Colliding with the ancient concept that chemotherapeutics may delay (and sometimes stop or even reverse) the advancement of cancer because they kill malignant cells, it has become clear that successful treatments with cytotoxicants (and presumably also with some of the so-called targeted agents) only have durable effects when they succeed in stimulating an anticancer immune response. This discovery was spurred by preclinical experiments, unraveling that anthracyclines and other cytotoxicants are much more efficient in controlling the growth of tumors evolving in immunocompetent (as opposed to immunodeficient) mice and then validated in cancer patients in which chemotherapy-induced changes in the immune infiltrate predict the therapeutic response.Citation1,Citation2 One major mechanism through which chemotherapy induces clinically relevant anticancer immunity resides in their capacity to induce immunogenic cell death (ICD), meaning that they kill tumor cells in a way that they become recognizable to the immune system.
Unfortunately, only a fraction of chemotherapeutic agents is capable of stimulating ICD, meaning that only some particularly efficient anticancer agents (such as anthracyclines for breast cancer or oxaliplatin for colon cancer) are able to do so.Citation3,Citation4 This observation has motivated us and others to define the particularities of pharmacological ICD inducers (as compared to non-ICD inducers), leading to the discovery that ICD inducers are endowed with the ability to stimulate a series of premortem stress responses that adjuvantize cancer cells, hence alerting innate immune effectors, in particular dendritic cells (DC) and their precursors. These peculiar ICD-associated stress responses involve autophagy (which facilitates the lysosomal release of ATP from dying cancer cells, causing the emission of a chemotactic signal for DC precursors) as well as the activation of the phosphorylation of eukaryotic initiation factor 2 alpha (eIF2α), a hallmark of endoplasmic reticulum (ER) stress (which facilitates the exposure of the normally ER-resident protein calreticulin to the cell surface, where calreticulin then serves as an ‘eat-me’ signal to facilitate the engulfment of tumor antigens by immature DC.Citation5 Of note, recent work reveals that eIF2α phosphorylation is also required for autophagy induction in some contexts,Citation6 suggesting that both hallmarks of ICD (ATP release and calreticulin exposure) may be mechanistically linked. Additional hallmarks of ICD include the induction of a type-1 interferon response (to stimulate the recruitment of cytotoxic T lymphocytes into the tumor immune infiltrate) as well as the release of annexin A1 and high mobility group A1 (HMGB1) protein from the cytoplasm and nuclei of dead cells respectively, to stimulate correct DC positioning and DC maturation in the tumor bed.Citation1,Citation4
Based on these insights, we have built medium-throughput high-content screening strategies in which human osteosarcoma U2OS cancer cells are equipped with suitable biosensors (to measure ATP release, calreticulin exposure, type-1 interferon signaling and HMGB1 release), then cultured with compound collections, and characterized for the induction of ICD characteristics, followed by validation experiments in vitro (with other methods and on other cell lines) and in vivo (to measure the effective induction of anticancer immune responses in mouse models).Citation7,Citation8 Several large screens including the measurement of additional stress responses (like all arms of the ER stress response) led to the generation of a data bank, instructing us on the facts that (i) induction of other types of ER stress than eIF2α phosphorylation was not activated upon ICD induction and (ii) that anticancer drugs must have a specific set of physicochemical characteristics that define their propensity to induce ICD, allowing to use artificial intelligence to create a mathematical model, which, based on molecular descriptors, yield a theoretical ‘ICD score’.Citation5 When computing this ICD score to a library of 50,000 agents, we found that one of the top hits was dactinomycin (DACT, best known as actinomycin D), an agent that has been used by myriads of molecular biologists to inhibit transcription but that is also employed for the chemotherapy of sarcomas. Intrigued by this observation, we used multiple tests to measure RNA synthesis (and downstream protein synthesis, downstream of RNA transcription) to conclude that most ICD inducers (including anthracyclines, oxaliplatin, lurbinectedin, crizotinib and thiostrepton) actually cause an inhibition of DNA-to-RNA transcription (and hence a subsequent inhibition of RNA-to-protein translation) and that this effect may cause a peculiar ER stress response consisting in the phosphorylation of eIF2α by eIF2α kinase 3 (EIF2AK3, better known as PERK) without any other signs of the unfolded stress response such as activation of the ATF6 and the IRE1-XBP1 axes.Citation8–Citation10 The aforementioned results suggest that a vast class of ICD inducers (with the exception of microtubular poisons such as vinca alkaloids and taxanes) is able to reduce RNA synthesis, which resembles a response to viral infection. This has two major implications. On one hand, it is possible to consider anticancer agents with known transcription-inhibitory properties as candidates for ICD induction.Citation9 On the other hand, it is relatively easy to measure inhibition of RNA synthesis in vitro, on cultured cells, for example using a chemically derivatized uridine analogue whose incorporation into nascent RNA, can be visualized by click chemistry to yield a fluorescent signal (). An alternative method to diagnose stalled transcription consists in detecting the separation of two proteins involved in RNA synthesis, nucleolin and fibrillarin, that normally (when RNA synthesis is active) colocalize in the nucleus, yielding an overlapping immunofluorescence staining ().Citation9
Figure 1. Principle of the measurement of transcription inhibition.
