ABSTRACT
The consensus Immunoscore is a routine assay quantifying the adaptive immune response within the tumor microenvironment. It has a prognostic value that has been confirmed in a phase 3 clinical trial (NCCTG N0147) in stage III colon cancers. Moreover, results from another phase 3 randomized trial revealed the predictive value of Immunoscore for response to adjuvant chemotherapy duration. These results highlight the clinical utility of Immunoscore. In its latest edition, the World Health Organization classification of Digestive System Tumors introduced for the first time the immune response as an essential and desirable diagnostic criterion for colorectal cancer. Within the tumor microenvironment, the immune response provides an important estimate of the risk of recurrence and death in colon cancer. The international validation of the prognostic value of the consensus Immunoscore together with its prognostic value in the N0147 trial and its predictive utility for response to chemotherapy in stage III patients provide valuable information for patient management.
Immune response and colon cancer classification
In patients with colorectal cancer (CRC), considerable intra-stage variability in clinical outcome is observed that is not predicted by the Tumor-Node-Metastasis (TNM) staging system.Citation1 The impact of the preexisting intratumoral adaptive immunity on tumor progression and invasion and on the patient’s clinical outcome has been demonstrated.Citation2 This led to a major paradigm shift in tumor immunology, and to the development of an immune-based scoring system named Immunoscore.Citation3-Citation5 Within the tumor microenvironment, the consensus Immunoscore is analyzed using standardized immunohistochemistry and a digital pathology-based assay that quantifies the densities of two lymphocytes populations, CD3+ and CD8+ T cells, in the tumor and its invasive margin. Immunoscore is prognostic in patients with colon cancer,Citation2 and in an international collaboration, it was confirmed to be highly reproducible and objective.Citation6 Recent phase 3 clinical trial results highlight the importance of the Immunoscore to risk-stratify patients with stage III colon cancer to aid in clinical decision-making.Citation7,Citation8
Figure 1. Clinical utility of Immunoscore. The tumor anatomy, including the tumor core, the invasive margin, and different T-cell subpopulations is illustrated. Immunoscore is a powerful prognosis marker and a predictive marker of response to chemotherapy for stage III colon cancer patients. CTL, cytotoxic T lymphocyte; TFH, T follicular-helper; TH1, T helper type 1.
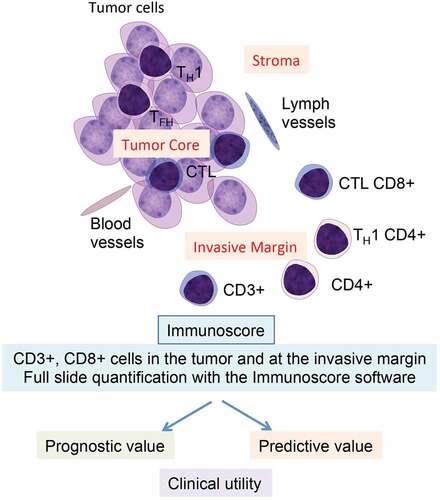
Phase 3 trials and clinical utility of the consensus Immunoscore
The phase 3 trial [NCCTG-N0147 (Alliance)] consists of stage III colon cancers from an adjuvant chemotherapy trial that found no survival benefit for the addition of cetuximab to standard FOLFOX chemotherapy. Tumor samples from 611 TNM stage III colon cancers from the FOLFOX arm were evaluated for immune markers.Citation8 Immunoscore (CD3+, CD8+) data were available on 559 patients after quality control. Clinical and pathological characteristics of the study population and recurrence/survival data were carefully collected, and median patient follow-up was 83.4 months. Patients whose tumors showed a high vs. medium or low Immunoscore had significantly better 3-y DFS rates of (82.6% vs. 63.8% at 3 y, hazard ratio [HRadj-Hi vs Lo] = 0.54 (95%CI 0.37–0.78), Padj. = 0.0032) after adjustment for KRAS and BRAF,V600E DNA Mistmatch Repair (MMR) status, and other covariates. A predefined five-category Immunoscore (I0-I4) revealed a statistically significant improvement in 3-y Disease-Free Survival (DFS) rates ranging from 63.8% for I0 patients to 100% for I4 patients. Strikingly, patients with a very high Immunoscore of I4 had no risk of recurrence for up to 8 y after tumor resection, similar to results from other cohorts.Citation7,Citation8 No relationship was found between the Immunoscore and the mutational status of KRAS or BRAFV600E genes, nor with patient body mass index or race.
