ABSTRACT
Pancreatic ductal adenocarcinoma (PDAC) is characterized by a prominent stromal reaction that has been variably implicated in both tumor growth and tumor suppression. B-lymphocytes have been recently implicated in PDAC progression but their contribution to the characteristic stromal desmoplasia has never been assessed before. In the present work, we aimed to verify whether B-lymphocytes contribute to stromal cell activation in PDAC. CD19+ B-lymphocytes purified from peripheral blood of patients with PDAC were cultivated in the presence of human pancreatic fibroblasts and cancer-associated fibroblasts. Released pro-fibrotic soluble factors and collagen production were assessed by ELISA and Luminex assays. Quantitative RT-PCR was used to assess fibroblast activation in the presence of B cells. The expression of selected pro-fibrotic and inflammatory molecules was confirmed on PDAC tissue sections by multi-color immunofluorescence studies. We herein demonstrate that B-cells from PDAC patients (i) produce the pro-fibrotic molecule PDGF-B and stimulate collagen production by fibroblasts; (ii) express enzymes implicated in extracellular matrix remodeling including LOXL2; and (iii) produce the chemotactic factors CCL-4, CCL-5, and CCL-11. In addition we demonstrate that circulating plasmablasts are expanded in the peripheral blood of patients with PDAC, stimulate collagen production by fibroblasts, and infiltrate pancreatic lesions. Our results indicate that PDAC is characterized by perturbations of the B-cell compartment with expansion of B-lymphocyte subsets that directly contribute to the stromal reaction observed at disease site. These findings provide an additional rationale for modulating B-cell activity in patients with pancreatic cancer.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive solid tumors and the fourth leading cause of cancer-related mortality worldwide.Citation1 Escape from immune-surveillance, resistance to standard chemotherapies and early metastatic potential are considered the main reasons for this dismal prognosis .Citation1
In apparent contrast with this aggressiveness, the majority of the tumor volume in PDAC is not made of malignant cells, but of a desmoplastic reaction consisting of cancer-associated fibroblasts (CAF) and immune cells .Citation2,Citation3 Pancreatic CAF are believed to originate from different cellular sources, including pancreatic stellate cells, mesenchymal stem cells (MSC) resident fibroblasts, and epithelial cells .Citation4,Citation5 Although CAF have been classically associated with tumor growth, immune suppression, and metastatic dissemination, recent evidences have challenged these tumor-promoting properties and showed more aggressive PDAC behavior in CAF deprived mouse models.Citation2–Citation8 These findings suggest a complex network of signals between PDAC and CAF that is not uniformly stimulatory or inhibitory, and possibly support the existence of different tumor-promoting and tumor-suppressing populations of CAF. Indeed, a previously unappreciated heterogeneity in PDAC fibroblasts has been recently observed, with tumor-promoting subsets of CAF expressing variable combinations of activation and mesenchymal stem cell markers such as the secreted protein acidic and rich in cysteine (SPARC), the encoding fibroblast activation protein (FAP), COL1A1, COL1A2, and COL3A1 collagen genes, CD73, and CD90.Citation9,Citation10 In the rapidly evolving field of PDAC, understanding of the molecular mechanisms driving CAF differentiation and activation could lead to the identification of novel mechanistic insights as well as to innovative therapeutic targets.
Based on our recent findings about B cells promoting tissue fibrosis in IgG4-related type-1 autoimmune pancreatitis (AIP), we here hypothesize that B lymphocytes might contribute to the prominent stromal reaction observed in PDAC by promoting CAF activation, collagen secretion, and extracellular matrix (ECM) remodeling.Citation11,Citation12 This previously overlooked hypothesis stems from multiple clinical and pathological analogies existing between type-1 AIP and PDAC. Both conditions, in fact, present with tumefactive lesions of the pancreatic gland, and display common histological features that typically complicate the differential diagnosis, such as a dense stromal reaction and an abundant lymphoplasmocytic infiltrate rich in IgG4+ plasma cells .Citation13–Citation17
Results
B lymphocytes from patients with pancreatic adenocarcinoma secrete soluble factors that stimulate collagen production by pancreatic MSC and CAF
To demonstrate pro-fibrotic properties of B lymphocytes in PDAC, we set up co-cultures of human pancreatic fibroblasts with CD19+ B cells isolated from the peripheral blood of five patients with PDAC, and performed transcriptomic studies of cultured fibroblasts. In particular, human pancreatic MSC and B cells from PDAC patients were first used to model in vitro some of the possible interactions occurring between these two cell types in vivo. B cells purified from the peripheral blood of five sex- and age-matched healthy donors were used as controls. Up-regulation of selected genes was assessed, based on our previous findings showing activation of epithelial-to-mesenchymal transition trascriptomic pathways in fibroblast co-cultured with B cells from patients with IgG4-related AIP.Citation12 As shown in , expression of COL1A1, COL1A2, and COL3A1 collagen genes, and α-Smooth Muscle Actin (ACTA2) in pancreatic MSC was significantly up-regulated in the presence of B cells. Accordingly, collagen production in the co-cultures of MSC with B cells from PDAC patients was significantly increased compared to MSC cultured alone (). The expression of COL1A1, COL1A2, and COL3A1 genes, but not of ACTA2, was also significantly increased in fibroblasts co-cultured with B cells from healthy donors ().
Figure 1. B lymphocytes from patients with PDAC secrete soluble factors that induce collagen production by human pancreatic fibroblasts.
