ABSTRACT
Background
Subgroup analysis of clinical trials of PD-1/PD-L1 inhibitors have reported ethnic differences in outcomes. We systematically collected published data and performed a meta-analysis to compare therapeutic efficacy in Asian and non-Asian patients receiving PD-1/PD-L1 inhibitors.
Methods
Eligible studies included phase II and III prospective clinical trials with available subgroup data on Asian versus non-Asian populations. Overall survival (OS) and progression-free survival (PFS) were used to evaluate differences in outcome between Asian versus non-Asian cancer patients.
Results
A total of 11,020 cancer patients from 19 prospective randomized controlled clinical trials were included. The overall estimated HR for OS was 0.69 with 95% CI of 0.61–0.77 in Asian versus 0.82 with 95% CI of 0.77–0.88 in non-Asian patients. The estimated hazard ratio (HR) for PFS measured 0.54 (95% CI, 0.32–0.76) and 0.69 (95% CI, 0.54–0.85) in Asian and non-Asian patients, respectively. Pooled ratios of OS HRs and PFS HRs reported in Asian versus non-Asian cancer patients were 0.84 (95% CI, 0.75–0.94) and 0.78 (95% CI, 0.59–0.97), respectively.
Conclusions
This meta-analysis shows for the first time that Asian cancer patients have a significantly improved survival benefit than non-Asian patients receiving PD-1/PD-L1 inhibitor-based therapy.
Introduction
Treatment modalities for cancer have undergone a drastic evolution changing from conventional drugs targeting the tumor itself to immune checkpoint inhibitors (ICIs) that typically modulate T-cell function, targeting relevant mechanisms of immuno-resistance, including inhibitory molecules.1,Citation2 The often-impressive efficacies of these anti-PD-1/PD-L1 agents have been tested across various cancer types.Citation3
Generally, clinical trials for approval are conducted in western countries first and then evaluated in other ethnicities subsequently. Drug efficacy and toxicity vary greatly among different ethnicities for a variety of both known and unknown reasons. Among the drugs approved by the Food and Drug Administration (FDA), more than 20% reported ethnic differences in pharmacokinetics, safety, efficacy or pharmacogenomics .Citation4 Ethnic differences in treatment response in the field of oncology are also known .Citation5 A meta-analysis compared ethnic differences in survival of advanced stage non-small cell lung cancer (NSCLC) patients receiving chemotherapy, and found that ethnic differences exist in terms of both survival and response rate, Citation6 and of course other treatments such as anti-EGFR therapeutics have effects based on differences of mutation/deletion prevalence.
Some clinical trials involving only Asian patients have reported a numerical longer PFS and OS than likewise designed trials in Caucasian patients. Due to different enrollment criteria and patient baselines amongst clinical trials, direct comparisons of median survival times and hazard ratios may not lead to correct conclusions. For example, an expansion cohort of Chinese subgroup in KEYNOTE-407 study has a longer survival data than the entire patient population.Citation7 However, in KEYNOTE-407, there is no allocation of α value and statistical comparisons for the Chinese sub-group, so data differences are only numerical and may not have significance. Researchers have understandably questioned whether Asian patients have a better prognosis than non-Asian patients receiving immune checkpoint inhibitors (ICIs).Citation8 Data are mixed and some clinical studies reported subgroup efficacies regarding ethnic differences in ICI treatment, but no comprehensive analysis is available at present. We propose here that pooled analyses of subgroup results of Asian versus non-Asian efficacy of the studies could provide a better understanding on the potential efficacy difference in Asian versus non-Asian cancer patients receiving PD-1/PD-L1 inhibitor. We systematically collected available published data and performed a study-level meta-analysis to compare efficacy outcomes in Asian versus non-Asian cancer patients enrolled in clinical trials of PD-1/PD-L1 inhibitors.
