ABSTRACT
Background
Immune checkpoint inhibitors (ICI) have become an important treatment option for non-small cell lung cancer (NSCLC). We aimed to evaluate the clinical impact of pseudoprogression (PsP) and treatment beyond RECIST1.1-defined progressive disease (TBP) on outcome in NSCLC patients treated with ICI.
Methods
NSCLC patients treated with ICI between Mar 2016 and July 2018 were recruited in a consecutive manner. Response was assessed every 8–12 weeks using RECIST1.1 and iRECIST. Based on iRECIST, PsP was defined as progressive disease (PD) on RECIST1.1 subsequently reset to non-PD categories. Using log-rank test, progression-free survival (PFS) was compared between patients with and without PsP, and overall survival (OS) was compared between patients treated with and without TBP. The impact of TBP on OS was evaluated through multivariate Cox proportional hazard models.
Results
Of the 189 patients, seven (3.7%) experienced PsP which mostly occurred approximately 3 months after baseline. The median PFS was significantly longer in patients with PsP (not reached) than those without PsP (3.8 months, P = .02). Among patients who demonstrated PD according to RECIST1.1, median OS was significantly longer in patients with TBP (17.2 months) than those without TBP (7.4 months, P < .001). On multivariate analysis adjusting other covariates, TBP (HR, 0.4; 95% CI, 0.2–0.7) remained as a significant protective factor for mortality.
Conclusion
PsP occurred in 3.7% of NSCLC patients under ICI treatment. Based on iRECIST scheme, PsP and TBP may be associated with survival benefit.
Introduction
Immune checkpoint inhibitors (ICI) targeting programmed cell death-1 (PD-1) or its ligand (PD-L1) have become an important treatment option for non-small cell lung cancer (NSCLC).Citation1 Recently, pembrolizumab, nivolumab, and atezolizumab have documented overall survival benefits in phase III trials.Citation2 Consequently, in the 2019 National Comprehensive Cancer Network (NCCN) guidelines for NSCLC, PD-1 and PD-L1 inhibitors are recommended as the first line or second line standard of treatment in patients with advanced/recurrent NSCLC.Citation3
Interestingly, tumor response with ICI treatment is featured by an atypical response pattern called pseudoprogression (PsP) that may not be fully captured by conventional response assessment schemes such as response evaluation criteria in solid tumors (RECIST) 1.1.Citation4 In a subset of patients treated with ICI, the tumor burden transiently increases and then decreases while the treatment is continued, because of an immune reaction between tumor cells and host immune cells. To reflect this phenomenon, the RECIST Working Group released the new criteria, iRECISTCitation5 for standardized response assessment in patients under ICI treatment.
The key differences between RECIST 1.1 and iRECIST are the terminology and the way to confirm of progressive disease (PD). The iRECIST-based assessments are denoted with the prefix “i,” which stands for “immune” as follows: iCR for complete response, iPR for partial response, iSD for stable disease, iUPD for unconfirmed progressive disease, and iCPD for confirmed progressive disease. When PD occurs according to RECIST 1.1, a status of iUPD is assigned according to iRECIST and this requires confirmation on subsequent imaging at 4–8 week intervals while treatment is continued; this is called treatment beyond progression (TBP), defined as the treatment past RECIST 1.1-defined PD. TBP is continued as long as the patient is clinically stable, without organ dysfunction, tolerates the therapy, and provides renewed consent.Citation6
Although iRECIST addresses new important issues in the field of immune-oncology (PsP and TBP), there has been sparse data for the impact of PsP and TBP on outcomes of ICI therapy.Citation7–Citation11 Furthermore, although ICIs are currently used in routine clinical practice after approval from regulatory agencies, the majority of prior studies have used immune-related Response Criteria (irRC) or irRECIST for evaluating the clinical impact of PsP and/or TBP only in the research setting. From this perspective, we aimed to evaluate the impact of PsP and TBP on outcome in NSCLC patients treated with ICI in the real-world setting.
Material and methods
This retrospective study was approved by the Institutional Review Board of our institution, and written informed consent was waived. The study was conducted in accordance with the Declaration of Helsinki with good clinical practices, as defined by the International Conference on Harmonization.
Patients
This study used data from a prospectively constructed immuno-oncology registry of patients with lung cancer at Asan Medical Center. Eligible patients were those treated with ICI for NSCLC, including adenocarcinoma, squamous cell carcinoma, and adenosquamous cell carcinoma. The registry includes patients treated with ICI as monotherapy in both the clinical practice setting and the clinical trial setting.
