 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The prediction of the response to Bacillus Calmette-Guerin (BCG) can help identify non-muscle-invasive bladder cancer (NMIBC) patients that may be better served with alternative therapy. Several cytokine profiles present promising results, but they are difficult to use in clinical practice. In this prospective, longitudinal study, we tried to identify reliable serum cytokines/chemokines to predict the response to BCG using samples collected before and during BCG induction therapy. We used the Bio-plex multiplex assays to identify potential BCG failure-related serum cytokines/chemokines in the discovery set (n = 13). After screening, we identified CCL27 as the top candidate biomarker for predicting the response to BCG (P = .003). In the validation set, we found that the AUC of the baseline CCL27 was 0.730 (95% CI 0.515–0.945, P = .040) along with 67% sensitivity, 78% specificity. The changes from baseline to last timepoint can also distinguish BCG responders from non-responders (AUC: 0.726, 95% CI 0.474–0.979, P = .044). Moreover, the combination score of serum CCL27 (CSCCL27), based on the baseline and changes of CCL27, could further improve the predictive accuracy with an AUC of 0.897 (95% CI 0.790–1.000, P < .001). The correlations between CCL27 and local/systemic immunologic parameters were further analyzed. The level of serum CCL27 was strongly correlated with regulatory T cells (Tregs) in the tumor microenvironment (P = .002), indicating that CCL27 may promote the recruitment of Tregs into the tumor microenvironment. Our results show that serum CCL27 may represent a practical and reliable marker for the prediction of the response to BCG in NMIBC.
Introduction
Bladder cancer are the tenth most predominant cancers worldwide, with an estimated 549,000 new cases and 200,000 deaths in 2018.Citation1 At the time of the diagnosis, approximately 75% of UCB present with a disease confined to the mucosa or submucosa, termed non-muscle invasive bladder cancer (NMIBC).Citation2 Intravesical administration of Bacillus Calmette-Guerin (BCG) is the gold-standard treatment for high risk NMIBC and is also recommended for intermediate-risk NMIBC following a transurethral resection of bladder tumors (TUR-BT) .Citation3 Despite its success, still 32.6%-42.1% of the cases are faced with disease recurrence following BCG immunotherapy .Citation4 The lack of simple, reliable criteria for the prediction of BCG response makes it difficult to identify patients who may be better served with new or alternative therapies.
It has been shown that cytokines and chemokines are associated with a potent pro-BCG immune response, including the stimulation of immune cell recruitment, differentiation of the immunological microenvironment, and direct tumor cytotoxicity .Citation5 Thus, multiple studies have investigated the possibility of predicting the BCG response using cytokines and chemokines. The majority of previous studies have focused on the local responses to BCG and they have developed urinary cytokine/chemokine profiles that display promising results by using high throughput techniques .Citation6,Citation7 However, these profiles are difficult to use in routine clinical practice. Moreover, the levels of urinary cytokines and chemokines could be transient and influenced by several factors such as sampling time or urinary infection .Citation4
It has been established that BCG immunotherapy induces both a local and systemic immune response.Citation5,Citation8 In contrast to the local responses in the urine, the predictive role of the systemic immune response following BCG therapy is still not well investigated. Therefore, we initiated a prospective study to identify a serum cytokine or chemokine that could be used to predict the treatment response to BCG immunotherapy using an enzyme-linked immunosorbent assay (ELISA) analysis.
To perform this study, we profiled 48 serum cytokines and chemokines in 13 patients treated with intravesical BCG, to screen differentially altered cytokines and chemokines between BCG responders and non-responders. According to the screening results, we further analyzed 148 serum samples from 37 patients who were with BCG immunotherapy at 4 different timepoints prior to or during BCG induction therapy. This study identified serum CCL27 as a potential cytokine for the prediction and monitoring of the response to BCG. Further investigations have demonstrated the potential involvement of CCL27 in the recruitment of regulatory T cells (Tregs). This suggests that CCL27 plays a detrimental role in BCG immunotherapy.