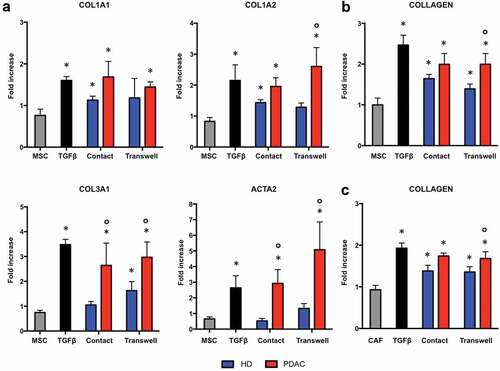
To assess whether contact between B cells and fibroblasts is required for collagen production, co-culture experiments were repeated using a semipermeable membrane to separate these two cell types. As shown in , co-culture with and without separation using semipermeable membranes (transwells) both led to a significant increase in collagen secretion compared to the incubation of fibroblasts without B cells. Collagen production in the co-cultures of fibroblasts with B cells from PDAC patients was significantly higher compared to co-cultures of fibroblasts with B cells from healthy donors. Similar results were obtained using CAF isolated from 5 PDAC patients co-cultured with B cells from healthy individuals and from patients with PDAC, suggesting that phenotypic differences between MSC and CAF (reported in Supplementary Figure 1) were not responsible for the different reactions observed with B-cells from healthy individuals and PDAC patients (). Taken together, these findings demonstrate that CD19+ B lymphocytes from patients with PDAC and, to lesser extent, from healthy individuals induce collagen production by pancreatic MSC and CAF through the secretion of soluble factors.
Platelet-derived growth factor-B is increased in the co-cultures of human fibroblasts with B lymphocytes from patients with pancreatic adenocarcinoma
To identify pro-fibrotic soluble mediators secreted by B lymphocytes in the co-culture system, we quantitated the level of cytokines and chemokines implicated in tissue fibrosis using a Luminex-based approach. As shown in , the concentrations of platelet-derived growth factor-B (PDGF-B) was significantly higher when MSC were challenged with B cells compared to MSC alone. The effect of the co-incubation was specific, since the concentration of other pro-fibrotic molecules such as IL-1α, IL-4, IL-5, IL-10, IL-13, IFNγ, TNFα, and TGFβ did not differ between MSC alone and MSC co-cultured with B cells from either PDAC patients or healthy donors. The chemokines CCL-4 (MIP1β), CCL-5 (RANTES), and CCL-11 (EOTAXIN) were also significantly increased in the presence of B cells from PDAC patients and from healthy subjects. In particular, CCL-4 and CCL-5 production was induced by the presence of B lymphocytes while low levels of PDGF-B and CCL-11 were constitutively secreted by untreated MSC. The levels of PDGF-B, CCL-4, CCL-5, and CCL-11 were similar in co-cultures of MSC with B cells from PDAC patients and from healthy donors, and in co-cultures in which B cells and MSC were separated or not by semipermeable membranes ().
Figure 2. PDGF-B, CCL-4, CCL-5, and CCL-11 are increased in the co-cultures of pancreatic MSC with B lymphocytes.
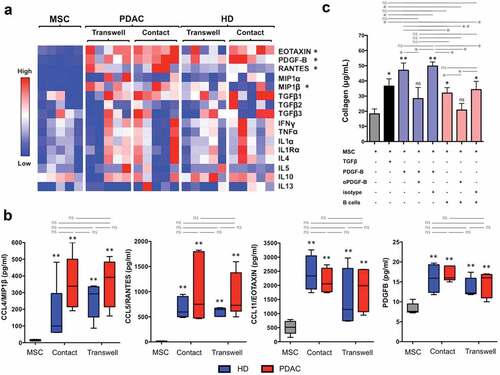
These results implicate PDGF-B as a mediator of the fibrotic action exerted by B lymphocytes but do not clarify whether PDGF-B is primarily secreted by B cells, by fibroblasts upon B-cell mediated activation, or by both cells. These results also suggest that MSC stimulated by B cells or perhaps B cells themselves, secrete chemokines that might contribute to the recruitment of inflammatory cells in vivo.
PDGF-B mediates B-lymphocyte dependent collagen production by human fibroblasts
In order to assess the biological relevance of PDGF-B in our model, we used an anti-PDGF-B antibody or an isotype control to interfere with collagen production. As shown in , the addition of anti-PDGF-B antibody, but not of irrelevant IgG of the same isotype, significantly reduced collagen secretion induced by B cells from patients with PDAC. Indeed, B lymphocytes might activate MSC either through the production of PDGF-B or by inducing the secretion of PDGF-B by activated fibroblasts, or by both mechanisms, thus perpetuating a paracrine/autocrine pro-fibrotic loop. Of note, B cells from PDAC patients induced significantly higher amounts of collagen in co-culture with pancreatic MSC compared to B cells from healthy individuals even if levels of PDGF-B in the supernatant were similar in the two conditions (). Thus factors other than PDGF-B might also contribute to the fibrotic effect of B cells from PDAC patients.