2. Materials and methods
2.1. Search strategy
A literature search was conducted using the following electronic databases, including PubMed, Embase, and Cochrane databases. The upper date limit of Feb 29th, 2020 was applied, with no lower date limit. Our search strategy included the following Medical Subject Headings (MeSH) terms and keywords: “cancer”, “tumor”, “PD-1”, “programmed death receptor 1”, “PD-L1”, “programmed death-ligand 1”, “nivolumab”, “pembrolizumab”, “avelumab”, “durvalumab”, “atezolizumab” and “cemiplimab”. Searches were performed using the filter “clinical trial”. We also reviewed abstracts and presentations from conference proceedings, including American Society of Clinical Oncology (ASCO), World Conference on Lung Cancer (WCLC), European Society for Medical Oncology (ESMO), European Lung Cancer Conference (ELCC), American Association for Cancer Research (AACR). The references cited by the included studies were also used to complete the search.
2.2. Study selection
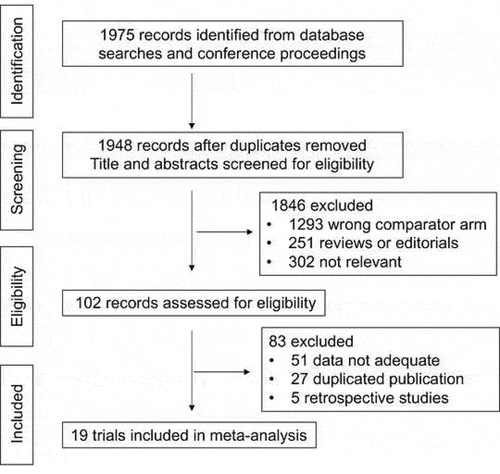
Eligible criteria for inclusion in this meta-analysis were:Citation1 prospective phase II and III randomized controlled clinical trials (RCTs) in patients with metastatic solid cancer;Citation2 random assignment of participants to treatment group containing PD-1 or PD-L1 inhibitor to a non- PD-1/PD-L1 inhibitor;Citation3 the language was restricted in English;Citation4 subgroup data available regarding Asian versus non-Asian population, andCitation5 in cases of duplicate publications, only the most complete, recent and updated report of the clinical trial were also included. Review articles, non-randomized trials, and observational studies, non-English studies were excluded from the analysis. Phase I trials and single arm trials were omitted due to lack of controls. The selection process is shown in .
Studies involving use of ICIs in hematologic malignancies were excluded. Abstracts of all candidate articles were read by two independent readers (LP and BDQ). Articles that could not be categorized based on title and abstract alone were retrieved for full-text review. Disagreements were resolved by consensus between two authors. To determine the issue of multiple publications from the same data sets, we confirmed clinical trial information such as the trial number and the time period of patient recruitment of the articles. Two further authors independently assessed the eligibility of the articles and abstracts identified by the search, and discrepancies were resolved by consensus.
2.3. Data extraction
Meta-analysis was performed based on outcomes coming from the included studies. Data extracted from eligible studies including the following items: study name, year of publication, publication source, tumor type, number of patients, treatment line, phase of trial, sample size, treatment arm and control arm. In the case of trials that did not include survival subgroup analysis by Asian versus non-Asian, we reviewed each published trial’s supplement. If data from any of the above categories were not reported in the study, items were treated as NR (not reported). The primary variables of interest were HRs with 95% confidence intervals (CIs) for OS or PFS in Asian and non-Asian patients.
2.4. Quality assessment of primary studies
The Jadad scoring system was used to assess the methodological quality of the included studies to evaluate the quality of factors. This provides a summary numeric quality score with a range of 0–5. Two authors (LP and BDQ) checked and confirmed the final results, and any disagreements were resolved by consensus with a third-party author (SX).