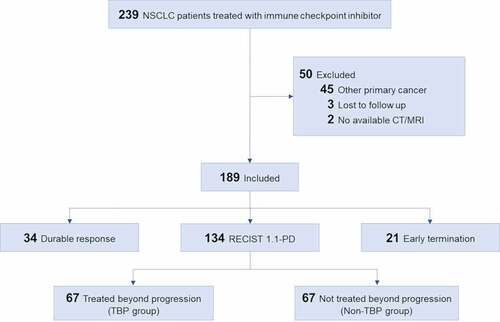
From the registry, we included 239 consecutive patients that met the inclusion criteria, as follows: (a) patients who were treated with ICI for NSCLC at Asan Medical Center between March 2016 and July 2018 and (b) patients >18 y of age. Among the 239 patients, 50 patients were eliminated according to the following exclusion criteria: (a) patients with synchronous double-primary cancers or patients with prior history of other primary malignancy (n = 45); (b) patients that were lost to follow-up (n = 3); and (c) patients with no available follow-up computed tomography (CT) or magnetic resonance imaging (MRI) data for evaluation of RECIST 1.1/iRECIST (n = 2). Ultimately, a total of 189 patients were included (). The characteristics of the patients including Eastern Cooperative Oncology Group (ECOG) performance status and treatment regimen were evaluated.
Image acquisition
The primary imaging modality was contrast-enhanced CT of the chest and abdomen/pelvis. If the patient showed renal dysfunction or had a history of adverse reactions on contrast agents, then the imaging modality was replaced with non-enhanced CT at the discretion of the investigator. Baseline CT scans were generally performed within 1 month before the start of treatment with ICI. Restaging CT scans were performed every 8–12 weeks. Unscheduled scans and confirmatory scans were performed according to the investigators’ judgment and iRECIST. If necessary, other potential sites of disease were scanned using site-specific imaging modalities: e.g., brain MRI, bone scans, or fluorodeoxyglucose-positron emission tomography.
Assessment using RECIST 1.1 and iRECIST
Tumor response was assessed by an imaging team at our institutional imaging core lab (Asan Image Metrics, www.aim-aicro.com) using both RECIST 1.1Citation4 and iRECIST.Citation5 The team was composed of an experienced radiologist (K.W.K., with 11 y of experience in cancer imaging), a qualified image analyst (S.E.W., with 2 y of experience in assessment of treatment response using RECIST 1.1 and iRECIST), and an imaging data manager (S.I.B., with 4 y of experience in clinical data management). The radiologist reviewed the images, and the image analyst completed the electronic case report forms for RECIST 1.1 and iRECIST. Then, an imaging data manager verified the source data of the completed electronic case report forms. If there was any potential error, the data manager informed it all members of the imaging response team so that they could re-review the case and correct the report if necessary. For the whole image response assessment process, our clinical trial image management system (AiCRO) was used.
The overall tumor response was determined according to both RECIST 1.1 (CR, PR, SD, or PD) and iRECIST (iCR, iPR, iSD, iUPD, or iCPD) at each time point. If there was any difficulty in response assessment, a value of “not evaluable” was assigned at that time point. The time to progression or death (i.e., from the initiation of ICI treatment to the date of progression or death from any cause) was also assessed based on RECIST 1.1 and iRECIST in order to calculate progression-free survival (PFS) according to each tumor response assessment criteria.
Pseudoprogression
Cases of PsP are those that were defined as PD on RECIST 1.1 but were reset as iSD, iPR, or iCR on subsequent imaging according to iRECIST. The incidence of PsP was evaluated, and the characteristics of patients with PsP were also analyzed. The impact of PsP on clinical outcome was evaluated by comparing PFS between patients with PsP and those without PsP. PFS was also compared between assessments according to RECIST 1.1 and iRECIST.
Response based on RECIST 1.1
According to the response based on RECIST 1.1, patients were divided into three groups, as follows: (a) durable response group, which included patients that showed SD, PR, or CR during the treatment period; (b) RECIST 1.1-PD group, which included patients that showed progression based on RECIST 1.1; and (c) the early termination group, which included patients that stopped ICI treatment before the first restaging CT scan due to early death or toxicity. Outcomes in these three response groups were evaluated using the overall survival (OS), which was defined as the time from initiation of ICI treatment to death from any cause.
Treatment beyond PD
The RECIST 1.1-PD group was further divided into two groups according to the presence of TBP. Outcomes in the TBP group and the non-TBP group were evaluated using the OS. The patient characteristics including demographic characteristics, ECOG performance status at baseline and at the time of RECIST 1.1-PD, and the change in tumor burden between baseline and the time of RECIST 1.1-PD were evaluated. The change in tumor burden was assessed in a semi-quantitative manner including all target, non-target, and new lesions and graded from 1 to 4, as follows: 1, 20–50% increase; 2, 50–100% increase; 3, 100–200% increase; and 4, >200% increase.