B lymphocytes and fibroblasts infiltrating pancreatic adenocarcinoma express PDGF-B, CCL-4, CCL-5, and CCL-11
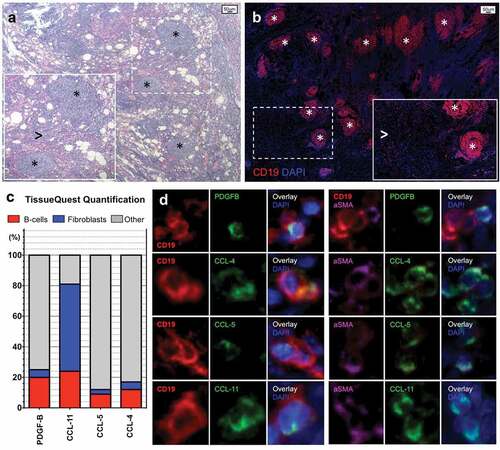
To determine whether B cells or pancreatic fibroblasts represent the cellular source of PDGF-B, CCL-4, CCL-5, and CCL-11 in our co-culture system and to confirm the relevance of these molecules to the characteristic stromal reaction observed in PDAC, we performed multicolor immunofluorescence and quantitative analysis on tissue sections of 5 PDAC patients. Infiltrating CD19+ B cell was either organized in tertiary lymphoid structures or spread throughout the tissue (). Quantification analysis showed that CD19+ B lymphocytes expressing either PDGF-B, CCL-4, CCL-5, or CCL-11 represented on average 20.9% (range 10.2–21.0), 12.2% (range 9.2–15.4), 9.2% (range 5.4–16.4), and 24.1% (range 19.1–31.6), of total PDGF-B, CCL-4, CCL-5, CCL-11 expressing cells, respectively (). α-SMA+ fibroblasts expressing either PDGF-B, CCL-4, CCL-5, or CCL-11 represented on average 4.9% (range 4.3–9.5), 5.0% (range 3.4–7.1), 3.1% (range 0.9–5.1), and 57.9% (range 47.5–62.4) of total PDGF-B, CCL-4, CCL-5, and CCL-11 expressing cells, respectively (). These findings indicate that the pro-fibrotic molecule PDGF-B and the inflammatory chemokines CCL-4, CCL-5, and CCL-11 are produced by both B lymphocytes and myofibroblasts infiltrating PDAC lesions in vivo. In particular, while B-lymphocytes seem capable of expressing relevant amounts of all four cytokine and chemokines in vivo, fibroblasts primarily express large amounts of CCL-11. These results also suggest that B lymphocytes represent a source of PDGF-B, CCL-4, CCL-5, and CCL-11 in the co-culture studies described above, and mirror the relatively minor contribution of fibroblasts to the secretion of CCL-4 and CCL-5 (). Indeed, transcriptomic analysis performed on circulating B-cells from PDAC patients and on pancreatic MSC from the co-cultures revealed that B-lymphocytes constitutively express PDGF-B and that they significantly up-regulate PDGF-B expression by co-cultured fibroblasts compared to fibroblasts alone (Supplementary Figure 2).
Figure 3. B-lymphocytes and fibroblasts infiltrating pancreatic adenocarcinoma express PDGF-B, CCL-4, CCL-5, and CCL-11.
Circulating plasmablasts are expanded in patients with pancreatic adenocarcinoma and induce collagen production by human fibroblasts
As shown in , B lymphocytes from both PDAC patients and healthy subjects were found to increase collagen production in co-cultures with pancreatic MSC but collagen concentration was higher in the presence of B cells from PDAC patients than from healthy subjects. Similar results were obtained by using B cells from patients with IgG4-RD AIP and were attributed to previously unanticipated pro-fibrotic properties of circulating plasmablasts.Citation12 Indeed, plasmablasts, the precursors of tissue resident plasma cells, are oligoclonally expanded in the blood of patients with IgG4-RD AIP and activate fibroblasts in co-culture experiments.Citation12,Citation18-Citation20 We therefore hypothesized the existence of fibrogenic B-lymphocyte subpopulations in PDAC as well and compared the levels of circulating plasmablasts, naive and memory B cells in 20 patients with resectable pancreatic cancer (mean age 66; range 56–75; male to female ratio = 12:8) and 20 age- and sex-matched healthy controls by flow cytometry. As shown in , circulating plasmablasts, but not naïve and memory B cells, were significantly higher in patients with PDAC than in healthy individuals both as absolute numbers () and as percentage of total CD19+ cells (Supplementary Figure 3a).
Figure 4. Pro-fibrotic role of plasmablasts/plasma cells in pancreatic adenocarcinoma.
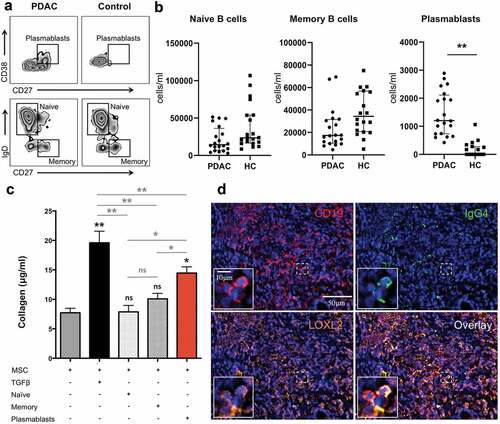
To assess the pro-fibrotic properties of circulating plasmablasts in the setting of PDAC, we set up co-cultures of pancreatic MSC with naïve B cells, memory B cells, and plasmablasts from PDAC patients isolated by sorting via flow-cytometry. Addition of circulating plasmablasts resulted in the production of significantly more collagen compared to that produced constitutively by MSC alone, or by fibroblasts challenged with naïve or with memory B cells ().