2.5. Statistical analysis
The primary endpoint of this study-level meta-analysis was the ratio of the HR for OS and PFS in Asian patients to the same HR in non-Asian patients, which showed the difference in efficacy of PD-1/PD-L1 inhibitor-based therapy between Asian versus non-Asian patients. Altogether, three meta-analyses were conducted. The first meta-analysis calculated the pooled HR for OS and PFS in the total population of the included studies. The second meta-analysis focused on the efficacy of PD-1/PD-L1 inhibitor-based therapy in Asian and non-Asian cancer patients, respectively. The third meta-analysis focused on the difference in efficacy of PD-1/PD-L1-based therapy versus control arm between patients in Asian versus non-Asian patients based on the pooled trial-specific ratios of HRs. We calculated the ratio of HR from each study based on the HR reported of Asian versus non-Asian cancer patients. Then, we combined the trial-specific ratios of HRs across trials using a fixed-effects model.Citation9,Citation10 If the pooled-HR ratio was <1, it indicated a better treatment effect in Asian patients; if it was >1, it showed a better treatment effect in non-Asian patients.
Between-study heterogeneity was estimated using the χ2-based Q statistic .Citation11 Heterogeneity was considered statistically significant when P < .10 or ICitation2 > 50%. In the absence of heterogeneity, a fixed effects model was used. If heterogeneity existed, data were analyzed using a random effects model. To calculate the pooled hazard ratio, an inverse variance statistical method with a standard P < .05 considered significant. To assess the stability of results, sensitivity analysis was carried out by sequential omission of individual studies. The presence of publication bias was evaluated by using the Begg’s and Egger’s tests.Citation12,Citation13 All calculations were performed by STATA version 14.0 (Stata Corporation, College Station, TX).
3. Results
3.1. Study selection and characteristics
Our search strategy yielded a total of 1,975 potentially relevant articles. After initial exclusion of duplicate and non-randomized studies, 19 original studies were considered eligible for the meta-analysis, comprising 11,020 patients for final analysis (). The major baseline characteristics of the 19 eligible studies were represented in , all of which being phase III clinical trials. Thirteen of them were involved with first-line treatment, and the rest 6 trials were performed at second or later lines. Studies involving anti-CTLA4 were excluded.
Table 1. Main characteristics and results of the eligible studies.
All 19 RCTs investigated the efficacy of PD-1/PD-L1 inhibitors in cancer. Ten studies focused on a PD-1 inhibitor, while the other 9 focused on a PD-L1 inhibitor. Nine RCTs were conducted in patients with NSCLC, 4 RCTs in gastric cancer, 2 RCTs with small-cell lung cancer (SCLC), 1 RCT in head and neck cancer, 1 RCT in breast cancer, and 1 RCT in hepatocellular carcinoma (HCC). Thirteen RCTs compared the efficacy of PD-1/PD-L1 against control group in cancer, and 5 RCTs compared immunotherapy plus chemotherapy with control, and 1 RCT compared PD-1 plus CTLA-4 inhibitor. The sample size of the included studies ranged from 204 to 1,274 patients (median sample size, 585 patients). The studies were published between 2016 and 2020. Studies were chosen and systemically reviewed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.Citation32 The Jadad scores ranged from 3 to 5. No trial received a low-quality Jadad score (i.e., 1–2), validating our selection criteria.
3.2. Pooled HRs for PD-1/PD-L1 inhibitors in the overall patient population
Sixteen and 6 studies investigated the efficacy in terms of OS and PFS of PD-1/PD-L1 inhibitor monotherapy or combination stratified by Asian versus non-Asian, respectively. The HR of the individual studies and the combined results based on the random-effects models are summarized in . The ratios presented compare PD-1/PD-L1 inhibitor-based therapy against control arm in the total population. The overall estimated, random-effects HR for OS is 0.79 with 95% CI of 0.72–0.86 with significant heterogeneity (ICitation2 = 57.9%, P = .002, ). Based on the selected trials, there is evidence of a statistically significant 21% reduction in the hazard of death with PD-1/PD-L1 inhibitor-based therapy compared with control. In Asian patients, the meta-analysis showed that PD-1/PD-L1 inhibitor-based therapy could decrease the risk of death of Asian patients by 31%, and the pooled HR for PFS was 0.69 (95% CI 0.61–0.77) without heterogeneity (ICitation2 = 23.0%, P = .193; ). Similarly, in non-Asian patients, the analysis demonstrated that PD-1/PD-L1 inhibitor-based therapy could decrease the risk of death by 18% (HR = 0.82; 95% CI, 0.77–0.88; ) without heterogeneity (ICitation2= 28.9%, P = .134).