Statistical analysis
Comparisons of the mean values of continuous variables between groups were performed using Student’s t-test. Comparisons of the distribution of categorical variables between groups were performed using Chi-square test. Time-dependent survival probabilities were determined by the non-parametric Kaplan–Meier method, and log-rank test was used to compare the survival function across subgroups.Citation12,Citation13
Univariate and multivariate Cox proportional hazard models were used to estimate hazard ratios (HRs) for TBP, adjusting for other covariates such as patients’ age and sex, ECOG performance status at baseline, and the increase in tumor burden between baseline and the time of first RECIST 1.1-PD. In the multivariate analysis, a stepwise selection process was used. The statistical analysis was performed using R, version 4.3.1, with the package ‘survival’.Citation14,Citation15
Results
Patient characteristics at baseline
The baseline characteristics of the patients are presented in , which shows the entire patient population as well as patients grouped according to response. The mean age of all patients was 64.6 y (range, 22–87; 140 men and 49 women). Regarding the ECOG performance status, 143 patients (75.6%) showed grade 0 or 1, and 46 patients (24.4%) showed grade 2, 3, or 4. The median follow-up duration was 6.7 months (range, 0.3–32.5 months), and 108 patients (57.1%) were alive at the time of analysis.
Table 1. Baseline characteristics of patients.
Pseudoprogression
The incidence of PsP was 3.7% (7/189). In all seven patients, there was a discrepancy in the determination of PD between RECIST 1.1 and iRECIST, because each of the patients showed an initial PD on RECIST 1.1 that was then reset as SD (n = 3) or PR (n = 4) upon subsequent imaging based on iRECIST. Except one patient who experienced PsP at 29.3 months, the time from baseline to PsP of six pseudoprogressors was approximately 3 months (average, 3.2 months; range, 1.9–5.0 months). The time from PsP to reset of all seven pseudoprogressors was approximately 3 months (average, 2.4 months; range, 1.2–5.8 months). The detailed patterns of PsP for each patient are summarized in and . Representative cases are presented in the Supplementary Figures (Fig. S1 – Fig. S4).
Table 2. Patterns and outcomes of patients with pseudoprogression.
Figure 2. Spider plot of tumor burden changes during ICI treatment in seven patients with PsP. Asterisks indicate the increase of non-target lesions (PsP2, PsP4, PsP6) or the occurrence of new lesion (PsP1) at the time of 1st PD per RECIST 1.1. Except one patient who experienced PsP at 29.3 months (PsP1), the time from baseline to PsP was approximately 3 months (average, 3.2 months; range, 1.9–5.0 months). The time from PsP to reset of all seven pseudoprogressors was approximately 3 months (average, 2.4 months; range, 1.2–5.8 months).
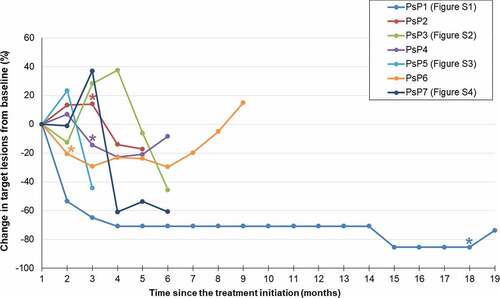
Of the seven patients with PsP, after reset, two patients showed iUPD by iRECIST (2nd PD by RECIST 1.1). One patient showed iUPD (2nd PD by RECIST 1.1) at 9.6 months and died at 17.0 months after starting treatment, and another patient showed iUPD (2nd PD by RECIST 1.1) at 6.8 months after starting treatment but was alive as of the last follow-up visit at 7.2 months. The other five patients did not show progression after reset at the last follow-up visit. The PFS based on iRECIST was significantly longer in the seven patients who experienced PsP (median, not reached) than in the 182 patients without PsP (median, 3.8 months [95% CI, 3.9–4.6 months], P = .02), as illustrated in .
Figure 3. Impact of PsP on progression-free survival in patients treated with immune checkpoint inhibitors. (a) Kaplan–Meier curves of PFS in patients with PsP (n = 7) and without PsP (n = 182). PFS was significantly longer in the seven patients with PsP (median, not reached) than in the 182 patients without PsP (median, 3.8 months; 95% CI, 3.9–4.6 months, P = .02) (b) Kaplan–Meier curves of PFS according to RECIST 1.1 and iRECIST. There was no significant difference between PFS according to RECIST 1.1 (median, 3.8 months; 95% CI, 3.1–4.8 months) and that according to iRECIST (median, 4.1 months; 95% CI, 3.1–5.4 months) (P = .58).