Materials and methods
Study population
In this prospective, longitudinal study, all serum samples were collected from patients with high-risk NMIBC enrolled in a multicenter, phase 4 trial (ChiCTR-IIR-16008357). The design of our study was shown in Supplementary Figure 1. Our study was comprised of two successive cohorts from the Sun Yat-Sen Memorial Hospital from January 2015 to December 2017. The initial 13 patients were enrolled in the discovery set, and based on the initial results, 37 patients were recruited in the validation set. Patients with T1 tumor would receive a restaging TUR-BT according to our institutional protocol and patients’ intention. All of the patients in our study started BCG induction therapy consisting of six weekly intravesical instillations. According to the protocol of the multicenter clinical trial, patients enrolled were randomized to two BCG maintenance schedules: (1) 3 weekly instillations at 3, 6 and 12 months after the initial induction course (9-instillation schedule); (2) 10 monthly instillations after the induction course (10-instillation schedule). Clinicopathological data including gender, age, body mass index (BMI), previous illness, tumor characteristics and treatment-related variables were collected. Follow-up were performed per standard of care, consisting of urine cytology and cystoscopy per 3 months, and upper tract CT urography per year. In case of positive cytology and/or visible tumors, bladder biopsy and/or TUR-BT was performed to confirm the presence of disease recurrence or progression.
The primary endpoint of our study was the response status to BCG immunotherapy (responder/non-responder). Specifically, BCG non-responders were subdivided according to European Urology Association Guideline :Citation3 BCG unresponsive (high-grade T1 disease recurrence within 3 months or high-grade recurrence with or without CIS within 6 months after last exposure to BCG), BCG relapse (high grade recurrence after completion of BCG maintenance, despite an initial response to BCG), BCG progression (T2-4 disease recurrence after TUR-BT). Patients who had complete response without disease recurrence or progression were classified as “BCG responders”. The secondary endpoint was recurrence-free survival, defined as the time interval between the beginning of BCG therapy to the first disease recurrence based on follow-up cystoscopy and pathologic interpretation.
The protocol was approved by the ethic committee of the Sun Yat-sen Memorial Hospital and conducted in accordance with the Declaration of Helsinki. The patients analyzed in this study had consented for their serum samples to be used in our study.
Processing of the serum samples
Serum samples were collected from patients at four different time points: baseline (immediately prior to BCG therapy), as well as at early (second instillation), medium (fourth instillation), and last phase of BCG induction therapy. All serum samples were collected immediately prior to each instillation and obtained under standard conditions. All samples were stored at −80°C and assayed in batch form for each cohort.
Cytokines and chemokines analysis
For the discovery set, the levels of serum cytokines and chemokines were quantified using a Bio-Plex multiplex Reader system (Bio-rad, Milan, Italy) equipped with a Bio-Plex Manager software (BioRad) according to the manufacturer’s instructions. Measurements were performed using two panels (BioPlex Pro Human Cytokine 27-plex panel, Bio-rad and BioPlex Pro Human Cytokine Group II 21-plex panel, Bio-rad) consisting of 48 cytokines and chemokines. All samples were run in triplicate and analyzed according to the manufacturer’s instructions. Serum samples from different time points for the same patient were included on the same assay plate. All of the assays were performed blinded to both the clinicopathologic and prognostic data.
Based on the initial results, serum CCL27 was selected for evaluation in the validation set. For further validation, the human CTACK/CCL27 DuoSet ELISA kit (R&D system, #DY376) was used to determine the concentration of CCL27. Each sample was run in triplicate, and changes from the baseline level to the subsequent individual timepoints were calculated for each cytokine.
Immunohistochemistry (IHC) staining and quantification
IHC staining was performed on formalin-fixed, paraffin-embedded (FFPE) 4-μm serial sections from pre-BCG treatment tumor tissues from patients in the validation set as previously described .Citation9,Citation10 Briefly, tissue sections were deparaffinized with Xylene and rehydrated in ethanol. Endogenous peroxidase inactivation was conducted in 3% H2O2. After antigen retrieval and blocking nonspecific binding, primary antibodies against human CD8 (C8/144B, Thermo Fisher Scientific), CD4 (UMAB64, Zhongshan Golden Bridge Biotechnology), Foxp3 (236A/E7, Abcam), GATA-3 (D13C9, CST), T-bet (D6N8B, Cell Signaling Technology), CD68 (PG-M1, Dako) and CD66b (G10F5, BD Biosciences) were incubated with tissue slides overnight at 4°C. Next, the slides were incubated with a corresponding secondary antibody for 30 minutes at room temperature and visualized by using a DAKO EnVision Detection System (Dako).
For the evaluation of IHC staining, up to five individual regions of interest (ROI) were selected according to the highest expression within the stromal cells for each marker (×400 magnification; 0.07 mm2 per field). The cell densities were counted using a computer-assisted cell counting method. Data are expressed as the mean ± SD number of cells per field. The median counts of each marker were used as the cutoff point to stratify the high and low groups. All specimens were examined by two independent pathologists in a blinded manner.