Figure 2. Hazard ratios of OS in patients receiving PD-1/PD-L1 inhibitor-based therapy versus control in the overall population, Asian and non-Asian patients.
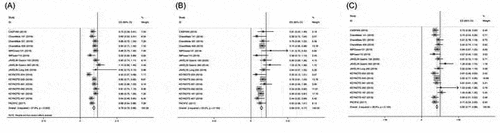
The HR for PFS of the individual studies and the combined results based on the random-effects models are summarized in . The overall estimated, random-effects HR is 0.72 with 95% CI of 0.55–0.90 (). Based on the selected trials, there is evidence of a statistically significant, 28% reduction in the hazard of a PFS event with PD-1/PD-L1 compared with control arm. In consistent with OS, the analysis also demonstrated that PD-1/PD-L1 inhibitor-based therapy could significantly prolong PFS in Asian and non-Asian cancer patients (HR = 0.54; 95% CI 0.32–0.76 for Asian and HR = 0.69; 95% CI, 0.54–0.85 for non-Asian patients, respectively) (.
Figure 3. Hazard ratios of PFS in patients receiving PD-1/PD-L1 inhibitor-based therapy versus control in the overall population, Asian and non-Asian patients.

3.3. Pooled HR ratios for patients in Asian versus non-Asian patients
The pooled ratio of OS HRs reported in Asian cancer patients versus non-Asian patients in each trial was 0.84 (95% CI, 0.75–0.94) (). This indicated a greater OS benefit from PD-L1/PD-L1-based therapy compared with control. The same results could also be observed in the PFS HR ratio: the pooled ratio was 0.78 (95% CI, 0.59–0.97) (), suggesting a greater benefit from PD-1/PD-L1-based therapy between Asian versus non-Asian cancer patients. When grouped according to tumor type and PD-1/PD-L1 drug, a similar trend was observed in lung cancer and other tumor types, as well as different PD-1/PD-L1 drugs (.
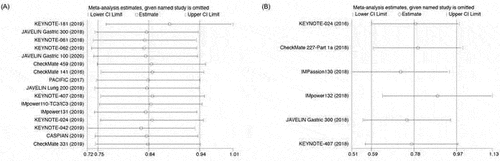
3.4. Sensitivity analysis
We performed sensitivity analyses to examine the stability and reliability of the pooled HRs by sequential omission of individual studies. Our results of OS and FPS are statistically stable (.
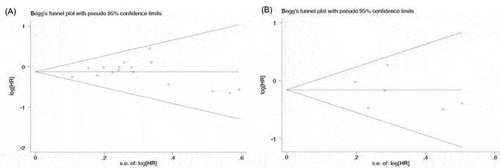
3.5. Publication bias
Begg’s funnel plot and Egger’s test were performed to evaluate the publication bias of the eligible studies. Altogether, 16 and 6 studies investigating OS and PFS yielded an Egger’s test scores of P = .860 and P = .594, respectively, indicating the absence of publication bias in the studies (.
4. Discussion
The majority of clinical trials of ICIs have been undertaken in Caucasians patients, by understandable virtue of the fact they are performed in English-speaking countries. Clinical trials of multiple races usually included a small portion of Asian patients, and results were calculated for the intent-to-treat cohort without any ethno-specific conclusions. Due to the unique biology of the host and the tumors from different sub-populations, it is critical to confirm a given therapy fits all relevant patient populations in the real world.Citation33
One study investigated population-based differences in the outcome of metastatic NSCLC, and found that Asian population had a better adjusted OS in the stage IV lung cancer patients.Citation34 Intriguingly, it is also reported that adverse events between ethnicities exist, such as the tyrosine kinase inhibitors.Citation35 Nowadays, use of anti-PD-1/PD-L1 agents in Asian populations have been gradually increased, for both domestically trialed molecules and the larger international pharmaceutical ones. Subgroup analysis such as this provides timely information whether different ethnic groups have similar survival trend of the entire population receiving immunotherapy.