LOXL2 is expressed by plasmablasts/plasma cells in pancreatic adenocarcinoma
As we previously reported, plasmablasts represent a subset of B lymphocytes with intrinsic fibrotic properties because, in contrast to naïve and memory B cells, in addition to PDGF-B, they express a set of genes implicated in positive regulation of fibroblast proliferation such as COL1A1 and COL1A2 collagen genes, insulin-like growth factor-1 (IGF-1), lysyl oxidase homolog 2 (LOXL2), and gremlin-1 (GREM-1).Citation8 In particular, LOXL2, a member of the lysyl oxidases family, controls ECM stiffness by crosslinking collagen fibers and supports the pathologic stromal microenvironment in various solid tumors and fibrotic diseases.Citation21–Citation29 To demonstrate LOXL2 expression by B-lymphocytes infiltrating PDAC we performed multicolor immunofluorescence studies and found that CD19+ cells accounted for up to 12.6% of total LOXL2 expressing cells in PDAC (average 8.9%; range 7.3%–12.6%) (Supplementary Figure 3b). LOXL2 expression by class-switched plasmablasts or plasma cells was further confirmed by additional immunofluorescence for CD19 and IgG4 double-positive cells, a subset of B lymphocytes that has been reported in PDAC biopsiesCitation9 (). Of note, plasmablasts from PDAC patients also expressed PDGF-B on RNA-seq analysis, suggesting multiple intrinsic pro-fibrotic properties of this antigen experienced class-switched B-cell subpopulation (Supplementary Figure 1b)
Discussion
The disproportion between stromal elements and the less prevalent neoplastic epithelial cells represents a hallmark pathological feature of PDAC but the contribution of this characteristic desmoplastic reaction to cancer progression remains lively debated.Citation2 While stromal cells have been classically thought to favor tumor growth and dissemination, recent evidence have shown that CAF can also hamper PDAC development by restraining angiogenetic phenomenaCitation2-Citation8,Citation30–Citation33 In addition, CAF seem to exert diverse effects on pancreatic cancer progression – either inhibitory or stimulatory – depending on their relative proportion to neoplastic cells.Citation34 Accordingly, although selective blockade of signals associated with CAF activation improves survival in animal models, interference with these signals has not proven as effective when translated into clinical trials on pancreatic and non-pancreatic cancers.Citation2,Citation7,Citation30 All together, these findings indicate that, besides being tightly integrated and probably redundant, tumor-stroma interactions are not uniformly suppressive or supportive. Understanding the biology of the stromal compartment in PDAC is, therefore, key to intercept relevant pathways implicated in tumor spreading and immune-surveillance.
In the present work we describe for the first time that B lymphocytes actively contribute to fibrogenesis in PDAC and introduce a novel player involved in CAF differentiation and activation. In addition, we demonstrate that circulating plasmablasts with fibrogenic properties are expanded in the peripheral blood of patients with PDAC and in neoplastic lesions, reflecting unexpected perturbations of the B-cell compartment in pancreatic cancer. Indeed, B lymphocytes have been already shown to infiltrate neoplastic lesions and pre-malignant pancreatic intraepithelial neoplasia (PanIN), but the role of tumor-infiltrating B cells (TIL-Bs) in PDAC did not receive much attention until recent .Citation35–Citation38 B cells infiltrating PDAC, in fact, have been traditionally assumed to bear a regulatory phenotype and to support malignant cells growth by hampering antitumor immunity in analogy with other solid tumors.Citation35–Citation39 Compelling evidence for the involvement of TIL-Bs in pancreatic tumorigenesis came only recently, when B-cells were shown to contribute to PDAC progression through diverse mechanisms, including paracrine secretion of IL-35 – a proliferative stimulus for transformed epithelial cells – and suppression of cytotoxic T-cells. Accordingly, prevention of B-cell infiltration into the tumor by blocking the B-cell chemo attractant CXCL13, by inhibiting B-cell activity using a Bruton tyrosine kinase inhibitor, or by simple depletion of B cells, all reduced tumor progression in preclinical models of PDAC.Citation40–Citation42
By demonstrating a direct interaction with pancreatic MSC and CAF, our results suggest an additional and previously overlooked contribution of B lymphocytes to PDAC pathophysiology via direct orchestration of stromal activation. In particular, B lymphocytes (i) induced epithelial-to-mesenchymal transition of myofibroblast precursors; (ii) stimulated collagen production by pancreatic fibroblasts through PDGF-B; (iii) regulated extracellular matrix stiffness through specialized enzymes such as LOXL2; and (iv) secreted chemotactic factors for putative fibrogenic immune-cells (). Of note, production of both PDGF-B and LOXL2 by malignant cells has been already demonstrated in PDAC lesions, while secretion of these molecules by TIL-Bs has never been observed before.Citation21–Citation26,Citation43,Citation44 PDGF-B is a member of the PDGF family and contributes to tissue fibrosis by stimulating fibroblast proliferation and collagen production through its tyrosine kinases receptor.Citation45 Depending on its expression level, PDGF-B has been also found to regulate neoplastic cell proliferation, vascular sprouting, and metastatic potential in a variety of solid tumors.Citation43,Citation44 LOXL2 is an enzyme that catalyzes the cross-linking of collagen components in the ECM, a process that controls the structure of the ECM, the tensile strength of collagen bundles, and the differentiation of quiescent fibroblasts into activated myofibroblasts.Citation24–Citation29,Citation46 LOXL2 has been also implicated in carcinogenesis and invasiveness of pancreatic cancer cells, and its inhibition has been shown to reduce tumor volume and metastases in preclinical studies.Citation21,Citation22 In addition, increased LOX2 activity has been shown to promote resistance to gemcitabine, and its expression in PDAC biopsies has been associated with a poorer prognosis.Citation22 B cells also secreted CCL-4, CCL-5, and CCL-11, chemotactic factors for cells with fibrogenic properties that are typically found in PDAC lesions, such as T lymphocytes, macrophages, and eosinophils.Citation47–Citation50 CCL-4 is a chemokine involved in monocyte recruitment and activation by engaging the chemokine receptor CCR5;Citation51 CCL-5 attracts T cells, eosinophils, and basophils, by interacting with CCR1, CCR3, and CCR5 ;Citation52 and CCL-11 selectively recruits eosinophils through CCR2, CCR3, and CCR5.Citation53 Moreover, according to our previous findings, B cells might stimulate fibroblasts secretion of these same chemokines, thus amplifying the recruitment of inflammatory cells at tumor site and further sustaining the desmoplastic reaction.Citation12 Finally, B cells from PDAC patients induced the expression of genes associated with a pro-tumorigenic stroma such as COL1A1, COL1A2, and COL3A1 collagen genes. Indeed, overexpression of COL1A1, COL1A2, COL3A1 – in addition to SPARC and FAP that we previously found overexpressed in pancreatic MSC exposed to B lymphocytes from patients with IgG4-RD AIP – has been identified by Moffitt and colleagues as a characteristic gene signature of “activated” tumor-promoting stroma in human PDAC.Citation9,Citation12,Citation54 If confirmed on further in vivo studies, these preliminary findings may suggest that B lymphocytes contribute to the complex interplay between CAFs and tumor cells by favoring the acquisition of pro-tumorigenic properties by pancreatic fibroblasts.
