ABSTRACT
In its latest edition, the WHO classification of the Digestive System Tumors introduced for the first time the immune response as essential and desirable diagnostic criteria for colorectal cancer. The immune response within the tumor microenvironment is therefore clinically relevant. The consensus Immunoscore has a prognostic value that has been confirmed in a meta-analysis on more than 10,000 patients, and it provides a reliable estimate of the recurrence risk in colon cancer. The international validation of the prognostic value of the consensus Immunoscore for time to recurrence, disease-free survival and overall survival in colon cancer together with its predictive value of response to chemotherapy provides valuable information for patient care management.
Current classification of colon cancer
The American Joint Committee on Cancer/Union Internationale Contre le Cancer (AJCC/UICC) TNM staging system is currently the gold-standard classification system for colon cancer. This TNM system relies on histopathological criteria of tumor invasion, namely the extent of the primary tumor (T) and the spread to nearby lymph nodes (N) or to distant metastases (M). This classification has room for improvement in terms of prognostic prediction, as patients within the same stage grouping may have different clinical outcomes. Strikingly, none of the cancer classification systems currently used in clinical practice include immune-based parameters, despite the clear consensus that the state of the immune system of cancer patients strongly influences their survival. The first glimmer of hope for a paradigm shift in the colon cancer classification came recently with the latest (5th) edition of the WHO Digestive System Tumors, which introduced the immune response as essential and desirable diagnostic criteria for colorectal cancer, and cited the consensus Immunoscore assay as the evidence of the immune response prognostic power in colon cancer.Citation1 A meta-analysis of the prognostic value of Immunoscore on more than 10,000 patients also confirmed that the consensus Immunoscore provided a reliable estimate of the recurrence risk in colon cancer.Citation2
The clinical utility of the consensus immunoscore
The Immunoscore is an immunohistochemistry and digital pathology-based scoring system assessing the densities of two lymphocyte populations, the CD3+ and CD8+ T cells, in the tumor and its invasive margin. Briefly, two adjacent slides of formalin-fixed, paraffin-embedded tumor blocks are stained with anti-CD3 and anti-CD8 antibodies in an autostainer. The slides are then scanned and the digital images are used to quantify the densities of the cells of interest with a digital pathology software. The densities are finally translated into an Immunoscore, ranging from Low Immunoscore (I0) to High Immunoscore (I4). We demonstrated that a high density of lymphocytes in both tumor regions correlated with a favorable clinical outcome,Citation3 leading to a major paradigm shift in tumor-immunology.Citation4-Citation6
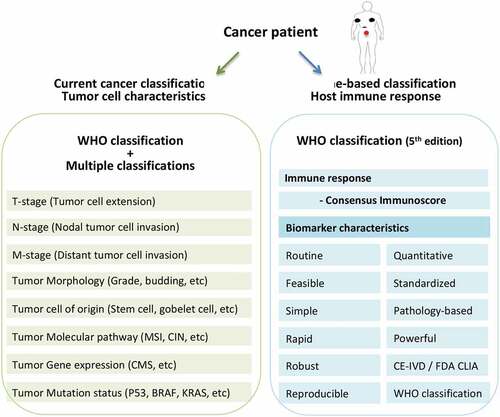
The Immunoscore assay was then standardized and a consortium of 14 pathology expert centers in 13 countries of North America, Europe and Asia, was created to validate the consensus Immunoscore in patients with TNM stage I–III colon cancer. The analytical performances of the consensus Immunoscore assay were tested and demonstrated the feasibility, robustness, reproducibility and accuracy of the assay. The consensus Immunoscore has all the characteristics of a good biomarker, being pathology-based, routine, simple, rapid quantitative, powerful, standardized, CE-IVD, and FDA CLIA-certified (). The international study conducted on more than 2500 patients demonstrated that patients with High Immunoscore had the lowest risk of recurrence, the longest overall survival and the longest disease-free survival. Most importantly, the consensus Immunoscore was shown to predict the risk of relapse with a prognostic value independent and superior to the traditional TNM classification for stage I–III colon cancer.Citation7
Figure 1. Cancer classification systems. All currently used cancer classifications (WHO, AJCC-TNM, UICC-TNM, NCCN, CAP, Asian guidelines, ESMO guidelines, …) are tumor cell-based. The consensus Immunoscore is the first immune-based classification system. It holds all characteristics of a good biomarker, and such immune response has been recommended in the latest edition (5th) of the WHO for colon cancer classification.
Several studies then focused on the prognostic value of the consensus Immunoscore for each stage of colon cancer specifically. A sub-analysis of the stage II patients of the above-described SITC study demonstrated that a high Immunoscore was associated with better clinical outcome.Citation7 Interestingly, this was true as well for the high-risk stage II patients defined as having a tumor with unfavorable histopathological characteristics such as a T4 tumor. Notably, high-risk stage II patients with a high Immunoscore had a risk of recurrence similar to the low-risk stage II patients, suggesting that these patients, usually treated with adjuvant chemotherapy, could be left untreated.
The consensus Immunoscore was also evaluated in stage III colon cancer patients, in a sub-analysis of the SITC study, and was recently validated using cohorts of two randomized phase 3 clinical trials, the N0147Citation8 and the IDEA trials.Citation9 In all cohorts, the Immunoscore was able to stratify stage III colon cancer patients based on their risk of recurrence or death. Strikingly, the patients with a very-high Immunoscore I4, hence a highly infiltrated tumor, had no risk of recurrence for up to 8 y after tumor resection. The Immunoscore is therefore a powerful prognostic marker for stage III colon cancer patients. Additionally, the predictive value of the Immunoscore was evaluated in the IDEA cohort, where patients received either 3 months or 6 months adjuvant FOLFOX chemotherapy. High Immunoscore was shown to significantly predict response to 6 months chemotherapy in all stage III patients. Furthermore, Immunoscore was also predictive of response to 6 months FOLFOX chemotherapy within Low-risk (T1-3 and N1) and high-risk (T4 or N2) tumors.
