ABSTRACT
Why PD-1 blockade is ineffective in the vast majority of colorectal cancers (CCs) lacking microsatellite instability but harboring high densities of tumor infiltrating T cells and follicular T helper (TFH) and B cells remained so far an open conundrum. In a recent report published in Nature Medicine, we bring evidence in mice and patients that ileal microbiota turns tolerogenic apoptosis of ileal intestinal epithelial cells (IEC) into immunogenic cell demise capable of eliciting IL-1β-dependent TFH responses that benefit from anti-PD1 antibodies.
Chemotherapy induced an immunogenic apoptosis of ileal crypts
We monitored apoptosis of ileal cells by performingCitation1-Citation4 immunohistochemical stainings of cleaved caspase-3 in the healthy colon and ileal mucosae to better understand the immunogenicity of chemotherapy in proximal CC.Citation1-Citation4 These tissues were harvested during right hemicolectomy for proximal colon cancer (pCC) in patients receiving or not pre-operative oxaliplatin (OXA)-based chemotherapy. Surprisingly, apoptosis of IEC was detectable only in the crypts of ilea (not in the villi nor lamina propria), and not in healthy colons, mainly after chemotherapy, in two independent patient cohorts from different institutions. Ileal apoptosis dictated the prognosis in as much as stage IV pCC patients presenting with higher cleaved caspase 3 levels in ileal crypts had a prolonged progression free survival. We reproduced these findings in MC38 and CT26 colon cancer bearing mice. OXA(which induced a T cell-dependent tumor regression) promoted the ileal (but not colonic) crypt apoptosis. Importantly, the ability of OXA to treat established colon cancers was compromised in Casp3/7ΔIEC animals where the executioner caspases were disabled only in intestinal villi.
Figure 1. Proposed mechanism of action how the gut microbiota impacts the immunogenic apoptosis of ileal IEC during oxaliplatin based chemotherapy. Chemotherapy acts not only on the tumor cells but on dividing cells, in particular the IEC of the ileal crypts, resulting in their Casp3-deficient cell death. The immunogenic potential of IEC death relies on the profile of the ileal microbiota. Local immunity is then modulated by migration of CD103+CD11b− (Batf3+) DCS to the mLN where they trigger a local immune response characterized by the IL-1β and IL-12 dependent activation of TFH cells. TFH accumulate in the tumor microenvironment and shift the balance toward anti-tumor immunity in colorectal cancer, restoring responsiveness to PD-1 blockade.
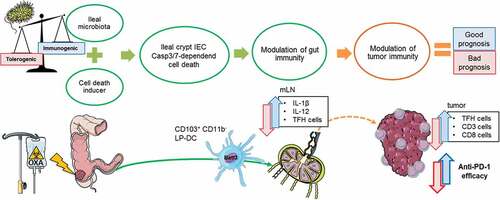
Moreover, dying ileal IEC (crypt-derived, not villus-derived, and not colon-derived) harvested right after OXA could immunize naive animals against MC38 (or CT26) but not unrelated sarcoma or breast cancers or cholangiocarcinomas, suggesting that stem cells from normal crypt shared antigens with colon cancers. Taken together, these findings indicate that intestinal apoptosis of ileal crypts is indispensable for the efficacy of OXA-mediated immunogenic cell death and anticancer effects of chemotherapy.
Role of ileal microbiome as adjuvant for ileal crypt cell demise
Ileal crypt IEC lost their immunogenicity when derived from germ-free animals or TLR2/4 knock-out (KO) mice. Similarly, small intestinal crypt stem cell derived-enteroids failed to mediate any anticancer protection. Some patients’ ileal microbiome restored the immunogenic potential of IEC. Culturomics and 16S rRNA gene sequencing-based screening of all 12 ileal mucosae-associated microbiomes used to compensate the lack of immunogenicity of enteroids in vivo led us to discover that distinct commensals (such as Erysipelatotrichaceae family members or non-enterotoxigenic B. fragilis) conveyed immunogenicity while others (such as Fusobacterium nucleatum or Prevotellaceae family members) conferred tolerance. Cause-effect relationship could be brought up by admixing to crypt derived-enteroids exposed to OXA, distinct species (and strains) belonging to immunogenic (such as B. fragilis) or tolerogenic (such as F. nucleatum or P. clara) families that could turn tolerogenic enteroids into immunogenic vaccines (for B. fragilis) or failed to do so (F. nucleatum or P. clara).