While the Immunoscore was developed in retrospective case series of CRC patients across all tumor stages and with varying treatment and follow-up, the Immunoscore results from the N0147 clinical trial are from colon cancer patients of uniform stage (III) and treatment (FOLFOX). High Immunoscore, both as a continuous variable or as di-chotomous, tri-chotomous, or penta-chotomous categorization, predicted better postsurgical survival after adjuvant FOLFOX. Specifically, patients whose tumors had a high vs. low three-level Immunoscore had significantly better DFS and Overall Survival (OS) rates even after adjustment for covariates and molecular features. This finding indicates that the tumor containment by the host immune system is mediated, at least in part, by intratumoral CD3+ and CD8 + T-cells. Conversely, patients whose tumors had a low or intermediate Immunoscore were more likely to experience disease recurrence or death. Importantly, Immunoscore remained significantly prognostic after excluding patients with deficient DNA Mismatch repair (dMMR) tumors, confirming that its association with prognosis was not driven by dMMR. In a multivariate model, Immunoscore remained statistically significant for DFS in contrast to MMR status. Furthermore, we found that Immunoscore enhances prognostication beyond that of established prognostic variables in stage III patients.
A risk classification strategy proposed by the phase 3 clinical trial International Duration Evaluation of Adjuvant chemotherapy (IDEA)Citation9 which divided patients into low-risk (T1-3, N1) and high-risk (T4 or N2) groups based on histopathological criteria. We found that the three-level Immunoscore can further risk-stratify stage III patients for DFS within these risk groups in the N0147 trial. Specifically, we identified a high-Immunoscore subset within the T1-3, N1 group that had a 91.8% 3-y DFSCitation8 that is similar to or above the 87% and 84.7% 3-y DFS rates reported for stage II patients treated with FOLFOX in the MOSAIC or NSABP-C-08 adjuvant trials, respectively.
Strengths of our study include a patient population from a clinical trial cohort of uniform tumor stage and treatment with molecular annotation and rigorously collected patient outcome data. Furthermore, a centralized Immunoscore evaluation was performed assuring uniformity in its determination. Since all patients received adjuvant chemotherapy, a limitation is the inability to examine the predictive impact of immune markers for chemotherapy response, which was recently evaluated in the IDEA phase 3 randomized trial. The IDEA randomized phase 3 trial evaluated the noninferiority of 3 vs. 6 months of adjuvant chemotherapy in patients with resected stage III colon cancer. The primary objective of the study was not reached. However, data suggested that risk categories could be used to inform the duration of adjuvant chemotherapy. Component drugs in the FOLFOX regimen include 5-fluorouracil that may partially deplete or transiently inactivate inhibitory immune cells and oxaliplatin that may increase cytotoxic T-cell infiltration and are shown to induce immunogenic cell death.Citation10 High Immunoscore significantly predicted response to a 6- month duration of a fluoropyrimidine–oxaliplatin chemotherapy in all stage III patients in the IDEA phase 3 clinical trial.Citation7 Furthermore, Immunoscore predicted response to 6 months of FOLFOX chemotherapy within patients with low-risk (T1-3 and N1) and high-risk (T4 or N2) tumors. These data highlight the utility of Immunoscore for guiding treatment decisions in adjuvant settings ().Citation7 In separate studies, Immunoscore within colon cancer metastases was also shown to predict the risk of patient relapse and death.Citation11
Immunoscore for patient management decisions
The tumor and immune interaction indicated by the Immunoscore enables risk stratification of stage III colon cancer patients. These data provide a validation of the Immunoscore assay in a clinical trial cohort and underscore the limitations of T and N staging which are further illustrated by the ability of Immunoscore to refine prognostication among predetermined low- and high-risk T and N groups of stage III patients. Furthermore, data suggest that Immunoscore can be used to inform the duration of adjuvant chemotherapy in stage III patients.