Statistical analysis
Descriptive data were presented as frequencies and percentages. Continuous parametric variables were presented in the form of means ± standard deviation (SD). Nonparametric variables were expressed as the median (interquartile range). Comparisons of categorical variables were conducted using a Pearson chi-square test or Fisher exact test. Mann-Whitney U tests or student T test were performed for continuous variables. A repeated measures design (General Linear Model) with four serum samples measurements as within subject factors and the BCG response as a between subject factor were used to evaluate the changes of cytokines and chemokines over time, as well as the differences in the cytokines and chemokines between patients stratified by the BCG response. The predictive values of serum CCL27 were evaluated by the receiver operator characteristic (ROC) and areas under curves (AUCs). The cutoff values for estimating sensitivity and specificity was decided using Youden’s Index. The Kaplan–Meier log-rank analysis and Cox proportional hazards model were used to estimate recurrence-free survival. To evaluate the combined effect of monitoring the candidate cytokine, logistic regression model was used to build a predictive classifier. A two-sided P < .05 was considered to indicate statistical significance. Statistical analyses were performed using R software (Version 3.6.1). The graphics were produced using R software (Version 3.6.1) and GraphPad Prism 5.0 (version 5.01).
Results
Identification of serum CCL27 in the discovery set
The discovery set consists of 12 males and 1 female, with an average age of 62 years. The set included nine BCG responders and four BCG non-responders. All the non-responders were classified as BCG unresponsive. Detailed characteristics and the response status for the 13 patients were listed in Supplementary Table 1. As a pilot study, we used a Bio-Plex multiplex cytokines assay system to analyze the chemokines and cytokines in the serum samples during BCG induction therapy. Among the 48 cytokines, six cytokines had several values below or above the assay detection limits, thus 42 cytokines were finally included (a).
Figure 1. Identification of serum CCL27 in the discovery set.
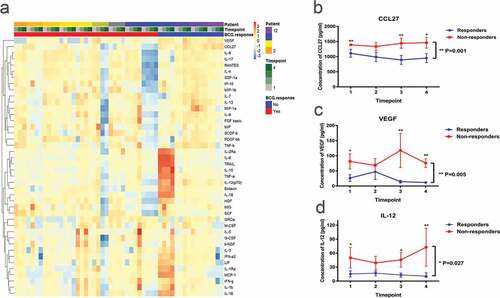
Predicting the BCG response before induction therapy would be a significant advance. Thus, to identify an actionable biomarker for the prediction of the BCG response, we first screened for the differential cytokines at baseline between BCG responders and BCG non-responders. Supplementary Table 2 illustrate the discriminability of serum cytokines at baseline for BCG response. This screening led to the identification of only three candidate cytokines or chemokines, interleukin (IL)-12(p70), vascular endothelial growth factor (VEGF) and CCL27. Of these cytokines, serum CCL27 was the top candidate biomarker (P = .003). Moreover, repeated measurement test at all of the four timepoints before and during therapy confirmed the top role of serum CCL27 (b-d, Supplementary Tables 2–5). Of note, the levels of serum CCL27 was nearly 100 folds higher than IL-12(p70) and 50 folds as to VEGF, which was much easier to be studied by means of ELISA analysis. We thus proceeded to evaluate the predictive role of CCL27 in validation set.
Baseline serum CCL27 predicts the BCG response in the validation set
In the validation set, a total of 37 patients (35 males and 2 females) with a median age 61 years (IQR 55.5–67.0) were enrolled. All of the patients were BCG naïve. Among five patients (13.5%) who had a prior history of bladder cancer, three patients had received intravesical epirubicin, one patient had received gemcitabine, and the other patient had received both epirubicin and mitomycin. After the induction therapy, 15 patients received 9-instillation maintenance schedule and 22 patients received 10-instillation maintenance schedule. After a median follow-up of 24 months, 9 patients had experienced disease recurrence and thus classified as non-responders. Notably, all of the non-responders developed recurrence within six months following BCG immunotherapy and were subdivided into BCG unresponsive. The other 27 recurrence-free patients were classified as “responders”. Of interest, no differences in age, gender, multifocality, histological grade, the peripheral neutrophil lymphocyte ratio, and maintenance schedule were identified between responders and non-responders (). However, recurrent tumors were positively associated with a poor response to BCG therapy (P = .046).
Table 1. Patients characteristics in validation cohort stratified by treatment response to BCG.