Role of TFH in the immunogenicity of ileal crypt apoptosis in the presence of a suitable ileal microbiome
Driven by other reports highlighting the key role of TFH and B cells during PD-1 blockade,Citation4-Citation10 we performed a comprehensive flow cytometric analyses of immune changes post-OXA in tumor and mesenteric lymph node (mLN) as well as in the vaccine-draining lymph-node. An accumulation of TFH was observed in the tumor draining lymph node (tdLN) of MC38 tumor bearers responding to therapy after 12–14 days of intraperitoneal OXA. Skin draining LN after sub-cutaneous (s.c.) vaccination of mice with OXA-treated ileal IEC revealed priming of CXCR5+PD1highBcl6+ TFH expressing high levels of membrane ICOS at day 7. Such TFH were also increased at day 7 post-OXA in mLN of WT (wild type) mice but not Casp3/7ΔIEC animals, suggesting the crucial role for ileal apoptosis in the activation of TFH.
The immunogenicity of vaccines composed of apoptotic ileal IEC was lost after depletion of CD4+ or CD8+ T cells from the host, in accord with the possibility that CD4+ helper T cells including TFH cells are mandatory to confer memory T cell capacities to CD8+ T lymphocytes. Next, to evaluate the functional impact of TFH on the immunogenicity of dying ileal intestinal epithelial cells, we utilized four approaches. We observed that OXA-exposed ileal IEC (“vaccine”) exhibited a reduced immunizing capacity in two TFH-deficient mouse models, namely, Bcl6fl/flCreCD4 mice where Bcl6 is conditionally deleted in CD4+ T cells and CXCR5-deficient (Cxcr5-/-) hosts, compared with WT counterparts. Moreover, neutralizing the CXCR5 ligand CXCL13 with specific antibodies affected protection by this vaccine. Given that TFH cells are pivotal regulators of the germinal center responses and humoral immunity, we explored humoral immune responses and the impact of B cell responses on the immunizing potential of dying ileal IEC. Elevated serum levels of IgG2b were monitored by 3 weeks post-immunization, and this effect was blunted by neutralization of CXCL13 and the depletion of CD4+ T cells. Systemic administration of anti-CD19, -CD20, -CD22 antibodies, which depleted circulating B cells, compromised the prophylactic antitumor effects of OXA- exposed ileal IEC.
In untreated CC patients, the immunoscore (based on the abundance of positive CD3, CD8 in the cancer core and invasive margins as well as B cells and TFH cells assessed by immunohistochemistry in paraffin embedded-tumor tissues) determines the prognosis of patients1. Chemotherapy could not increase the overall abundance of TILs (tumor-infiltrating lymphocytes) in stage IV individuals but enriched tumor beds with CD4+Bcl6+ TFH cells, the density of which correlated with that of TILs within the tumor core and was positively associated with ileal epithelial cell apoptosis. We conclude that TFH are indispensable for the full-blown efficacy of chemotherapy against CC and depend on ileal caspases 3/7 in mice while positively correlating with ileal apoptosis in patients.
Role of lamina propria dendritic cell in the priming of PD1high TFH
Distinct subsets of ileal antigen presenting cells activated through damage and microbe-associated molecular patterns are in charge of priming of TFH and crypt IEC-specific CD8+ T cell responses. After OXA, lamina propria (LP) DC subsets were progressively eliminated such as the cDC1, while the mLN got enriched in cDC1 in the mLN microenvironment enriched in IL-6, IL-21, IFNγ and IL-12p40. Indeed, Batf3 KO mice (lacking cDC1) failed to mount TFH following OXA. Moreover, immunogenic bacteria (B. fragilis, E. ramosum) could stimulate IL-1β and IL-12 coproduction by cDC1 cells in vitro while tolerogenic bacteria (F. nucleatum, P. clara) failed to trigger IL-12 in these conditions. We demonstrated that ileal crypt apoptosis lost its immunizing potential against CC when IL-1R1 or IL-12p70 were neutralized in vivo.