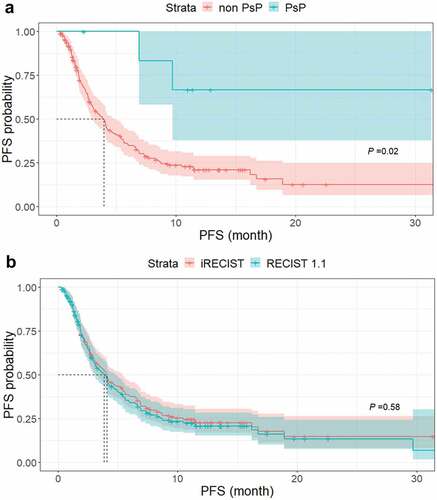
At the end of follow-up (median, 6.6 months), 134 patients (70.9%) had PD by RECIST 1.1, while 129 patients (68.3%) had iUPD by iRECIST. Although the median PFS was 3.8 months (95% CI, 3.1–4.8 months) according to RECIST 1.1 and 4.1 months (95% CI, 3.1–5.4 months) according to iRECIST, log-rank test indicated that this difference was not statistically significant (P = .58). Kaplan–Meier curves of PFS according to RECIST 1.1 and iRECIST are illustrated in .
Outcomes according to response
Of the 189 patients, there were 34 (18.0%) in the durable response group, 134 (70.9%) in the RECIST 1.1-PD group, and 21 (11.1%) in the early termination group. There were 81 (42.8%) deaths during follow-up. Kaplan–Meier curves of OS in these groups are shown in , and log-rank testing revealed significant differences among the three groups (P < .001).
Figure 4. Kaplan–Meier curves of OS in three groups of patients according to response to ICI. There were significant differences in OS among the durable response group (median, not reached), RECIST 1.1-PD group (median, 11.1 months; 95% CI, 9.3 months–not reached) and early termination group (median, 1.3 months; 95% CI, 0.8–1.7 months) (P < .001).
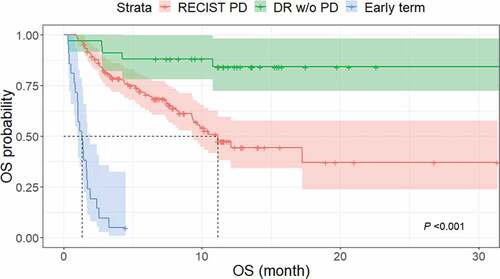
The durable response group had a favorable outcome, in that only 5 of 34 (14.7%) patients died during the follow-up period (median, 4.2 months). Thus, the median OS was not reached. In the RECIST 1.1-PD group, 56 of 134 (41.8%) patients died, resulting in a median OS of 11.1 months (95% confidence interval [CI], 9.3 months–not reached) and a 1-y OS rate of 44.3% (95% CI, 32.4–55.5). In the early termination group, 20 of 21 (95.2%) patients died before the first restaging CT scan, and one patient developed pneumonia lasting from 3 days to 3 weeks after the initiation of ICI treatment and ultimately discontinued the therapy. The median OS in this group was 1.3 months (95% CI, 0.8–1.7 months). The comparison of OS between durable response group and patients with PsP is shown as Figure S5.
Regarding the relationship between patients’ outcomes and characteristics of drug and tumor, there was no significant difference in OS and PFS according to the type of ICI, tumor histology, and mutation, as detailed in Table S1.
Outcomes according to treatment beyond progression
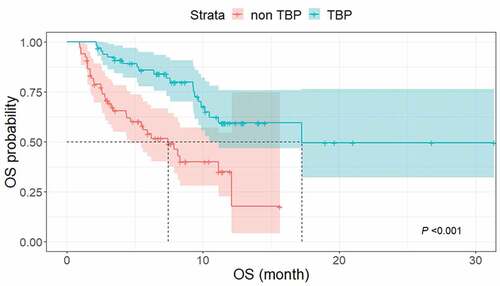
Of the 134 patients in the RECIST 1.1-PD group, 67 (50.0%) underwent TBP, whereas the other 67 (50.0%) stopped treatment at the time of PD by RECIST 1.1. The median OS was significantly longer in the TBP group (17.2 months; 95% CI, 10.2 months–not reached) than in the non-TBP group (7.4 months; 95% CI, 4.4–12.1 months) by log-rank testing (P < .001) ().