To validate the correlation between the levels of CCL27 and response status, we used a commercial human CCL27 ELISA kit for cytokine measurement. Prior to intravesical BCG, the level of serum CCL27 at baseline was significantly higher in non-responders compared to responders (Supplementary Figure 2, P = .036). The AUC of the baseline CCL27 was 0.730 (95% CI 0.515–0.945, P = .040) along with 67% sensitivity, 78% specificity (a). We further classified the patients into “high-baseline” and “low-baseline” subgroups according to the best cutoff value. Compared with the low-baseline group, more high-grade tumors were observed in the high-baseline group (91.7% vs 52.0%, P = .018); whereas no other clinicopathologic parameters displayed significant differences between the groups (). The rate of the six-month recurrence-free survival was lower among the 12 patients (50.0%) in high-baseline group compared to the 25 patients (87.8%) in the low-baseline group (P = .009, b). In a multivariate analysis that excluded age and recurrent tumor as confounding variables, the hazard ratio for recurrence among patients in high-baseline versus low-baseline group was 4.650 (95% CI 1.097–19.717, P = .037).
Table 2. Patients characteristics in validation set stratified by baseline level of serum CCL27.
Figure 2. Baseline levels and dynamic change of serum CCL27 may predict the treatment response to intravesical BCG immunotherapy in the validation set.
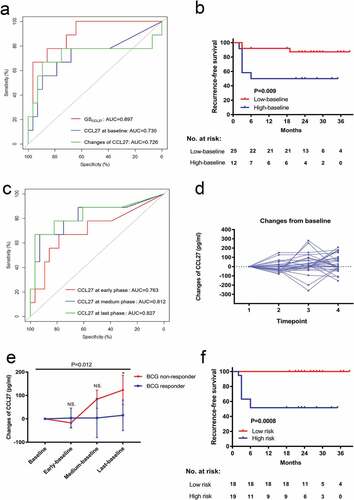
Dynamic monitoring of serum CCL27 increases the predictive accuracy in the validation set
After identifying the baseline level of CCL27 associated with a treatment response to BCG immunotherapy, we next explored whether monitoring serum CCL27 could increase predictive accuracy for the BCG response. The AUC of the serum CCL27 levels at early, medium and the last phase of induction therapy ranged from 0.706–0.827 (c). Regarding the changes in the level of CCL27, the changes from baseline to any single latter timepoint were calculated. Serum CCL27 changed heterogeneously between baseline and the latter timepoints in the validation set (d). Early changes (from baseline to second or fourth instillation) failed to correlate with the response to BCG (P > .05). However, the changes of serum CCL27 in non-responders was significantly higher within all timepoints during BCG therapy as compared with BCG responders (e, P = .012). An increase in serum CCL27 over 89.16 pg/ml from baseline to last timepoint was the best predictor of the BCG response, and the AUC was 0.726 (95% CI 0.474–0.979, P = .044) along with 89% sensitivity, 68% specificity (a).
We further evaluated the combined effects of the baseline and dynamic changes of serum CCL27 during BCG induction therapy. Using the logistic regression model, we calculated a combined score (GSCCL27) for each patient based on the serum CCL27 at baseline and the changes from baseline to last timepoint using the following formula:
The AUC of GSCCL27 was 0.897 (95% CI 0.790–1.000, P < .001) and the predictive accuracy of the GSCCL27 was significantly higher than that of baseline CCL27 or changes of CCL27 alone (a). Patients were divided into high-risk (n = 18) and low-risk (n = 19) groups, with the median GSCCL27 as the cutoff. Compared with the patients in the low-risk group, patients in high-risk group had a shorter recurrence-free survival (f, P < .001).
Serum CCL27 is strongly correlated with the Tregs in the tumor microenvironment
To address the putative involvement of CCL27 upon BCG therapy, we investigated the correlation between the baseline level of serum CCL27 and the local/systemic immunological parameters. For the systemic immune factors, there were no significant differences in the peripheral neutrophil lymphocyte ratio between the groups (P = .75). With regard to the Th1/Th2 immune response, there were no significant differences between serum CCL27 and the density of Th1-related T-bet+ cells (a, P = .10), as well as Th2-related Gata-3+ T cells in the tumor microenvironment (TME) (b, P = .93).
Figure 3. Immunohistochemistry analysis of each immune cell subtype in the tumor microenvironment and its correlation with serum CCL27.