The present meta-analysis has combined 19 publications, all of which are randomized controlled phase III trials. The first and second meta-analysis investigated the survival benefit of entire population, Asian and non-Asian populations in terms of OS and PFS. Both ethnic groups benefit from PD-1/PD-L1-based therapy compared with control group. However, pooled analyses HRs of Asian and non-Asian group cannot be compared directly. Therefore, the third meta-analysis compared the ratio of HRs of each study in terms of subgroup HR benefit. These data demonstrated that compared with non-Asian population, Asian cancer patients have an improved OS and PFS benefit receiving immunotherapy. Ratio of HRs of Asian versus non-Asian patients in terms of OS and PFS benefits are similar, indicating a consistent survival advantage. When the OS benefit was grouped according to tumor type and drug, different tumor type and different PD-1/PD-L1 inhibitor have a similar trend of OS benefit.
A study compared Asian versus non-Asian patients from 11 metastatic NSCLC trials which were submitted to FDA and found that Asian patients of metastatic NSCLC patients have a better survival than non-Asian patients, which corresponded to our meta-analysis.Citation36 Other than clinical trials, real-world studies have confirmed the benefit of Asian versus non-Asian patients receiving PD-1/PD-L1 patients. Real-world data of nivolumab in Asian population have a higher ORR than non-Asian cohorts.Citation37-Citation39 However, due to different baseline and other confounding factors, the numerical data again cannot be compared directly and of course one would caution inter-trial comparisons for this reason, thus necessitating our meta-analysis.
Although the exact mechanisms underlying the reasons why Asian populations might benefit more from immunotherapy are unclear, there are several possible explanations. An ancillary analysis of individual patient data in the OAK and POPLAR studies suggested that baseline characteristics and genetic mutations may account for the differences of outcome for Asian and non-Asian patients receiving atezolizumab.Citation40 Ethnic differences in somatic mutations such as STK11, TP53 and EGFR were associated with treatment responses or survival. For example, the mutation rate of STK11 differs among Asian (1.6%) and non-Asian patients (12.3%), which was reported previously to affect efficacy of ICI.Citation41,Citation42 Therefore, in NSCLC patients receiving atezolizumab, different clinic-pathologic features including tumor mutation profiles may explain the ethnic differences of efficacy though we are unaware of an “Asian signature”. Other purported mechanisms center round previous studies which have reported that PD-1 antibody clearance was a predictor of OS in several cancers .Citation43 According to population PK analyses, ethnicity (Asian vs Caucasian) is a key factor that may affect antibody clearanceCitation44 but others have suggested that efficacy in different ethnicities are unrelated to antibody clearance.Citation45
In our meta-analysis, we did not rule out the studies of non-significant trials, because treatment effects may still not be homogeneously absent across the study population.Citation44 However, the overall type I error could be substantially inflated. Further subgroup analyses of clinical trials are needed, as multiple distinct mechanisms could underscore reasons why Asian populations benefit more. Questions include whether this benefit relates to tumor type, PD-L1 expression and gene mutation profiling, amongst others. Other than reporting subgroup analysis data, clinical trials should also report interaction P values. Furthermore, underlying factors from individual patient data would give information of cofounding factors of different ethnic groups.
