ABSTRACT
Basophils play an important role in orienting Th2 immune response, and in the pathogenesis of allergic and inflammatory disorders. However, the mechanism by which basophils are kept in check remains unclear and hence we explored the role of regulatory T cells (Treg cells) in this process. We demonstrate that human Treg cells do not suppress rather induce activation of basophils, and promote Th2 responses by IL-3 and STAT5-dependent mechanism.
Basophils are rare granulocytes representing approximately 1% of peripheral blood leukocytes. Basophils are reported to play a role in regulating acquired immunity, particularly by promoting Th2 cell differentiation as well as amplifying humoral memory response.Citation1 Several studies have also uncovered a role for basophils in protective immunity to pathogens. But dysregulated functions of basophils are associated with many pathologies and in particular allergic and inflammatory diseases. Main reason for the exceptional role of basophils in various pathologies despite their low frequency, is the expression of an array of receptors including FcεRI and cytokine receptors that sense signals derived from diverse sources, and immediate release of inflammatory mediators including cytokines (IL-4, IL-13, IL-6, thymic stromal lymphopoietin and B-cell-activating factor), histamine and leukotriene that support hypersensitive and inflammatory responses.Citation1 However, the mechanisms by which basophils are kept in check remains unclear and hence recently we explored if CD4+CD25+Foxp3+ regulatory T cells (Treg cells) have the capacity to control the functions of human basophils.
Treg cells play a pivotal role in maintaining the peripheral tolerance by inhibiting autoimmune and inflammatory diseases. Treg cells also play a major role in transplantation tolerance and in suppressing tumor immunity.Citation2 The cellular targets of Treg-mediated suppressor functions include T cells, dendritic cells, B cells, macrophages, monocytes, mast cells, NK cells and NKT cells.Citation3 Treg cells exert their suppressive functions through several mechanisms that are mediated either by cell-cell contact through inhibitory surface molecules (cytotoxic T lymphocyte antigen-4 (CTLA-4) and lymphocyte-activation gene-3 (LAG-3)), or by secreting regulatory cytokines (TGF-β1 and IL-10) and cytolysis through cytotoxic molecules (granzyme and perforin).Citation4 As Treg cells suppress the activation of both innate and adaptive immune cells, they are the targets to boost protective immune response to cancer.Citation2
In order to explore the regulation of human basophils functions by Treg cells, we activated basophils with IL-3 and anti-IgE antibodies that induce basophil activation via IL-3 receptor (IL-3R, a heterodimer composed of IL-3-specific α-subunit and a common β-subunit) and FcεRI-mediated signaling respectively. Interestingly, we found that basophils were refractory to Treg cell-mediated suppression. Despite being under the influence of Treg cells, basophils had undergone activation and anti-IgE-induced degranulation as analyzed by the expression of surface markers (CD203c, CD69, CD63 and CD107a) and histamine in the culture supernatants.Citation5
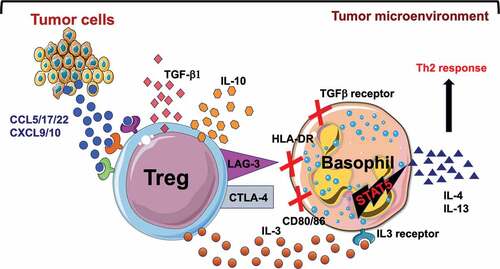
We were surprised by the results and hence investigated reasons for the refractoriness of human basophils toward Treg-mediated suppressive effects. As mentioned earlier, Treg cells mediate suppression of target cells by contact-dependent and – independent mechanisms. However, we found that human basophils either in the circulation or residing at secondary lymphoid organs lack CD80 and CD86, the receptors for CTLA-4; and HLA-DR, the receptor for LAG-3.Citation6 Furthermore, we also found that human basophils do not express TGF-βRII. On the other hand, a subset of human basophils express IL-10Rα, but we found that basophils are not responsive to IL-10.Citation5 Together, these results could explain underlying reasons for the refractoriness of human basophils toward Treg cell-mediated suppression.