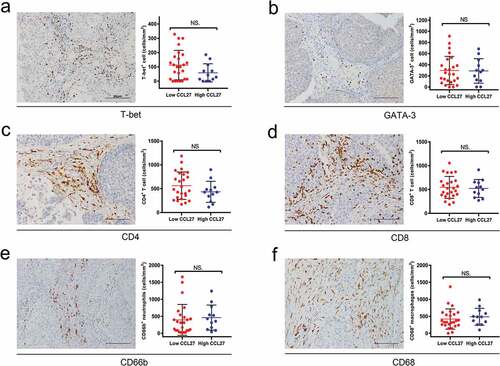
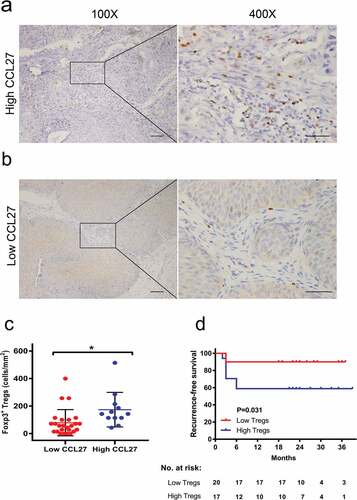
As a next step, we assessed the correlation between CCL27 and different immunoreactive cells including CD4, CD8, Tregs, CD66b, and CD68 in the TME. As shown in , the densities of tumor infiltrating CD4+ T cells (c, P = .18), CD8+ T cells (d, P = .76), CD66b+ neutrophils (e, P = .51) and CD68+ macrophages (f, P = .51) were not significantly correlated with baseline levels of serum CCL27. Previous studies have indicated that CCL27 could specifically recruit Tregs .Citation11 Consistently, we confirmed that serum CCL27 had strong positive correlations with the density of tumor infiltrating Tregs in NMIBC (, a-c, P = .015). Moreover, increasing levels of pre-treatment Foxp3 were significantly associated with a poor recurrence-free survival after BCG treatment (d, log-tank test, P = .031).
Figure 4. Association between serum CCL27 and regulatory T cells (Tregs) in the tumor microenvironment.
Discussion
In the current study, we performed a comprehensive analysis of the serum cytokines and chemokines in longitudinal serum samples to identify a biomarker for the response to BCG in patients with NMIBC. We identified and validated that the level of serum CCL27 at baseline was strongly associated with poor outcomes of intravesical BCG in NMIBC. Dynamic monitoring of serum CCL27 during BCG therapy may add predictive value and we developed a combined score: GSCCL27, which will enable physicians to make more informed treatment decisions. Moreover, we also found that the level of serum CCL27 was correlated with the density of Tregs in the TME of NMIBC. To our knowledge, this is the first study to investigate the predictive role of CCL27 in BCG immunotherapy for NMIBC. Our data show the potential for this novel biomarker to be translated into clinical tests.
Cytokines and chemokines have already been shown to be suitable for the detection and prediction of the BCG response.Citation12 Previous studies have developed multiple cytokine panels to predict the treatment response to BCG; however, they have limited clinical usefulness for the measurement techniques and costs .Citation4,Citation13 In an attempt to identify a robust predictive biomarker for potential clinical use, we evaluated 48 cytokines and chemokines involved in several stages of the mechanism of BCG immunotherapy and screened the cytokine or chemokine with most predictive value. CCL27, namely T-cell attracting chemokine (CTACK), has been reported to be involved in tumor progression, metastasis and immune escape in various cancers.Citation14,Citation15 Our study demonstrated a critical role of serum CCL27 in the clinical response of BCG immunotherapy. Indeed, the baseline serum CCL27 could identify BCG non-responders with an AUC of 0.73 prior to induction therapy. It is important to note that, although numerous predictive cytokines that belong to various immunologic components have been reported, most of the predicted outcomes were based on the changes over the entire induction therapy .Citation4 Predicting outcomes prior to the start of BCG therapy would be a significant advance, enabling more precise BCG therapy for the target population.
On the other hand, the level of serum CCL27 may serve as a stable and reproducible marker that can be applied at any time point prior to or during BCG therapy. Moreover, the changes in the level of serum CCL27 in each patient may provide additive information for the prediction of the BCG response. Kamat et al.Citation6 recently developed a nomogram (CyPRIT) using a panel of nine urinary cytokines, which resulted in an accuracy of 85.5% for discriminating between BCG responder and non-responders. Of interest, although the level of serum CCL27 at any single time point did not reach an AUC above 83%, dynamic monitoring of CCL27 can likely discriminate between the outcome groups more effectively than CyPRIT (AUC: 0.897). Nevertheless, these results should be interpreted with caution, and further validation in a larger cohort is warranted.