This is the first meta-analysis of racial disparities of efficacy in cancer patients receiving PD-1/PD-L1 inhibitor. Our meta-analysis has several limitations. First, the meta-analysis was based on published literature, not individual patient data. It is not possible for us to adjust for baseline factors and other differences existed among the clinical trials from which the data were pooled. Second, this meta-analysis aims to investigate if there is an overall benefit for Asian patients regardless of tumor type, however, published data are still limited other than lung and gastric cancer. In our meta-analysis, the clinical studies included only 2 SCLC trials, 1 head and neck, 1 breast, 1 HCC and 1 esophageal cancer. Due to limited trials in certain tumor types or histologies, further subgroup analysis is not possible. Third, in the original studies, ethnicity was self-reported, and there were no standardized definitions across trials. Some trials defined ethnicity based on country or region of accrual, which were analyzed according to primary study subgroup information.
In summary, our meta-analysis is the first study to systematically estimate the efficacy associated with racial difference in cancer patients receiving PD-1/PD-L1 inhibitor. Our data showed that Asian patients was associated with a higher OS and PFS benefit compared with non-Asians.
Authors’ contributions
Prof. Li-Ping Xie and Prof. Justin Stebbing had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Xie, Stebbing, Peng.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript: All authors.
Administrative, technical, or material support: Xie, Stebbing.
Supervision: Xie, Stebbing.
Acknowledgments
We are grateful to the authors of the primary studies.
Disclosure statement
JS’ conflicts can be found at: https://www.nature.com/onc/editors. None are relevant here. No other author declares a conflict.
Data availability statement
No primary data.
Additional information
Funding
References
- Hegde PS, Karanikas V, Evers S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin Cancer Res. 2016;22(8):1865–8. doi:10.1158/1078-0432.CCR-15-1507.
- Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48(3):434–452. doi:10.1016/j.immuni.2018.03.014.
- Kyi C, Postow MA. Immune checkpoint inhibitor combinations in solid tumors: opportunities and challenges. Immunotherapy. 2016;8(7):821–837. doi:10.2217/imt-2016-0002.
- Ramamoorthy A, Pacanowski MA, Bull J, Zhang L. Racial/ethnic differences in drug disposition and response: review of recently approved drugs. Clin Pharmacol Ther. 2015;97(3):263–273. doi:10.1002/cpt.61.
- Ozdemir BC, Dotto GP. Racial differences in cancer susceptibility and survival: more than the color of the skin? Trends Cancer. 2017;3(3):181–197. doi:10.1016/j.trecan.2017.02.002.
- Soo RA, Loh M, Mok TS, Ou SH, Cho BC, Yeo WL, et al. Ethnic differences in survival outcome in patients with advanced stage non-small cell lung cancer: results of a meta-analysis of randomized controlled trials. J Thorac Oncol. 2011;6(6):1030–1038. doi:10.1097/JTO.0b013e3182199c03.
- Cheng Y, Zhang L, Hu J, Wang D, Hu C, Zhou J, et al. KEYNOTE-407 China extension study: pembrolizumab (pembro) plus chemotherapy in Chinese patients with metastatic squamous NSCLC. Ann Oncol. 2019;30(suppl_9):ix183–ix202. doi:10.1093/annonc/mdz446.
- Peng L, Wu YL. Immunotherapy in the Asiatic population: any differences from Caucasian population? J Thorac Dis. 2018;10(Suppl 13):S1482–S93. doi:10.21037/jtd.2018.05.106.
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219. doi:10.1136/bmj.326.7382.219.
- Conforti F, Pala L, Bagnardi V, Viale G, De Pas T, Pagan E, et al. Sex-based heterogeneity in response to lung cancer immunotherapy: a systematic review and meta-analysis. J Natl Cancer Inst. 2019;111(8):772–781. doi:10.1093/jnci/djz094.
- Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28(2):123–137. doi:10.1002/gepi.20048.
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27(5):335–371. doi:10.1016/S0033-0620(85)80003-7.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi:10.2307/2533446.
- Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi:10.1056/NEJMoa1602252.
- Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa1606774.
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi:10.1056/NEJMoa1709937.
- Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus Ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–2104. doi:10.1056/NEJMoa1801946.
- Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–2121. doi:10.1056/NEJMoa1809615.