Figure 5. Kaplan–Meier curves of OS in patients with TBP and without TBP. The median OS was significantly longer in the TBP group (17.2 months; 95% CI, 10.2 months–not reached) than in the non-TBP group (7.4 months; 95% CI, 4.4–12.1 months) (P < .001).
The difference in demographics and the change in tumor burden between the TBP group and the non-TBP group are presented in . Notably, patients with a marked tumor burden increase (>200% from baseline) tended to stop treatment on the first PD by RECIST 1.1, while patients with a mild tumor burden increase (20–50% from baseline) were more likely to continue treatment beyond PD. The distribution of ECOG performance status was similar between the TBP group and the non-TBP group at baseline, but significantly different at the time of RECIST 1.1-PD. Most patients who had a higher ECOG performance status at the time of RECIST 1.1-PD than at baseline (i.e., patients who showed clinical deterioration) stopped ICI treatment.
Table 3. Characteristics between TBP and non-TBP patients.
Cox proportional hazard analysis revealed the impact of TBP on OS while adjusting for covariates of age and sex, ECOG performance status, and tumor burden increase at the time of RECIST 1.1-PD, as presented in . Univariate analyses of OS demonstrated that TBP was a significant protective factor for mortality (P < .001), while an ECOG performance status of 4 and a tumor burden increase of greater than 100% at the time of RECIST 1.1-PD were statistically significant risk factors for mortality (P < .001). TBP (HR, 0.4, 95% CI, 0.2–0.7) remained a statistically significant, independent protective factor upon multivariate analysis, while a tumor burden increase of greater than 50% at RECIST 1.1-PD (HRs: 2.6, 95% CI, 1.1–6.2; 2.5, 95% CI, 1.8–5.4; and 3.3, 95% CI, 1.5–7.3; for grades 2, 3, and 4, respectively) remained a statistically significant, independent risk factor for mortality. ECOG performance status at baseline, age, and sex did not remain as independent risk factors for mortality.
Table 4. Univariate and multivariate Cox proportional hazard analysis of OS in patients with RECIST 1.1-PD.
When we compared the survival outcomes between patients with PsP and non-pseudoprogressors with TBP, the median OS did not significantly differ (not reached vs. 17.2 months in patients with PsP and non-pseudoprogressors with TBP, respectively,P = .2) while the median PFS based on iRECIST was significantly different between the two groups (not reached vs. 3.4 months [95% CI, 2.6–4.2 months]) for patients with PsP and non-pseudoprogressor with TBP (P ≤ 0.001) ().
Figure 6. Kaplan–Meier curves of OS and PFS based on iRECIST to compare outcomes between patients with PsP (n = 7) and non-pseudoprogressors treated with TBP (n = 60). (a) The median OS did not significantly differ (not reached vs. 17.2 months, respectively, P = .2). (b) The median PFS based on iRECIST was significantly different between the two groups (not reached vs. 3.4 months [95% CI, 2.6–4.2 months]) for patients with PsP and non-pseudoprogressor with TBP (P < .001).
![Figure 6. Kaplan–Meier curves of OS and PFS based on iRECIST to compare outcomes between patients with PsP (n = 7) and non-pseudoprogressors treated with TBP (n = 60). (a) The median OS did not significantly differ (not reached vs. 17.2 months, respectively, P = .2). (b) The median PFS based on iRECIST was significantly different between the two groups (not reached vs. 3.4 months [95% CI, 2.6–4.2 months]) for patients with PsP and non-pseudoprogressor with TBP (P < .001).](/cms/asset/9e782db8-c2fc-4b01-99c5-b484d2da9961/koni_a_1776058_f0006_oc.jpg)
Discussion
In our study, the incidence of PsP was 3.7% (7/189) based on iRECIST. This is similar to the incidence of PsP in NSCLC patients from prior studies, ranging from 0.6% to 6.9%.Citation10,Citation16-Citation21 However, in previous studies, the definition and criteria to evaluate PsP have not been standardized. Unlike prior immune response criteria such as irRC and irRECIST, iRECIST has adopted the new concept of the “reset.” In iRECIST, if the first progression on RECIST 1.1 is not confirmed on subsequent imaging, but instead tumor shrinkage occurs, then the response is reset to the corresponding category of iCR, iPR, or iSD. This strategy reflects the PsP phenomenon well, and thus we defined PsP according to iRECIST as PD on RECIST 1.1 followed by reclassification as iSD, iPR, or iCR on subsequent imaging.