Despite its long-term use and FDA approval, the mechanisms involved in BCG failure remain poorly understood .Citation16 It has been well established that the Th1/Th2 milieu in TME is consequential for clinical response to BCG therapy.Citation17–Citation19 However, the immunosuppressive mechanisms are multifaceted and heterogeneous for BCG failure. Cumulative evidences support that, in addition to a Th1/Th2 imbalance, several other immune parameters can contribute to BCG failure .Citation20–Citation27 These mechanisms include insufficient immune cell infiltration, Citation22 the local balance between T lymphocytes and myeloid suppressor cells, Citation23 adaptive immune resistance,Citation24,Citation25 as well as accumulation of Tregs .Citation26,Citation27 Although CCL27 was usually regarded as a Th2 cytokine,Citation28 we were unable to confirm a link between serum CCL27 and Th2 predisposition in the TME. CCL27 may be non-redundant to Th1/Th2 response in immune regulation during BCG therapy.
It has been previously reported that CCL27, together with its receptor CCR10, is involved in Tregs recruitment .Citation11 Facciabene et al.Citation29 emphasized that CCR10-expressing Tregs in the TME were critical for promoting immune tolerance and angiogenesis. Two independent IHC-based studies have indicated that high Tregs counts are predictive of a shorter recurrence-free survival in NMIBC treated with BCG.Citation22,Citation26 In addition, a recent study demonstrated that both conventional and PD-L1-expressing Tregs were strongly enriched during intravesical BCG instillations and further favored rapid disease recurrence.Citation27 Consistent with these findings, we found the level of serum CCL27 was positively correlated with the density of Tregs in NMIBC. Moreover, we confirmed the correlation between the increased density of Tregs and unfavorable recurrence-free survival following BCG therapy. In light of our findings, CCL27 may contribute to the recruitment of CCR10+Tregs in NMIBC, thereby shortening disease recurrence survival following BCG therapy. In addition, intratumoral administration of anti-CCR10 immunotoxin has shown to attenuate Tregs accumulation into the TME and increase the antitumor immune response.Citation29 Given the identification of CCL27 and its potential role in Tregs recruitment, we hypothesize that a combination therapy of BCG and CCL27/CCR10 blockage may boost the immune response and improve BCG therapy.
Despite these remarkable results, our study has several inherent limitations. Though a prospective study, the results are limited by the small sample size in a single center. In the context of clinical applicability, the predictive role of serum CCL27 must be externally validated using larger cohorts and in other populations. Moreover, the mechanisms behand the association of CCL27 with Tregs in NMIBC were not clearly elucidated in the present study. Therefore in-depth in vivo experiments are required to explore their interaction during BCG therapy.
In summary, our results demonstrate for the first time that the level of serum CCL27 appears to be a practical and reproducible biomarker to predict the treatment outcome of BCG immunotherapy in patients with NMIBC. Furthermore, we have identified the potential role of CCL27 in the recruitment of Tregs to TME, thereby reducing the disease recurrence survival following BCG therapy. Thus, our findings may lead to a judicious use of intravesical BCG; however, further investigations using larger and multi-institutional cohorts are required.
Author contribution
Wenlong Zhong and Bo Wang designed the experiment, analyzed the data and wrote the manuscript; Hao Yu, Meihua Yang and Weibin Hou performed the cytokines and chemokines analysis; Hao Yu and Kun Xia collected the samples and clinic data; Junyu Chen, Meng Yang and Xiaofei Wang performed the immunohistochemical staining and evaluate the immunohistochemical makers of tissue sections, Lin T modified and revised the manuscript; Huang J supervised in the design of the study and finalized the manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download ()Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–10. doi:10.3322/caac.21492.
- Cambier S, Sylvester RJ, Collette L, Gontero P, Brausi MA, van Andel G, Kirkels WJ, Silva FCD, Oosterlinck W, Prescott S, et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1-3 years of maintenance bacillus Calmette-Guerin. Eur Urol. 2016;69(1):60–69. doi:10.1016/j.eururo.2015.06.045.
- Babjuk M, Burger M, Comperat EM, Gontero P, Mostafid AH, Palou J, van Rhijn BWG, Rouprêt M, Shariat SF, Sylvester R, et al. European association of urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) - 2019 update. Eur Urol. 2019;76(5):639–657. doi:10.1016/j.eururo.2019.08.016.