Next, we explored if Treg cells could suppress resting basophils. We co-cultured resting basophils with activated memory Treg cells in the absence of any exogenous stimulation or cytokines. Interestingly, we found that Treg cells induced activation of human basophils characterized by the enhanced expression of surface markers associated with basophil activation (CD203c, CD13 and CD69) and secretion of cytokines (IL-8, IL-13 and IL-4). However, Treg-induced basophil activation was not associated with degranulation. Trans-well experiments and blocking studies targeting the intercellular adhesion molecule-1 and inducible T cell costimulator ligand pathway in Treg cells failed to mitigate basophil activation. In contrast, culturing basophils with supernatants from the activated Treg cells recapitulated basophil activation similar to that observed in our co-culture studies.Citation5 These results suggested that soluble mediators released from the activated Treg cells but not cellular contact drive the activation of basophils.
Notably, we found that activated Treg cells secrete IL-3 that could potently activate basophils. To further investigate the role of IL-3 in Treg-induced basophils activation, we perturbed the IL-3-IL-3R pathway by blocking IL-3Rα (CD123) on basophils before culturing with Treg cells. We found that blocking of IL-3Rα abrogated Treg-induced basophil activation. Previous report has shown that IL-3 signaling activates STAT5 in human basophils. To further confirm that IL-3 signaling is important in Treg-induced basophil activation, we pre-treated basophils with STAT5 inhibitor (CAS 285986–31-4) before culturing with activated Treg cells. We found that STAT5 inhibition in basophils abrogated Treg-induced basophils activation.Citation5 Altogether, these results revealed that IL-3-derived from Treg cells is essential for inducing human basophils activation.
What is the importance of our results for tumor immunology? While it has been well established that protective anti-tumor immunity is associated with Th1 responses by activating CD8+ cytotoxic T lymphocytes and NK cell-mediated cytotoxicity, Th2 responses contributes to tumor survival and escape.Citation7 In this regard, basophils that play a key role in orienting Th2 immune response have been implicated in inhibiting anti-tumor immunity. Recent reports suggest that lymphoid infiltrate in pancreatic ductal adenocarcinomas (PDAC) is comprised mainly of Th2 cells and is predicted to be associated with a poor survival outcome in these patients.Citation8 Interestingly, basophils were identified as the major contributor of IL-4 in the tumor-draining lymph nodes. The tumor microenvironment is a complex ecosystem where malignant cells are surrounded by diverse nonmalignant cells (immune cells, stromal cells and endothelial cells) that coordinate and orchestrate the immune response to the malignant cells. Notably, tumor cells and innate immune cells secrete several chemokines such as CCL17/22, CCL5, and CXCL9/10 to establish a tolerogenic microenvironment by recruiting Treg cells.Citation9 In PDAC, chemokines such as CCL7 secreted by alternatively activated monocytes recruit basophils to the tumor draining-lymph nodes. IL-3 secreted by T cells in the tumor microenvironment further activates basophils to orchestrate a Th2 responses by secreting IL-4.Citation8 Based on our observation it appears that Treg cells might also promote tumor evasion by activating basophils to augment and sustain Th2 responses by secreting IL-3 ().
Figure 1. Tumor cells secrete chemokines that recruit Treg cells to the tumor microenvironment. Our data suggest that tumor-associated Treg cells promote tumor survival and its immune escape by several mechanisms including secretion of IL-3 to activate basophils and to orchestrate Th2 responses.
Thus, our data indicate that induction of basophil activation by Treg cells will act as double-edged sword. Promotion of basophil activation by Treg cells in physiology might support various functions of basophils like enhancement of humoral responses and protection against helminthic parasites. However, in case of cancer, it is possible that human basophils might cooperate with tumor-associated Treg cells to promote and sustain Th2 response, thus providing an additional layer of tumor evasion mechanism. Therefore, abrogating basophil functions or mitigating the IL-3/IL-3R axis might promote strong anti-tumor immune response and tumor eradication. In fact, drug-conjugated CD123 (IL-3Rα) monoclonal antibodies are under clinical evaluation for acute lymphoblastic leukemia to target CD123-positive leukemic blast and stem cells.Citation10
To conclude, human basophils are endowed with a mechanism to escape key checkpoints employed by Treg cells, but are rather licensed to undergo complete activation by IL-3 derived from Treg cells to promote Th2 responses.