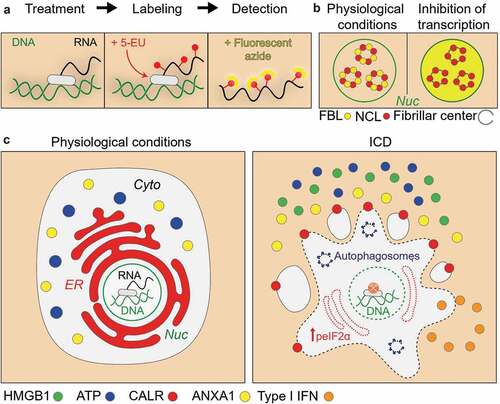
Hence, these types of tests might be easily added to the current compendium of assays to measure immunogenic stress events induced by anticancer agents (), adding yet another criterion to discriminate agents with a high potential of ICD induction from agents with a lower probability to kill cancer cells in an immunogenic fashion. Thus, this information could be fed into existing and yet-to-be-developed databanks to improve the algorithm calculating the ‘ICD score’, further refining the approach leading to the identification of ICD inducers.
Disclosure of Potential Conflicts of Interest
GK and OK are cofounders of Samsara Therapeutics.
Acknowledgments
GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Association “Le Cancer du Sein, Parlons-en!”; Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Gustave Roussy Odyssea, the European Union Horizon 2020 Project Oncobiome; Fondation Carrefour; High-end Foreign Expert Program in China (GDW20171100085), Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology (ANR-18-IDEX-0001); the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and the SIRIC Cancer Research and Personalized Medicine (CARPEM).
References
- Galluzzi L, Vitale I, Warren S, Adjemian S, Agostinis P, Martinez AB, Chan TA, Coukos G, Demaria S, Deutsch E, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer. 2020;8(1):e000337. doi:10.1136/jitc-2019-000337.
- Kroemer G, Senovilla L, Galluzzi L, Andre F, Zitvogel L. Natural and therapy-induced immunosurveillance in breast cancer. Nat Med. 2015;21(10):1128–4. doi:10.1038/nm.3944.
- Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482–491. doi:10.1038/onc.2009.356.
- Vacchelli E, Ma Y, Baracco EE, Sistigu A, Enot DP, Pietrocola F, Yang H, Adjemian S, Chaba K, Semeraro M, et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science. 2015;350(6263):972–978. doi:10.1126/science.aad0779.
- Bezu L, Sauvat A, Humeau J, Gomes-da-Silva LC, Iribarren K, Forveille S, Garcia P, Zhao L, Liu P, Zitvogel L, et al. eIF2alpha phosphorylation is pathognomonic for immunogenic cell death. Cell Death Differ. 2018;25:1375–1393. doi:10.1038/s41418-017-0044-9.
- Humeau J, Leduc M, Cerrato G, Loos F, Kepp O, Kroemer G. Phosphorylation of eukaryotic initiation factor-2α (eIF2α) in autophagy. Cell Death Dis. 2020; 11(6):433. doi10.1038/s41419-020-2642-6
- Liu P, Zhao L, Pol J, Levesque S, Petrazzuolo A, Pfirschke C, Engblom C, Rickelt S, Yamazaki T, Iribarren K, et al. Crizotinib-induced immunogenic cell death in non-small cell lung cancer. Nat Commun. 2019;10(1):1486. doi:10.1038/s41467-019-09415-3.
- Wang Y, Xie W, Humeau J, Chen G, Liu P, Pol J, Zhang Z, Kepp O, Kroemer G. Autophagy induction by thiostrepton improves the efficacy of immunogenic chemotherapy. J Immunother Cancer. 2020;8(1):e000462. doi:10.1136/jitc-2019-000462.
- Humeau J, Sauvat A, Cerrato G, Xie W, Loos F, Iannantuoni F, Bezu L, Lévesque S, Paillet J, Pol J, et al. Inhibition of transcription by dactinomycin reveals a new characteristic of immunogenic cell stress. EMBO Mol Med. 2020;12(5):e11622. doi:10.15252/emmm.201911622.
- Xie W, Forveille S, Iribarren K, Sauvat A, Senovilla L, Wang Y, Humeau J, Perez-Lanzon M, Zhou H, Martínez-Leal JF, et al. Lurbinectedin synergizes with immune checkpoint blockade to generate anticancer immunity. Oncoimmunology. 2019;8(11):e1656502. doi:10.1080/2162402X.2019.1656502.