Interestingly, patients with PsP showed significantly better outcomes than patients without PsP (median PFS, not reached versus 3.8 months). This observation is consistent with prior studiesCitation7–Citation11 that also reported better outcomes in patients showing PsP. The favorable outcome of patients with PsP may be related to the transient enlargement of the lesions not by increase in number of tumor cells but by infiltration of inflammatory cells, which is thought to be a mechanism of PsP. One case report demonstrated that cytotoxic T lymphocytes positive for CD3 and CD8 were infiltrated into the metastatic tumor in a patient who experienced PsP.Citation22 As T-lymphocyte infiltration in tumor and stroma has been reported as a promising prognostic factor in NSCLC patients,Citation23 the better outcome in PsP patients may in part be associated with the favorable effect of T-cell infiltration.
Our results suggest that a proportion of patients with NSCLC who receive ICI treatment beyond the first PD on RECIST 1.1 may derive survival benefit, as the median OS was significantly longer in patients who received TBP (17.2 months) than those who did not receive TBP (7.4 months). We tried to minimize the potential confounding effect of patients’ clinical characteristics on evaluating the clinical impact of TBP by adjusting the multiple covariates. The beneficial impact of TBP on OS (HR: 0.4) remained after adjusting for covariates including ECOG performance status at baseline, age, sex, and the increase in tumor burden between the baseline measurement and the time of the first PD by RECIST 1.1.
In our study, TBP was performed in 50.0% of patients who showed PD by RECIST 1.1, which was similar to other studies (21–80%).Citation19–Citation21,Citation24–Citation27 At our institution, it is allowed to continue treatment with ICI for patients after initial PD by RECIST 1.1 in both the clinical practice setting and the clinical trial setting, as long as patients are clinically stable, tolerating treatment, and expected to derive clinical benefit from treatment continuation. The decision to treat patients with ICI beyond progression has been based in part on patients’ clinical status, in that patients with poor clinical characteristics were unlikely to receive continued treatment. In our analysis, patients with a poor ECOG performance status at the time of the first PD on RECIST 1.1 and a markedly increased tumor burden (>200% from baseline) were less likely to be treated beyond progression than those without these characteristics. Our results are similar to those reported by Long et al.,Citation28 in that patients judged to be eligible for TBP on the basis of investigator-assessed clinical benefit without substantial adverse effects obtained clinical benefit. Further studies are necessary to identify appropriate candidates for TBP, in order to achieve better outcomes and avoid futile treatment.
The difference in the survival probability of PFS between iRECIST and RECIST 1.1 was not statistically significant. This finding is different from the results of the study by Nishino et al.,Citation29 in which time to progression was significantly longer with irRECIST than with RECIST 1.1. This is likely due to a difference in how the event date used for the calculation of PFS was defined: in iRECIST, if an iUPD is reassessed as an iUPD or an iCPD at a subsequent study, the date of the first iUPD is used as the event date,Citation5 whereas in Nishino’s study, the date on which the confirmation of PD occurred was defined as the time of PD according to the irRC and irRECIST scheme.Citation29,Citation30 The insignificant difference of PFS between iRECIST and RECIST 1.1 in our study might also be due to the low incidence of PsP (3.7%); if the incidence of PsP was higher, the difference in PFS between iRECIST and RECiST 1.1 may have become significant.
Early identification of pseudoprogressors at the time of tumor burden increase would be beneficial. However, distinguishing between PsP and true PD during cancer treatment has been a great challenge. Unfortunately, conventional CT and MRI have been known to be insensitive to the distinction of those two. Whole body PET/CT has also been suggested as a potential discriminator; however, the results have been conflicting. Recently, Immuno-PET is gaining attention as a new metabolic imaging strategy that combines monoclonal antibodies specific for PD-L1 with radiomaterials such as Zirconium-89 (89Zr).Citation31,Citation32 There are several ongoing clinical trials evaluating 89Zr-labeled immune-PET imaging for cancer immunotherapy. The results of these studies are highly anticipated to develop a reliable and noninvasive imaging technique capable of differentiating PsP from true progression with appropriate clinical application.
Currently, iRECIST provides a rationale to perform TBP with subsequent imaging follow-ups with a 4–8 week interval.Citation5 However, the benefits and risks of TBP still remain as a field of uncertainty. Therefore, the decision of TBP should be based on the patient selection criteria established in clinical protocols as well as the patient’s informed consent after providing potential benefits on survival and risks of futile treatment and delaying other treatment options.Citation28
Our study has some limitations. First, it was carried out only in NSCLC patients treated at a single institution, and additional data are needed to confirm the proportion of patients exhibiting PsP and TBP responses so that these results can be extrapolated to the wider NSCLC population and to other tumor types. Second, there were only seven patients (3.7%) experiencing pseudoprogression which might be a small number to base the clinical impact of pseudoprogression. Further, large-scale clinical studies might be necessary. Third, our study results might not be a strong evidence to justify TBP in patients treated with ICIs, because our results were based on analysis of uncontrolled real-world data. Further studies such as randomized trials or comparative effectiveness researches might be required. In addition, due to the retrospective nature of the study, various CT acquisition techniques were used according to different study protocols or clinical practices.