Figure 5. Contribution of B lymphocytes to pancreatic adenocarcinoma growth and dissemination.
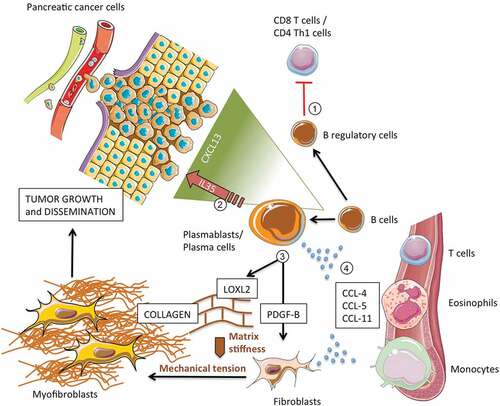
Another major finding of our study is the expansion of plasmablasts in the peripheral blood of patients with PDAC. Plasmablasts, identified as CD19+CD20−CD27+CD38+ cells by flow cytometry, may arise extra-folliculary or in germinal centers after affinity maturation from CD20+ naive precursors, enter the circulation and home to inflammatory niches or to the bone marrow, where they differentiate into antibody-secreting long-lived plasma cells.Citation18 Some plasmablasts may differentiate into short-lived plasma cells at the site of tissue reactivation, including the tumor milieu. Plasmablasts can circulate for prolonged periods in the setting of chronic antigenic stimulation or autoimmune diseases, but are generally infrequent in the peripheral blood of healthy individuals.Citation55 As opposite to naïve and memory B cells, circulating plasmablasts from patients with PDAC increased collagen production in co-culture with human fibroblasts, thus confirming the intrinsic pro-fibrotic properties of this B-cell population that we previously reported in another fibrotic disorder.Citation12 In this sense, it is tempting to speculate that the difference in collagen production observed in the co-cultures of fibroblasts with total B cells from PDAC patients and controls is likely due to the significant increase in circulating plasmablasts in patients with pancreatic cancer. Finally, in analogy with what observed in tissues affected by IgG4-related AIP, LOXL2 expressing class-switched plasmablasts/plasma cells were also found to infiltrate tumor lesions in PDAC, supporting their direct contribution to stromal activation at disease site. Whether plasmablasts also bear a regulatory phenotype, down-modulate antitumor immune-response, and promote neoplastic cell proliferation remains to be elucidated.
Our study has both strengths and weaknesses. A major potential strength is the adoption of human samples for co-culture experiments. On the one hand, MSC and CAF may have allowed us to recapitulate some of the possible interactions occurring between mesenchymal cells and B cells in vivo in PDAC lesions. On the other hand, by using freshly isolated B lymphocytes from healthy donors and patients with PDAC we focused on direct pro-fibrotic interactions occurring between B cells and fibroblasts, and avoided confounding factors. However, despite we generated compelling evidence for the involvement of B cells in the stromal reaction associated with PDAC, we also recognize that our study is limited to in vitro and histological data and lacks in vivo validation. In addition, our co-culture system based on B cells and fibroblasts excludes the possibility of other cell types being relevant to the stromal reaction occurring at tissue level.
In conclusion, our work provides novel insights into the pathophysiology of the stromal reaction associated with PDAC by unveiling perturbations of the B cell compartment and unexpected pro-fibrotic properties of B lymphocytes in patients with pancreatic cancer. These findings indicate that B cells might contribute to PDAC progression over and beyond the prevalent view of TIL-Bs functioning primarily as immunosuppressive cells and promoters of epithelial cell transformation. In addition, our study paves the way for further researches aimed at clarifying the role of B cells in the complex interplay between stromal elements and tumor cells and in the differentiation of pancreatic fibroblasts into tumor-promoting or tumor-suppressing CAF. Although much work needs to be done to decipher tumor-stroma interactions in PDAC, our data reinforce the notion that future therapeutic strategies for PDAC should not be limited to neoplastic cells or the stromal compartment, but should rather combine agents that target the tumor, the stroma, and immune-regulatory mechanisms.
Materials and methods
Patients
B cells from 35 patients with PDAC and 25 sex- and age-matched healthy donors were studied. For in vitro experiments, peripheral blood mononuclear cells (PBMC) were obtained before surgery, chemotherapy, or radiotherapy from 20 patients with histologically proven resectable T3M0 PDAC referred to the Division of Pancreatic Surgery of San Raffaele Scientific Institute (Milan, Italy) between September 2015 and September 2019. Five sex-and age-matched healthy donors were included as controls. Pancreatic glands from n = 5 patients with PDAC from the Pathology unit of Massachusetts General Hospital were used for immunofluorescence studies. For flow-cytometry quantification of B cell subsets we used blood samples from 20 patients with resectable T3M0 PDAC and 20 age- and sex-matched healthy individuals.Citation56,Citation57 All subjects enrolled provided written informed consent for the analyses performed. The study was conducted according to the Declaration of Helsinki and approved by the Ethical Committees of the San Raffaele Scientific Institute and Massachusetts General Hospital.