- Papadimitrakopoulou V, Cobo M, Bordoni R, Dubray-Longeras P, Szalai Z, Ursol G, et al. IMpower132: PFS and safety results with 1L Atezolizumab + Carboplatin/Cisplatin + Pemetrexed in Stage IV non-squamous NSCLC. J Thoracic Oncol. 2018;13(10):S332–S3. doi:10.1016/j.jtho.2018.08.262.
- Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29(10):2052–2060. doi:10.1093/annonc/mdy264.
- Shitara K, Ozguroglu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–133. doi:10.1016/S0140-6736(18)31257-1.
- Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi:10.1056/NEJMoa1810865.
- Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–1939. doi:10.1016/S0140-6736(19)32222-6.
- Reck M, Vicente D, Ciuleanu T, Gettinger S, Peters S, Horn L, et al. Efficacy and safety of nivolumab (nivo) monotherapy versus chemotherapy (chemo) in recurrent small cell lung cancer (SCLC): results from checkMate 331. Ann Oncol. 2018;29(suppl_10):x39–x43. doi:10.1093/annonc/mdy511.
- Yau T, Park JW, Finn RS, Cheng A, Mathurin P, Edeline J, et al. CheckMate 459: a randomized, multi-center phase 3 study of Nivolumab (NIVO) vs Sorafenib (SOR) as First-Line (1L) treatment in patients (pts) with advanced Hepatocellular Carcinoma (aHCC). Ann Oncol. 2019;30(suppl_5):v874–v875. doi:10.1093/annonc/mdz394.029.
- Spigel D, Marinis F, Giaccone G, Reinmuth N, Vergnenegre A, Barrios CH, et al. IMpower110: interim overall survival (OS) analysis of a phase III study of atezolizumab (atezo) vs platinum-based chemotherapy (chemo) as first-line (1L) treatment (tx) in PD-L1–selected NSCLC. Ann Oncol. 2019;30(suppl_5):v851–v934. doi:10.1093/annonc/mdz394.
- Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Abreu DR, Hussein M, et al. IMpower131: final OS results of Carboplatin + Nab-Paclitaxel ± Atezolizumab in advanced squamous NSCLC. J Thoracic Oncol. 2019;14(10):S243–S4. doi:10.1016/j.jtho.2019.08.484.
- Mok TSK, Wu Y, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Final analysis of the phase III KEYNOTE-042 study: pembrolizumab (Pembro) versus platinum-based chemotherapy (Chemo) as first-line therapy for patients (Pts) with PD-L1–positive locally advanced/metastatic NSCLC. Ann Oncol. 2019;30(Supplement_2):ii38–ii68. doi:10.1093/annonc/mdz063.
- Tabernero J, Eric Van Cutsem Y-JB, Fuchs CS, Wyrwicz L, Lee KW, Kudaba I, et al. Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: the phase III KEYNOTE-062 study. J Clin Oncol. 2019;37(18_suppl):LBA4007–LBA. doi:10.1200/JCO.2019.37.18_suppl.LBA4007.
- Kojima T, Muro K, Francois E, Hsu C-H, Moriwaki T, Kim S-B, et al. Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: phase III KEYNOTE-181 study. J Clin Oncol. 2019;37(4_suppl):2. doi:10.1200/JCO.2019.37.4_suppl.2.
- Moehler MH, Dvorkin M, Ozguroglu M, Ryu M-H, Muntean AS, Lonardi S, et al. Results of the JAVELIN Gastric 100 phase 3 trial: avelumab maintenance following first-line (1L) chemotherapy (CTx) vs continuation of CTx for HER2− advanced gastric or gastroesophageal junction cancer (GC/GEJC). J Clin Oncol. 2020;38(4_suppl):278. doi:10.1200/JCO.2020.38.4_suppl.278.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097.
- Nazha B, Mishra M, Pentz R, Owonikoko TK. Enrollment of racial minorities in clinical trials: old problem assumes new urgency in the age of immunotherapy. Am Soc Clin Oncol Educ Book. 2019;39(39):3–10. doi:10.1200/EDBK_100021.