In conclusion, a subset of NSCLC patients under ICI treatment may experience PsP based on iRECIST scheme, which is not captured by the RECIST 1.1. PsP may have a clinical impact by improving PFS, and TBP according to iRECIST may provide a survival benefit. Although our results favor TBP, its benefits on survival and risks of inefficacious treatment delaying other treatment options should be explored through further researches. In addition, more evidence-based standardized criteria to select appropriate patients for TBP should be established based on accumulated evidence and international consensus.
Author contribution
Conception and design of this article: Sang Eun Won, Hyo Jung Park, Sangil Byun, Junhee Pyo, Jwa Hoon Kim, Chang-Min Choi, Jae Cheol Lee, Dae Ho Lee, Sang-We Kim, Shinkyo Yoon, and Kyung Won Kim. Providing study material or patients: Sang Eun Won, Hyo Jung Park, Sangil Byun, Shinkyo Yoon, Kyung Won Kim. Collecting and/or assembling data: Sang Eun Won, Hyo Jung Park, Shinkyo Yoon, Kyung Won Kim. All authors participated in data analysis and interpretation, writing the article, and approved the final version.
Precis
Among patients with non-small cell lung cancer treated with immune checkpoint inhibitors, 3.7% (7/189) experienced pseudoprogression based on iRECIST scheme.
Pseudoprogression and treatment beyond RECIST 1.1-defined disease progression may be associated with survival benefit in patients with non-small cell lung cancer treated with immune checkpoint inhibitors.
Supplemental Material
Download ()Acknowledgments
None
Disclosure statement
The authors have declared that no competing interests, including financial interests, exist.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–10. doi:10.1038/nrc3239.
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi:10.1056/NEJMoa1200690.
- National Comprehensive Cancer Network. Non-small cell lung cancer version 7.2019 [ accessed 2019 Jan 01]. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026.
- Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152. doi:10.1016/S1470-2045(17)30074-8.
- Oxnard GR, Morris MJ, Hodi FS, Baker LH, Kris MG, Venook AP, Schwartz LH. When progressive disease does not mean treatment failure: reconsidering the criteria for progression. J Natl Cancer Inst. 2012;104(20):1534–1541. doi:10.1093/jnci/djs353.
- Fujimoto D, Yoshioka H, Kataoka Y, Morimoto T, Hata T, Kim YH, Tomii K, Ishida T, Hirabayashi M, Hara S, et al. Pseudoprogression in previously treated patients with non-small cell lung cancer who received nivolumab monotherapy. J Thorac Oncol. 2019;14(3):468–474. doi:10.1016/j.jtho.2018.10.167.
- Tazdait M, Mezquita L, Lahmar J, Ferrara R, Bidault F, Ammari S, Balleyguier C, Planchard D, Gazzah A, Soria JC, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer. 2018;88:38–47. doi:10.1016/j.ejca.2017.10.017.
- Nishino M, Giobbie-Hurder A, Manos MP, Bailey N, Buchbinder EI, Ott PA, Ramaiya NH, Hodi FS. Immune-related tumor response dynamics in melanoma patients treated with pembrolizumab: identifying markers for clinical outcome and treatment decisions. Clin Cancer Res. 2017;23(16):4671–4679. doi:10.1158/1078-0432.CCR-17-0114.
- Katz SI, Hammer M, Bagley SJ, Aggarwal C, Bauml JM, Thompson JC, Nachiappan AC, Simone CB, Langer CJ. Radiologic pseudoprogression during Anti-PD-1 therapy for advanced non-small cell lung cancer. J Thorac Oncol. 2018;13(7):978–986. doi:10.1016/j.jtho.2018.04.010.
- Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman DM, et al. Overall survival and long-term safety of nivolumab (Anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004–2012. doi:10.1200/JCO.2014.58.3708.
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. doi:10.1080/01621459.1958.10501452.
- Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35(1):1–39. doi:10.1038/bjc.1977.1.
- Therneau TM, Grambsch PM. Modeling survival data: extending the cox model. New York (NY): Springer Science & Business Media; 2013.
- Therneau TM, Li H. Computing the cox model for case cohort designs. Lifetime Data Anal. 1999;5(2):99–112. doi:10.1023/A:1009691327335.
- Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, Mazieres J, Zalcman G, Brosseau S, Le Moulec S, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4(11):1543–1552. doi:10.1001/jamaoncol.2018.3676.
- Matsuo N, Azuma K, Hattori S, Ohtake J, Kawahara A, Ishii H, Tokito T, Yamada K, Shibata Y, Shimokawaji T, et al. Association between soluble immune mediators and tumor responses in patients with nonsmall cell lung cancer treated with anti-PD-1 inhibitor. Int J Cancer. 2019;144(5):1170–1179. doi:10.1002/ijc.31923.
- Nishino M, Dahlberg SE, Adeni AE, Lydon CA, Hatabu H, Jänne PA, Hodi FS, Awad MM. Tumor response dynamics of advanced non-small cell lung cancer patients treated with PD-1 inhibitors: imaging markers for treatment outcome. Clin Cancer Res. 2017;23(19):5737–5744. doi:10.1158/1078-0432.CCR-17-1434.
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi:10.1056/NEJMoa1507643.
- Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi:10.1056/NEJMoa1504627.
- Kazandjian D, Keegan P, Suzman DL, Pazdur R, Blumenthal GM. Characterization of outcomes in patients with metastatic non-small cell lung cancer treated with programmed cell death protein 1 inhibitors past RECIST version 1.1-defined disease progression in clinical trials. Semin Oncol. 2017;44(1):3–7. doi:10.1053/j.seminoncol.2017.01.001.
- Kim HK, Baek SW, Jeong Y, Yang Y, Kwon J, Han HS, An J-Y, Woo CG, Lee O-J, Lee TG, et al. Pseudoprogression presenting as intestinal perforation in non-small cell lung cancer treated with anti-PD-1: A case report. Mol Clin Oncol. 2019;11(2):132–134. doi:10.3892/mco.2019.1871.
- Barnes TA, Amir E. HYPE or HOPE: the prognostic value of infiltrating immune cells in cancer. Br J Cancer. 2017;117(4):451–460. doi:10.1038/bjc.2017.220.
- Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, Patnaik A, Ribas A, Robert C, Gangadhar TC, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34(13):1510–1517. doi:10.1200/JCO.2015.64.0391.
- Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi:10.1158/1078-0432.CCR-09-1624.
- George S, Motzer RJ, Hammers HJ, Redman BG, Kuzel TM, Tykodi SS, Plimack ER, Jiang J, Waxman IM, Rini BI, et al. Safety and efficacy of nivolumab in patients with metastatic renal cell carcinoma treated beyond progression: a subgroup analysis of a randomized clinical trial. JAMA Oncol. 2016;2(9):1179–1186. doi:10.1001/jamaoncol.2016.0775.
- Escudier B, Motzer RJ, Sharma P, Wagstaff J, Plimack ER, Hammers HJ, Donskov F, Gurney H, Sosman JA, Zalewski PG, et al. Treatment beyond progression in patients with advanced renal cell carcinoma treated with nivolumab in checkMate 025. Eur Urol. 2017;72(3):368–376. doi:10.1016/j.eururo.2017.03.037.
- Long GV, Weber JS, Larkin J, Atkinson V, Grob -J-J, Schadendorf D, Dummer R, Robert C, Márquez-Rodas I, McNeil C, et al. Nivolumab for patients with advanced melanoma treated beyond progression: analysis of 2 phase 3 clinical trials. JAMA Oncol. 2017;3(11):1511–1519. doi:10.1001/jamaoncol.2017.1588.
- Nishino M, Ramaiya NH, Chambers ES, Adeni AE, Hatabu H, Jänne PA, Hodi FS, Awad MM. Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. J Immunother Cancer. 2016;4(1):84. doi:10.1186/s40425-016-0193-2.
- Nishino M, Gargano M, Suda M, Ramaiya NH, Hodi FS. Optimizing immune-related tumor response assessment: does reducing the number of lesions impact response assessment in melanoma patients treated with ipilimumab? J Immunother Cancer. 2014;2(1):17. doi:10.1186/2051-1426-2-17.
- Marciscano AE, Thorek DLJ. Role of noninvasive molecular imaging in determining response. Adv Radiat Oncol. 2018;3(4):534–547. doi:10.1016/j.adro.2018.07.006.
- Mayer AT, Natarajan A, Gordon SR, Maute RL, McCracken MN, Ring AM, Weissman IL, Gambhir SS. Practical immuno-PET radiotracer design considerations for human immune checkpoint imaging. J Nucl Med. 2017;58(4):538–546. doi:10.2967/jnumed.116.177659.