- Kamat AM, Li R, O’Donnell MA, Black PC, Roupret M, Catto JW, Comperat E, Ingersoll MA, Witjes WP, McConkey DJ, et al. Predicting response to intravesical bacillus Calmette-Guérin immunotherapy: are we there yet? a systematic review. Eur Urol. 2018;73(5):738–748. doi:10.1016/j.eururo.2017.10.003.
- Pettenati C, Ingersoll MA. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat Rev Urol. 2018;15(10):615–625. doi:10.1038/s41585-018-0055-4.
- Kamat AM, Briggman J, Urbauer DL, Svatek R, Nogueras Gonzalez GM, Anderson R, Grossman HB, Prat F, Dinney CP. Cytokine panel for response to intravesical therapy (CyPRIT): nomogram of changes in urinary cytokine levels predicts patient response to bacillus Calmette-Guérin. Eur Urol. 2016;69(2):197–200. doi:10.1016/j.eururo.2015.06.023.
- Salmasi A, Elashoff DA, Guo R, Upfill-Brown A, Rosser CJ, Rose JM, Giffin LC, Gonzalez LE, Chamie K. Urinary cytokine profile to predict response to intravesical BCG with or without HS-410 therapy in patients with non-muscle-invasive bladder cancer. Cancer Epidem Biomar. 2019;28(6):1036–1044. doi:10.1158/1055-9965.EPI-18-0893.
- Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer-a current perspective. Nat Rev Urol. 2014;11(3):153–162. doi:10.1038/nrurol.2014.15.
- Wang B, Pan WW, Yang MH, Yang WJ, He W, Chen X, Bi J, Jiang N, Huang J, Lin T, et al. Programmed death ligand-1 is associated with tumor infiltrating lymphocytes and poorer survival in urothelial cell carcinoma of the bladder. Cancer Science. 2019;110(2):489–498. doi:10.1111/cas.13887.
- Wang B, Xie S, Bi J, Liu Z, Zeng H, Huang H, Xue M, He Z, Yang M, Yu H, et al. Elevated pre-existing lymphocytic infiltrates in tumour stroma predict poor prognosis in resectable urothelial carcinoma of the bladder. Histopathology. 2019;75(3):354–364. doi:10.1111/his.13807.
- Baumgartner-Nielsen J, Vestergaard C, Thestrup-Pedersen K, Deleuran M, Deleuran B. Glucocorticoid-induced tumour necrosis factor receptor (GITR) and its ligand (GITRL) in atopic dermatitis. Acta Derm Venereol. 2006;86(5):393–398. doi:10.2340/00015555-0118.
- Qu K, Gu J, Ye Y, Williams SB, Dinney CP, Wu X, Kamat A. High baseline levels of interleukin-8 in leukocytes and urine predict tumor recurrence in non-muscle invasive bladder cancer patients receiving bacillus Calmette–Guerin therapy: A long-term survival analysis. Oncoimmunology. 2017;6(2):e1265719. doi:10.1080/2162402X.2016.1265719.
- Jallad S, Thomas P, Newport MJ, Kern F. Baseline cytokine profiles of tuberculin-specific CD4(+) T cells in non-muscle-invasive bladder cancer may predict outcomes of BCG immunotherapy. Cancer Immunol Res. 2018;6(10):1212–1219. doi:10.1158/2326-6066.Cir-18-0046.
- Karnezis T, Farnsworth RH, Harris NC, Williams SP, Caesar C, Byrne DJ, Herle P, Macheda ML, Shayan R, Zhang Y-F, et al. CCL27/CCL28-CCR10 chemokine signaling mediates migration of lymphatic endothelial cells. Cancer Res. 2019;79(7):1558–1572. doi:10.1158/0008-5472.CAN-18-1858.
- Simonetti O, Goteri G, Lucarini G, Filosa A, Pieramici T, Rubini C, Biagini G, Offidani A. Potential role of CCL27 and CCR10 expression in melanoma progression and immune escape. Eur J Cancer. 2006;42(8):1181–1187. doi:10.1016/j.ejca.2006.01.043.
- Crispen PL, Kusmartsev S. Mechanisms of immune evasion in bladder cancer. Cancer Immunol Immunother. 2020;69(1):3–14. doi:10.1007/s00262-019-02443-4.
- Martinez R, Tapia G, De Muga S, Hernandez A, Cao MG, Teixido C, Urrea V, García E, Pedreño-López S, Ibarz L, et al. Combined assessment of peritumoral Th1/Th2 polarization and peripheral immunity as a new biomarker in the prediction of BCG response in patients with high-risk NMIBC. Oncoimmunology. 2019;8(8):8. doi:10.1080/2162402x.2019.1602460.