Overall, the Immunoscore is a significant prognostic consensus marker of survival in stage I–III colon cancer patients, as well as specifically for stage II patients and stage III patients. The Immunoscore is also predictive of response to chemotherapy for stage III patients, highlighting its utility for guiding treatment decision. Importantly, at the metastatic stage, there is not a total immune escape,Citation10 and for stage IV metastatic colon cancer patients, the Immunoscore within metastasis could also predict the risk of relapse and death.Citation11
Toward a new classification of colon cancer
The Immunoscore constitutes the first immune-based scoring system for cancer, and is the first biomarker with a prognostic value superior to the TNM staging system. Being quantitative, reproducible and powerful, the consensus Immunoscore assay gathers the desired features of a reliable biomarker applicable in routine.Citation7 These results, as well as the latest WHO guidelines for digestive cancer classification, advocate for the implementation of the Immunoscore in clinical practice and for the introduction of a new TNM-I classification system taking into account the intra-tumoral adaptive immune system for a better patient stratification. The consensus Immunoscore assay has been developed as an in vitro diagnostic test (CE-IVD) to help guide treatment strategies, and is available in FDA CLIA-certified laboratories for routine use. The Immunoscore, therefore, has the potential to revolutionize colon cancer patient care management. Additional studies are ongoing to investigate its utility in other solid cancer types.
Disclosure of potential conflicts of interest
JG and FP have patents associated with the immune prognostic biomarkers. JG is the co-founder of HalioDx biotech company. Immunoscore® a registered trademark owned by the National Institute of Health and Medical Research (INSERM).
Additional information
Funding
References
- Quezada-Marin J, Lam AK, Ochiai A, Odze R, Washington MK, Fukuyama M, Rugge M, Kimstra DS, Nagtegaal ID, Tan PH, et al. Gastrointestinal tissue-based molecular biomarkers: A practical categorization based on the 2019 WHO classification of epithelial digestive tumours. Histopathology. 2020. PMID: 32320495. doi:10.1111/his.14120.
- Zhang X, Yang J, Du L, Zhou Y, Li K. The prognostic value of immunoscore in patients with cancer: A pooled analysis of 10,328 patients. Int J Biol Markers. 2020:1724600820927409. PMID: 32538254. doi:10.1177/1724600820927409.
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–3. PMID: 17008531. doi:10.1126/science.1129139.
- Galon J, Fox BA, Bifulco CB, Masucci G, Rau T, Botti G, Marincola FM, Ciliberto G, Pages F, Ascierto PA, et al. Immunoscore and immunoprofiling in cancer: an update from the melanoma and immunotherapy bridge 2015. J Transl Med. 2016;14:273. PMID: 27650038. doi:10.1186/s12967-016-1029-z.
- Kirilovsky A, Marliot F, El Sissy C, Haicheur N, Galon J, Pagès F. Rational bases for the use of the Immunoscore in routine clinical settings as a prognostic and predictive biomarker in cancer patients. Int Immunol. 2016;28:373–382. PMID: 27121213. doi:10.1093/intimm/dxw021.
- Pages F, Galon J, Fridman WH. The essential role of the in situ immune reaction in human colorectal cancer. J Leukoc Biol. 2008;84:981–987. PMID: 18559950. doi:10.1189/jlb.1107773.
- Pages F, Mlecnik B, Marliot F, Bindea G, Ou F-S, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, et al. International validation of the consensus immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–2139. PMID: 29754777. doi:10.1016/S0140-6736(18)30789-X.
- Sinicrope FA, Shi Q, Hermitte F, Zemla TJ, Mlecnik B, Benson AB, Gill S, Goldberg RM, Kahlenberg MS, Nair SG, et al. Contribution of immunoscore and molecular features to survival prediction in stage III colon cancer. JNCI Cancer Spectr. 2020;4:pkaa023. PMID: 32455336. doi:10.1093/jncics/pkaa023.
- Pagès F, André T, Taieb J, Vernerey D, Henriques J, Borg C, Marliot F, Ben Jannet R, Louvet C, Mineur L, et al. Prognostic and predictive value of the immunoscore in stage III colon cancer patients treated with oxaliplatin in the prospective IDEA France PRODIGE-GERCOR cohort study. Ann Oncol. 2020;31:921–929. PMID: 32294529. doi:10.1016/j.annonc.2020.03.310.
- Angelova M, Mlecnik B, Vasaturo A, Bindea G, Fredriksen T, Lafontaine L, Buttard B, Morgand E, Bruni D, Jouret-Mourin A, et al. Evolution of metastases in space and time under immune selection. Cell. 2018;175:751–765 e716. PMID: 30318143. doi:10.1016/j.cell.2018.09.018.
- Mlecnik B, Van den Eynde M, Bindea G, Church SE, Vasaturo A, Fredriksen T, Lafontaine L, Haicheur N, Marliot F, Debetancourt D, et al. Comprehensive Intrametastatic Immune Quantification And Major Impact Of Immunoscore On Survival. J Natl Cancer Inst. 2018;110:97–108. PMID: 28922789. doi:10.1093/jnci/djx123.