Flow cytometry and microscopy
A detailed description of the methods used for PBMC isolation, flow cytometry, cell sorting, immunohistochemistry, and immunofluorescence is provided in the Methods section of this article’s Online Repository.
Co-culture experiments
Co-culture experiments were carried out with B cells and B-cell subsets from patients with PDAC, primary human pancreatic fibroblasts, and CAF. Polycarbonate semipermeable membranes (Nunc Dominique Dutscher, Brumath, France) were used to investigate soluble factors or contact-mediated pro-fibrotic mechanisms. Soluble collagen concentration in the supernatant of fibroblasts/CD19 + B cells co-cultures was evaluated using Sircol collagen assay (Biocolor Ltd. Interchim, Montluçon, France). Soluble collagen concentration in the supernatant of fibroblasts/B-cells subsets co-cultures was evaluated using the Pro-collagen Iα1 ELISA kit (R&D Systems Inc., Minneapolis, MN, USA). Cytokines and chemokines in the supernatant of fibroblasts/CD19 + B cells co-cultures were measured using the Bio-Plex Pro Human Cytokine Grp I Panel 27-plex assay (Bio-Rad, Hercules, CA, USA). A detailed description of the methods used for the establishment of primary human pancreatic fibroblasts lines and of co-cultures with total B cells and B-cell subsets is provided in the Methods section of this article’s Online Repository.
Quantitative real-time polymerase chain reaction analysis
Total RNA was extracted from cultured fibroblasts using ReliaPrep™ RNA Cell Miniprep System (Promega) and reverse transcribed with random hexameric primers. Real-time quantitative PCR for human GAPDH, COL1A1, COLA1A2, COL3A1, ACTA2, and CD19 genes was performed with the KAPA SYBR® FAST qPCR Master Mix (2X) Kit (Bio-Rad, Hercules, CA, USA) using a Roche LightCycler® (Roche Molecular Diagnostic, Pleasanton, CA, USA).
Statistical analysis
Statistical analysis was performed using GraphPad Prism software 6.0 (La Jolla, CA, USA). Normal distribution of continuous variables was assessed with the D’Agostino & Pearson omnibus normality test. Non-normally distributed variables were compared using the Mann-Whitney U-test. Normally distributed variables were compared using Unpaired t-test. A p-value < 0.05 was considered significant. Continuous variables are expressed as mean ± standard deviation (SD), unless otherwise specified.
Disclosure
The authors have not received any financial support or other benefits from commercial sources for the work reported in the manuscript, or any other financial interests that could create a potential conflict of interest or the appearance of a conflict of interest with regard to the work.
Supplemental Material
Download ()Disclosure statement
Nothing to report.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–10. doi:10.1016/S0140-6736(10)62307-0.
- Rucki AA, Zheng L. Pancreatic cancer stroma: understanding biology leads to new therapeutic strategies. World J Gastroenterol. 2014;20(9):2237–2246. doi:10.3748/wjg.v20.i9.2237.
- Neesse A, Algül H, Tuveson DA, Gress TM. Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut. 2015;64(9):1476–1484. doi:10.1136/gutjnl-2015-309304.
- Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, Mc-Intyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–1461. doi:10.1126/science.1171362.
- Bruun Nielsen MF, Mortensen MB, Detlefsen S. Key Players in Pancreatic Cancer-Stroma Interaction: cancer-associated Fibroblasts, Endothelial and Inflammatory Cells. World J Gastroenterol. 2016;22(9):2678–2700. doi:10.3748/wjg.v22.i9.2678.
- Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–1616. doi:10.1126/science.1198443.
- Heinemann V, Reni M, Ychou M, Richel DJ, Macarulla T, Ducreux M. Tumour-stroma interactions in pancreatic ductal adenocarcinoma: rationale and current evidence for new therapeutic strategies. Cancer Treat Rev. 2014;40(1):118–128. doi:10.1016/j.ctrv.2013.04.004.
- Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva E, Saunders T, Becerra C, Tattersall I, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25(6):735–747. doi:10.1016/j.ccr.2014.04.021.
- Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, Rashid NU, Williams LA, Eaton SC, Chung AH, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47(10):1168–1178. doi:10.1038/ng.3398.
- Mathew E, Brannon AL, Del Vecchio A, Garcia PE, Penny MK, Kane KT, Vinta A, Buckanovich RJ, Di Magliano MP. Mesenchymal Stem Cells Promote Pancreatic Tumor Growth by Inducing Alternative Polarization of Macrophages. Neoplasia. 2016;18(3):142–151. doi:10.1016/j.neo.2016.01.005.
- Della-Torre E, Feeney E, Deshpande V, Mattoo H, Mahajan V, Kulikova M, Wallace ZS, Carruthers M, Chung RT, Pillai S, et al. B-cell depletion attenuates serological biomarkers of fibrosis and myofibroblast activation in IgG4-related disease. Ann Rheum Dis. 2015;74(12):2236–2243. doi:10.1136/annrheumdis-2014-205799.
- Della-Torre E, Rigamonti E, Perugino CA, Sain SB, Sun N, Kaneko N, Maehara T, Rovati L, Ponzoni M, Milani R, et al. B lymphocytes directly contribute to tissue fibrosis in IgG4-Related Disease. J Allergy Clin Immunol. 2020;145(3):968–981.e14. doi:10.1016/j.jaci.2019.07.004.
- Zhang X, Liu X, Joseph L, Zhao L, Hart J, Xiao SY. Pancreatic ductal adenocarcinoma with autoimmune pancreatitis-like histologic and immunohistochemical features. Hum Pathol. 2014;45(3):621–627. doi:10.1016/j.humpath.2013.08.027.