- Varlotto JM, Voland R, McKie K, Flickinger JC, DeCamp MM, Maddox D, et al. Population-based differences in the outcome and presentation of lung cancer patients based upon racial, histologic, and economic factors in all lung patients and those with metastatic disease. Cancer Med. 2018;7(4):1211–1220. doi:10.1002/cam4.1430.
- Liu X, Fiocco M, Swen JJ, Guchelaar HJ. Assessment of ethnic differences in sunitinib outcome between Caucasian and Asian patients with metastatic renal cell carcinoma: a meta-analysis. Acta Oncol. 2017;56(4):582–589. doi:10.1080/0284186X.2016.1265666.
- Chang E, Gong Y, Vallejo JJ, Liu Q, Mathieu LN, Booth B,et al. FDA analysis of outcomes in Asian patients (pts) with metastatic non-small cell lung cancer (mNSCLC) receiving immune checkpoint inhibitors (ICI). J Clin Oncol. 2019;37(15_suppl):e20690–e. doi:10.1200/JCO.2019.37.15_suppl.e20690.
- Juergens RA, Mariano C, Jolivet J, Finn N, Rothenstein J, Reaume MN, et al. Real-world benefit of nivolumab in a Canadian non-small-cell lung cancer cohort. Curr Oncol. 2018;25(6):384–392. doi:10.3747/co.25.4287.
- Garassino MC, Gelibter AJ, Grossi F, Chiari R, Soto Parra H, Cascinu S, et al. Italian Nivolumab expanded access program in nonsquamous non-small cell lung cancer patients: results in never-smokers and EGFR-mutant patients. J Thorac Oncol. 2018;13(8):1146–1155. doi:10.1016/j.jtho.2018.04.025.
- Song P, Zhang J, Shang C, Zhang L. Real-world evidenceand clinical observations of the treatment of advanced non-small cell lung cancer with PD-1/PD-L1 inhibitors. Sci Rep. 2019;9(1):4278. doi:10.1038/s41598-019-40748-7.
- Qian J, Nie W, Lu J, Zhang L, Zhang Y, Zhang B, et al. Racial differences in characteristics and prognoses between Asian and white patients with nonsmall cell lung cancer receiving atezolizumab: an ancillary analysis of the POPLAR and OAK studies. Int J Cancer. 2020;146(11):3124–3133. doi:10.1002/ijc.32717.
- Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 2018;8(7):822–835. doi:10.1158/2159-8290.CD-18-0099.
- Schabath MB, Welsh EA, Fulp WJ, Chen L, Teer JK, Thompson ZJ, et al. Differential association of STK11 and TP53 with KRAS mutation-associated gene expression, proliferation and immune surveillance in lung adenocarcinoma. Oncogene. 2016;35(24):3209–3216. doi:10.1038/onc.2015.375.
- Feng Y, Wang X, Bajaj G, Agrawal S, Bello A, Lestini B, et al. Nivolumab exposure-response analyses of efficacy and safety in previously treated squamous or nonsquamous non-small cell lung cancer. Clin Cancer Res. 2017;23(18):5394–5405. doi:10.1158/1078-0432.CCR-16-2842.
- Bajaj G, Wang X, Agrawal S, Gupta M, Roy A, Feng Y. Model-based population pharmacokinetic analysis of nivolumab in patients with solid tumors. CPT Pharmacometrics Syst Pharmacol. 2017;6(1):58–66. doi:10.1002/psp4.12143.
- Desnoyer A, Broutin S, Delahousse J, Maritaz C, Blondel L, Mir O, et al. Pharmacokinetic/pharmacodynamic relationship of therapeutic monoclonal antibodies used in oncology: part 2, immune checkpoint inhibitor antibodies. Eur J Cancer. 2020;128:119–128. doi:10.1016/j.ejca.2020.01.003.