The American-Joint-Committee-on-Cancer/Union-Internationale-Contre-le-Cancer TNM staging system is currently the gold standard classification system for colon cancer. The first paradigm shift that translated into guidelines came recently with the latest (5th) edition of the World Health Organization (WHO) Digestive System Tumors, which introduced “the immune response as essential and desirable diagnostic criteria for colorectal cancer”. The consensus Immunoscore assay was cited as evidence that the immune response can improve prognostication in colon cancer.Citation1 The N0147 results, together with the introduction into the WHO guidelines, highlight the benefit of implementing Immunoscore into clinical practice and strongly advocate for the introduction of a new TNM-Immune classification system. The consensus Immunoscore assay has been developed as an in vitro diagnostic test to help guide treatment strategies, and is available in Clinical Laboratory Improvement Amendments by the Food and Drug Administration regulation (FDA-CLIA)-certified laboratories for routine use. Accordingly, Immunoscore could significantly modify colon cancer patient management and may also provide benefits in other cancer types.
Disclosure of potential conflicts of interest
JG has patents associated with the immune prognostic biomarkers. JG is co-founder of HalioDx biotech company. Immunoscore® a registered trademark from the National Institute of Health and Medical Research (INSERM).
Additional information
Funding
References
- Quezada-Marin J, Lam AK, Ochiai A, Odze RD, Washington KM, Fukayama M, Rugge M, Klimstra DS, Nagtegaal ID, Tan P-H, et al. Gastrointestinal tissue-based molecular biomarkers: A practical categorization based on the 2019 WHO classification of epithelial digestive tumours. Histopathology. 2020. PMID: 32320495. doi:10.1111/his.14120.
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–3. PMID: 17008531. doi:10.1126/science.1129139.
- Galon J, Fox BA, Bifulco CB, Masucci G, Rau T, Botti G, Marincola FM, Ciliberto G, Pages F, Ascierto PA, et al. Immunoscore and immunoprofiling in cancer: an update from the melanoma and immunotherapy bridge 2015. J Transl Med. 2016;14:273. PMID: 27650038. doi:10.1186/s12967-016-1029-z.
- Kirilovsky A, Marliot F, El Sissy C, Haicheur N, Galon J, Pagès F. Rational bases for the use of the Immunoscore in routine clinical settings as a prognostic and predictive biomarker in cancer patients. Int Immunol. 2016;28:373–382. PMID: 27121213. doi:10.1093/intimm/dxw021.
- Pages F, Galon J, Fridman WH. The essential role of the in situ immune reaction in human colorectal cancer. J Leukoc Biol. 2008;84:981–987. PMID: 18559950. doi:10.1189/jlb.1107773.
- Pages F, Mlecnik B, Marliot F, Bindea G, Ou F-S, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–2139. PMID: 29754777. doi:10.1016/S0140-6736(18)30789-X.
- Pagès F, André T, Taieb J, Vernerey D, Henriques J, Borg C, Marliot F, Ben Jannet R, Louvet C, Mineur L, et al. Prognostic and predictive value of the Immunoscore in stage III colon cancer patients treated with oxaliplatin in the prospective IDEA France PRODIGE-GERCOR cohort study. Ann Oncol. 2020;31:921–929. PMID: 32294529. doi:10.1016/j.annonc.2020.03.310.
- Sinicrope FA, Shi Q, Hermitte F, Zemla TJ, Mlecnik B, Benson AB, Gill S, Goldberg RM, Kahlenberg MS, Nair SG, et al. Contribution of Immunoscore and molecular features to survival prediction in stage III colon cancer. JNCI Cancer Spectr. 2020;4:pkaa023. PMID: 32455336. doi:10.1093/jncics/pkaa023.
- Grothey A, Venook AP. Optimizing adjuvant therapy for localized colon cancer and treatment selection in advanced colorectal cancer. J Natl Compr Canc Netw. 2018;16:611–615. PMID: 29784738. doi:10.6004/jnccn.2018.0038.
- Vacchelli E, Galluzzi L, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, Kroemer G. Trial watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2012;1:179–188. PMID: 22720239. doi:10.4161/onci.1.2.190262011ONCOIMM0105.
- Mlecnik B, Van den Eynde M, Bindea G, Church SE, Vasaturo A, Fredriksen T, Lafontaine L, Haicheur N, Marliot F, Debetancourt D, et al. Comprehensive intrametastatic immune quantification and major impact of Immunoscore on survival. J Natl Cancer Inst. 2018;110:97–108. PMID: 28922789. doi:10.1093/jnci/djx123.