- Bledsoe JR, Della-Torre E, Rovati L, Deshpande V. IgG4-related disease: review of the histopathologic features, differential diagnosis, and therapeutic approach. APMIS. 2018;126(6):459–476. doi:10.1111/apm.12845.
- Della-Torre E, Stone JH. “How I manage” IgG4-related disease. J Clin Immunol. 2016;36(8):754–763. doi:10.1007/s10875-016-0331-0.
- Wallace ZS, Khosroshahi A, Carruthers MD, Perugino CA, Choi H, Campochiaro C, Culver EL, Cortazar F, Della‐torre E, Ebbo M, et al. An international multispecialty validation study of the IgG4-related disease responder index. Arthritis Care Res (Hoboken). 2018;70(11):1671–1678. doi:10.1002/acr.23543.
- Della-Torre E, Mattoo H, Mahajan VS, Deshpande V, Krause D, Song P, Pillai S, Stone JH. IgG4-related midline destructive lesion. Ann Rheum Dis. 2014;73(7):1434–1436. doi:10.1136/annrheumdis-2014-205187.
- Lanzillotta M, Della-Torre E, Stone JH. Roles of Plasmablasts and B Cells in IgG4-Related Disease: implications for Therapy and Early Treatment Outcomes. Curr Top Microbiol Immunol. 2017;401:85–92. doi:10.1007/82_2016_58.
- Lanzillotta M, Della-Torre E, Milani R, Bozzolo E, Bozzalla-Cassione E, Rovati L, Arcidiacono PG, Partelli S, Falconi M, Ciceri F, et al. Effects of glucocorticoids on B-cell subpopulations in patients with IgG4-related disease. Clin Exp Rheumatol. 2019;37(3):159–166.
- Lanzillotta M, Della-Torre E, Milani R, Bozzolo E, Bozzalla-Cassione E, Rovati L, Arcidiacono PG, Partelli S, Falconi M, Ciceri F, et al. Increase of circulating memory B cells after glucocorticoid-induced remission identifies patients at risk of IgG4-related disease relapse. Arthritis Res Ther. 2018;3(1):222. doi:10.1186/s13075-018-1718-5.
- Tanaka N, Yamada S, Sonohara F, Suenaga M, Hayashi M, Takami H, Niwa Y, Hattori N, Iwata N, Kanda M, et al. Clinical Implications of Lysyl Oxidase-Like Protein 2 Expression in Pancreatic Cancer. Sci Rep. 2018;8(1):9846. doi:10.1038/s41598-018-28253-9.
- Park JS, Lee JH, Lee YS, Kim JK, Dong SM, Yoon DS. Emerging role of LOXL2 in the promotion of pancreas cancer metastasis. Oncotarget. 2016;7(27):42539–42552. doi:10.18632/oncotarget.9918.
- Le Calvé B, Griveau A, Vindrieux D, Maréchal R, Wiel C, Svrcek M, Gout J, Azzi L, Payen L, Cros J, et al. Lysyl oxidase family activity promotes resistance of pancreatic ductal adenocarcinoma to chemotherapy by limiting the intratumoral anticancer drug distribution. Oncotarget. 2016;7(22):32100–32112. doi:10.18632/oncotarget.8527.
- Kagan HM, Li W. Lysyl oxidase: properties. specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–672.
- Ikenaga N, Peng ZW, Vaid KA, Liu SB, Yoshida S, Sverdlov DY, Mikels-Vigdal A, Smith V, Schuppan D, Popov YV, et al. Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal. Gut. 2017;66(9):1697–1708. doi:10.1136/gutjnl-2016-312473.
- Santos A, Lagares D. Matrix Stiffness: the Conductor of Organ Fibrosis. Curr Rheumatol Rep. 2018;20(1):2. doi:10.1007/s11926-018-0710-z.
- Aumiller V, Strobel B, Romeike M, Schuler M, Stierstorfer BE, Kreuz S. Comparative analysis of lysyl oxidase (like) family members in pulmonary fibrosis. Sci Rep. 2017;10;7(1):149. doi:10.1038/s41598-017-00270-0.
- Millanes-Romero A, Herranz N, Perrera V, Iturbide A, Loubat-Casanovas J, Gil J, Jenuwein T, García de Herreros A, Peiró S. Regulation of heterochromatin transcription by Snail1/LOXL2 during epithelial-to-mesenchymal transition. Mol Cell. 2013;52(5):746–757. doi:10.1016/j.molcel.2013.10.015.
- Cuevas EP, Eraso P, Mazón MJ, Santos V, Moreno-Bueno G, Cano A, Portillo F. LOXL2 drives epithelial-mesenchymal transition via activation of IRE1-XBP1 signalling pathway. Sci Rep. 2017;23(7):44988. doi:10.1038/srep44988.
- Zhang D, Li L, Jiang H, Li Q, Wang-Gillam A, Yu J, Head R, Liu J, Ruzinova MB, Lim K-H, et al. Tumor-Stroma IL1β-IRAK4 Feedforward Circuitry Drives Tumor Fibrosis, Chemoresistance, and Poor Prognosis in Pancreatic Cancer. Cancer Res. 2018;78(7):1700–1710. doi:10.1158/0008-5472.CAN-17-1366.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013.
- Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2(1):16022. doi:10.1038/nrdp.2016.22.
- Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy S, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25(6):719–734. doi:10.1016/j.ccr.2014.04.005.
- Ligorio M, Sil S, Malagon-Lopez J, Nieman LT, Misale S, Di Pilato M, Ebright RY, Karabacak MN, Kulkarni AS, Liu A, et al. Stromal Microenvironment Shapes the Intratumoral Architecture of Pancreatic Cancer. Cell. 2019;178(1):160–175. doi:10.1016/j.cell.2019.05.012.