Abbreviations
| CCL17 | = | Chemokine (C-C motif) ligand 17 |
| CTLA-4 | = | Cytotoxic T lymphocyte antigen-4 |
| CXCL9 | = | C-X-C Motif Chemokine Ligand 9 |
| IL-3R | = | IL-3 receptor |
| IL-10 | = | Interleukin 10 |
| LAG-3 | = | Lymphocyte-activation gene-3 |
| NK cells | = | Natural killer cells |
| PDAC | = | Pancreatic ductal adenocarcinomas |
| Treg cells | = | Regulatory T cells |
| STAT5 | = | Signal transducer and activator of transcription 5 |
| TGF-β1 | = | Transforming growth factor beta 1 |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank all the lab members and collaborators who contributed to the data discussed in this article.
Additional information
Funding
References
- Karasuyama H, Miyake K, Yoshikawa S, Yamanishi Y. Multifaceted roles of basophils in health and disease. J Allergy Clin Immunol. 2018;142:370–3. PMID: 29247714. doi:10.1016/j.jaci.2017.10.042.
- Pere H, Tanchot C, Bayry J, Terme M, Taieb J, Badoual C, Adotevi O, Merillon N, Marcheteau E, Quillien VR, et al. Comprehensive analysis of current approaches to inhibit regulatory T cells in cancer. Oncoimmunology. 2012;1:326–333. PMID: 22737608. doi:10.4161/onci.18852.
- Romano M, Fanelli G, Albany CJ, Giganti G, Past LG. Present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front Immunol. 2019;10:43. PMID: 30804926. doi:10.3389/fimmu.2019.00043.
- Wing JB, Tanaka A, Sakaguchi S. Human FOXP3(+) regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity. 2019;50:302–316. PMID: 30784578. doi:10.1016/j.immuni.2019.01.020.
- Sharma M, Das M, Stephen-Victor E, Galeotti C, Karnam A, Maddur MS, Bruneval P, Kaveri SV, Bayry J. Regulatory T cells induce activation rather than suppression of human basophils. Sci Immunol. 2018;3:eaan0829. PMID: 29802207. doi:10.1126/sciimmunol.aan0829.
- Stephen-Victor E, Das M, Sharma M, Galeotti C, Fohrer-Ting H, Sendid B, Darnige L, Terris B, Badoual C, Bruneval P, et al. Demystification of enigma on antigen-presenting cell features of human basophils: data from secondary lymphoid organs. Haematologica. 2017;102:e233–e237. PMID: 28209657. doi:10.3324/haematol.2016.163451.
- De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469–478. PMID: 21339327. doi:10.1084/jem.20101876.
- De Monte L, Wörmann S, Brunetto E, Heltai S, Magliacane G, Reni M, Paganoni AM, Recalde H, Mondino A, Falconi M, et al. Basophil recruitment into tumor-draining lymph nodes correlates with Th2 inflammation and reduced survival in pancreatic cancer patients. Cancer Res. 2016;76:1792–1803. PMID: 26873846. doi:10.1158/0008-5472.CAN-15-1801-T.
- Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008;9:970–980. PMID: 18711434. doi:10.1038/ni.f.213.
- Li F, Sutherland MK, Yu C, Walter RB, Westendorf L, Valliere-Douglass J, Pan L, Cronkite A, Sussman D, Klussman K, et al. Characterization of SGN-CD123A, A potent CD123-directed antibody-drug conjugate for acute myeloid leukemia. Mol Cancer Ther. 2018;17:554–564. PMID: 29142066. doi:10.1158/1535-7163.MCT-17-0742.
