomarkers of benefit from cetuximab-based therapy in metastatic colorectal cancer: interaction of EGFR ligand expression with RAS/RAF, PIK3CA genotypes. BMC Cancer. 2013;13:49. doi:10.1186/1471-2407-13-49.
- Yoshida M, Shimura T, Sato M, Ebi M, Nakazawa T, Takeyama H, Joh T. A novel predictive strategy by immunohistochemical analysis of four EGFR ligands in metastatic colorectal cancer treated with anti-EGFR antibodies. J Cancer Res Clin Oncol. 2013;139:367–378. doi:10.1007/s00432-012-1340-x.
- Gonzalez G, Crombet T, Neninger E, Viada C, Lage A. Therapeutic vaccination with epidermal growth factor (EGF) in advanced lung cancer: analysis of pooled data from three clinical trials. Hum Vaccin. 2007;3:8–13. doi:10.4161/hv.3.1.3537.
- Oji Y, Hashimoto N, Tsuboi A, Murakami Y, Iwai M, Kagawa N, Chiba Y, Izumoto S, Elisseeva O, Ichinohasama R, et al. Association of WT1 IgG antibody against WT1 peptide with prolonged survival in glioblastoma multiforme patients vaccinated with WT1 peptide. Int J Cancer. 2016;139:1391–1401. doi:10.1002/ijc.30182.
- Hansen GL, Gaudernack G, Brunsvig PF, Cvancarova M, Kyte JA. Immunological factors influencing clinical outcome in lung cancer patients after telomerase peptide vaccination. Cancer Immunol Immunother. 2015;64:1609–1621. doi:10.1007/s00262-015-1766-5.