Combining immunogenic commensals with OXA triggered the efficacy of PD1 blockade against MSS colon cancers
We finally showed-in various settings of MSI (microsatellite instable, CT26) or MSS (microsatellite stable, MC38)-colon cancers which responded poorly or modestly to the combination of OXA+anti-PD1 Abs, that oral gavage with B. fragilis (or E. ramosum) but not P. clara or F. nucleatum boosted IgG2b responses and markedly ameliorated the anticancer effects of the combinatorial therapeutic regimen.
Conclusions
These data (summarize in ) suggest potential new therapeutic avenues, including ileum-targeting cell death inducers and bacterial adjuvants with the purpose of inducing immune responses against CC (self-) antigens and of reinstating clinical responses to PD1 blockade in MSS tumors.
Abbreviations
| CC | = | colon cancer |
| pCC | = | proximal colon adenocarcinoma |
| Ab | = | antibody |
| Casp | = | caspase |
| IEC | = | intestinal epithelial cells |
| KO | = | knock-out |
| mLN | = | mesenteric lymph node |
| MSI/MSS | = | microsatellite instability/stability |
| OXA | = | oxaliplatin |
| tdLN | = | tumor draining lymph node |
| TFH | = | T follicular helper cell |
| TIL | = | tumor-infiltrating lymphocytes |
| TLR | = | Toll-like receptor |
| WT | = | wild type. |
Disclosure of potential conflicts of interest
LZ is cofounder of EverImmune, a biotech company devoted to the use of commensal bacteria for the treatment of cancers.
Additional information
Funding
References
- Pagès F, Mlecnik B, Marliot F, Bindea G, Ou F-S, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–3. doi:10.1016/S0140-6736(18)30789-X.
- Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482–491. doi:10.1038/onc.2009.356.
- Ciardiello D, Vitiello PP, Cardone C, Martini G, Troiani T, Martinelli E, Ciardiello F. Immunotherapy of colorectal cancer: challenges for therapeutic efficacy. Cancer Treat Rev. 2019;76:22–32. doi:10.1016/j.ctrv.2019.04.003.
- Roberti MP, Yonekura S, Duong CPM, Picard M, Ferrere G, Tidjani Alou M, Rauber C, Iebba V, Lehmann CHK, Amon L, et al. Chemotherapy-induced ileal crypt apoptosis and the ileal microbiome shape immunosurveillance and prognosis of proximal colon cancer. Nat Med. 2020 May 25. (published online ahead of print). DOI:10.1038/s41591-020-0882-8.
- Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf A, Angell H, Fredriksen T, Lafontaine L, Berger A, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi:10.1016/j.immuni.2013.10.003.
- Gu-Trantien C, Migliori E, Buisseret L, de Wind A, Brohée S, Garaud S, Noël G, C.V. LD, Lodewyckx J-N, Naveaux C, et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight. 2017;2:e91487. doi:10.1172/jci.insight.91487.
- Garaud S, Buisseret L, Solinas C, Gu-Trantien C, de Wind A, Van den Eynden G, Naveaux C, Lodewyckx J-N, Boisson A, Duvillier H, et al. Tumor-infiltrating B cells signal functional humoral immune responses in breast cancer. JCI Insight. 2019;4:e129641. doi:10.1172/jci.insight.129641.
- Hollern DP, Xu N, Thennavan A, Glodowski C, Garcia-Recio S, Mott KR, He X, Garay JP, Carey-Ewend K, Marron D, et al. B cells and T follicular helper cells mediate response to checkpoint inhibitors in high mutation burden mouse models of breast cancer. Cell. 2019;179:1191–1206.e21. doi:10.1016/j.cell.2019.10.028.
- Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–555. doi:10.1038/s41586-019-1922-8.
- Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, Johansson I, Phung B, Harbst K, Vallon-Christersson J, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577:1–5. doi:10.1038/s41586-020-2155-6.