- Pichler R, Gruenbacher G, Culig Z, Brunner A, Fuchs D, Fritz J, Gander H, Rahm A, Thurnher M. Intratumoral Th2 predisposition combines with an increased Th1 functional phenotype in clinical response to intravesical BCG in bladder cancer. Cancer Immunology, Immunotherapy. 2017;66(4):427–440. doi:10.1007/s00262-016-1945-z.
- Nunez-Nateras R, Castle EP, Protheroe CA, Stanton ML, Ocal TI, Ferrigni EN, Ochkur SI, Jacobsen EA, Hou Y-X, Andrews PE, et al. Predicting response to bacillus Calmette-Guérin (BCG) in patients with carcinoma in situ of the bladder. Urol Oncol. 2014;32(1):45e23–30. doi:10.1016/j.urolonc.2013.06.008.
- Yuk HD, Jeong CW, Kwak C, Kim HH, Ku JH. Elevated neutrophil to lymphocyte ratio predicts poor prognosis in non-muscle invasive bladder cancer patients: initial intravesical bacillus Calmette-Guerin treatment after transurethral resection of bladder tumor setting. Front Oncol. 2018;8:642. doi:10.3389/fonc.2018.00642.
- Takayama H, Nishimura K, Tsujimura A, Nakai Y, Nakayama M, Aozasa K, Okuyama A, Nonomura N. Increased infiltration of tumor associated macrophages is associated with poor prognosis of bladder carcinoma in situ after intravesical bacillus Calmette-Guerin instillation. Journal of Urology. 2009;181(4):1894–1900. doi:10.1016/j.juro.2008.11.090.
- Pichler R, Fritz J, Zavadil C, Schafer G, Culig Z, Brunner A. Tumor-infiltrating immune cell subpopulations influence the oncologic outcome after intravesical Bacillus Calmette-Guerin therapy in bladder cancer. Oncotarget. 2016;7(26):39916–39930. doi:10.18632/oncotarget.9537.
- Chevalier MF, Trabanelli S, Racle J, Salome B, Cesson V, Gharbi D, Bohner P, Domingos-Pereira S, Dartiguenave F, Fritschi A-S, et al. ILC2-modulated T cell-to-MDSC balance is associated with bladder cancer recurrence. J Clin Invest. 2017;127(8):2916–2929. doi:10.1172/Jci89717.
- Kates M, Matoso A, Choi W, Baras AS, Daniels MJ, Lombardo K, Brant A, Mikkilineni N, McConkey DJ, Kamat AM, et al. Adaptive immune resistance to intravesical BCG in non-muscle invasive bladder cancer: implications for prospective BCG-unresponsive trials. Clin Cancer Res. 2020;26(4):882–891. doi:10.1158/1078-0432.CCR-19-1920.
- Inman BA, Sebo TJ, Frigola X, Dong H, Bergstralh EJ, Frank I, Fradet Y, Lacombe L, Kwon ED. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109(8):1499–1505. doi:10.1002/cncr.22588.
- Miyake M, Tatsumi Y, Gotoh D, Ohnishi S, Owari T, Iida K, Ohnishi K, Hori S, Morizawa Y, Itami Y, et al. Regulatory T cells and tumor-associated macrophages in the tumor microenvironment in non-muscle invasive bladder cancer treated with intravesical bacille Calmette-Guerin: a long-term follow-up study of a Japanese cohort. Int J Mol Sci. 2017;18(10). doi:10.3390/ijms18102186
- Chevalier MF, Schneider AK, Cesson V, Dartiguenave F, Lucca I, Jichlinski P, Nardelli-Haefliger D, Derré L. Conventional and PD-L1-expressing regulatory T cells are enriched during BCG therapy and may limit its efficacy. Eur Urol. 2018;74(5):540–544. doi:10.1016/j.eururo.2018.06.045.
- Kagami S, Saeki H, Tsunemi Y, Nakamura K, Kuwano Y, Komine M, Nakayama T, Yoshie O, Tamaki K. CCL27-transgenic mice show enhanced contact hypers ensitivity to Th2, but not Th1 stimuli. Eur J Immunol. 2008;38(3):647–657. doi:10.1002/eji.200737685.
- Facciabene A, Peng XH, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T-reg cells. Nature. 2011;475(7355):226–U141. doi:10.1038/nature10169.