- Zhang Y, Morgan R, Podack ER, Rosenblatt J. B cell regulation of anti-tumor immune response. Immunol Res. 2013;57(1–3):115–124. doi:10.1007/s12026-013-8472-1.
- Nelson BH. CD20+B Cells: the Other Tumor-Infiltrating Lymphocytes. J Immunol. 2010;185(9):4977–4982. doi:10.4049/jimmunol.1001323.
- de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7(5):411–423. doi:10.1016/j.ccr.2005.04.014.
- Yuen GJ, Demissie E, Pillai S. B lymphocytes and cancer: a love-hate relationship. Trends Cancer. 2016;2(12):747–757. doi:10.1016/j.trecan.2016.10.010.
- Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, Bergsland E, Steinhoff M, Li Y, Gong Q, et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25(6):809–821. doi:10.1016/j.ccr.2014.04.026.
- Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, Gorjestani S, Liudahl SM, Truitt M, Olson P, et al. Bruton Tyrosine Kinase-Dependent Immune Cell Cross-talk Drives Pancreas Cancer. Cancer Discov. 2016;6(3):270–285. doi:10.1158/2159-8290.CD-15-0827.
- Lee KE, Spata M, Bayne LJ, Buza EL, Durham AC, Allman D, Vonderheide RH, Simon MC. Hif1a Deletion Reveals Pro-Neoplastic Function of B Cells in Pancreatic Neoplasia. Cancer Discov. 2016;6(3):256–269. doi:10.1158/2159-8290.CD-15-0822.
- Pylayeva-Gupta Y, Das S, Handler JS, Hajdu CH, Coffre M, Koralov SB, Bar-Sagi D. IL35-Producing B Cells Promote the Development of Pancreatic Neoplasia. Cancer Discov. 2016;6(3):247–255. doi:10.1158/2159-8290.CD-15-0843.
- Hosaka K, Yang Y, Seki T, Nakamura M, Andersson P, Rouhi P, Yang X, Jensen L, Lim S, Feng N, et al. Tumour PDGF-BB expression levels determine dual effects of anti-PDGF drugs on vascular remodelling and metastasis. Nat Commun. 2013;4(1):2129. doi:10.1038/ncomms3129.
- Demoulin JB, Essaghir A. PDGF receptor signaling networks in normal and cancer cells. Cytokine Growth Factor Rev. 2014;25(3):273–283. doi:10.1016/j.cytogfr.2014.03.003.
- Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22(10):1276–1312. doi:10.1101/gad.1653708.
- Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–1040. doi:10.1038/nm.2807.
- Della-Torre E, Bozzalla-Cassione E, Sciorati C, Ruggiero E, Lanzillotta M, Bonfiglio S, Mattoo H, Perugino CA, Bozzolo E, Rovati L, et al. A CD8α- Subset of CD4+ SLAMF7+ Cytotoxic T Cells is Expanded in Patients with IgG4-Related Disease and Decreases following Glucocorticoid Treatment. Arthritis Rheumatol. 2018;70(7):1133–1143. doi:10.1002/art.40469.
- De Monte L, Wörmann S, Brunetto E, Heltai S, Magliacane G, Reni M, Paganoni AM, Recalde H, Mondino A, Falconi M et al. Basophil Recruitment into Tumor-Draining Lymph Nodes Correlates with Th2 Inflammation and Reduced Survival in Pancreatic Cancer Patients. Cancer Res. 2016 Apr 1;76(7):1792–1803. doi:10.1158/0008-5472.CAN-15-1801-T.
- De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208(3):469–478. doi:10.1084/jem.20101876.
- Zhang A, Qian Y, Ye Z, Chen H, Xie H, Zhou L, Shen Y, Zheng S. Cancer-associated fibroblasts promote M2 polarization of macrophages in pancreatic ductal adenocarcinoma. Cancer Med. 2017;6(2):463–470. doi:10.1002/cam4.993.
- Sherry B, Tekamp-Olson P, Gallegos C, Bauer D, Davatelis G, Wolpe SD, Masiarz F, Coit D, Cerami A. Resolution of the two components of macrophage inflammatory protein 1, and cloning and characterization of one of those components, macrophage inflammatory protein 1 beta.. J Exp Med. 1988;168(6):2251–2259. doi:10.1084/jem.168.6.2251.
- Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270(5243):1811–1815. doi:10.1126/science.270.5243.1811.
- Jose PJ, Griffiths-Johnson DA, Collins PD, Walsh DT, Moqbel R, Totty NF, Truong O, Hsuan JJ, Williams TJ. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J Exp Med. 1994;179(3):881–887. doi:10.1084/jem.179.3.881.
- Cohen SJ, Alpaugh RK, Palazzo I, Meropol NJ, Rogatko A, Xu Z, Hoffman JP, Weiner LM, Cheng JD. Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas. 2008;37(2):154–158. doi:10.1097/MPA.0b013e31816618ce.
- Hiepe F, Dörner T, Hauser AE, Hoyer BF, Mei H, Radbruch A. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat Rev Rheumatol. 2011;7(3):170–178. doi:10.1038/nrrheum.2011.1.
- Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, et al. AJCC Cancer Staging Manual.Eight Ediction 2016.Springer: Switzerland. ISBN 978-3-319-40617-6.
- Soloff EV, Zaheer A, Meier J, Zins M, Tamm EP. Staging of pancreatic cancer: resectable, borderline resectable, and unresectable disease. Abdom Radiol (NY). 2018;43(2):301–313. doi:10.1007/s00261-017-1410-2.
