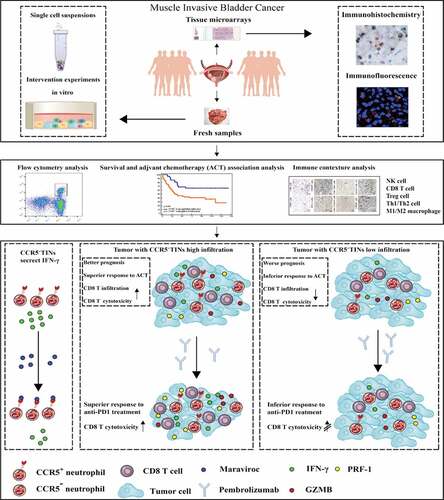
ABSTRACT
Our previous studies revealed tumor-infiltrating neutrophils (TINs) played dichotomous roles in different cancers, indicating diverse TINs subtypes might orchestrate anti-tumor immunity or immune evasion, respectively. This study aimed to investigate the clinical significance and immune characteristics of CCR5+TINs in muscle-invasive bladder cancer (MIBC). Two hundred and fifty-seven MIBC patients from two clinical centers and 95 fresh MIBC samples were included. CCR5+TINs were stained by immunohistochemistry, and the relationship between patients’ clinic-pathological features and prognosis was evaluated, respectively. Immunohistochemistry and flow cytometry were applied to assess the immune features of CCR5+TINs and their correlations with other immune cells. In vitro study was conducted to estimate immune characteristics of CCR5+TINs and their predictive potential for pembrolizumab therapeutic response. In the two MIBC cohorts, we found that high CCR5+TINs infiltration could predict better overall survival (OS, P= .032, 0.039) and recurrence-free survival (RFS, P= .001, 0.006) and be associated with survival benefit from adjuvant chemotherapy (ACT, P< .001 for OS and P= .022 for RFS, respectively) in merely pT2N0 MIBC. Maraviroc could partly reduce IFN-γ secretion by CCR5+TINs (P< .001). CCR5+TINs correlated with higher expression of effector molecules within CD8+T cells. Notably, pembrolizumab treatment could only elevate the apoptosis status of tumor cells in the CCR5+TINs high subgroup (P < .001), other than CCR5+TINs low subgroup (P= .481). Our results indicate that CCR5+TINs could prime anti-tumor immune response through autonomous IFN-γ release, thus leading to favorable prognosis and superior therapeutic response to ACT and immunotherapy in MIBC.
Introduction
Muscle-invasive bladder cancer (MIBC) is a frequently occurring malignancy all around the world,Citation1 which poses a significant risk of metastasis with grim survival. The cisplatin-based combination chemotherapy remains the standard first-line treatment for patients with advanced or metastatic urothelial cancer (UC) who are fit to tolerate cisplatin, while only a subset of patients can benefit from this treatment.Citation2 Immune checkpoint inhibitors (ICIs) represent an exciting advancement over previous standard treatments for advanced or metastatic UC.Citation3 However, the overall objective response rates (ORRs) of five ICIs targeting PD-1 or PD-L1 were relatively low (< 30%).Citation4,Citation5 Therefore, urgent works including better understanding of the underlying the complex mechanisms of UC immune microenvironment and precise stratification for patients who might have better adjuvant chemotherapy (ACT) response and ICIs response are posed at the forefront desk of the clinical cancer research.
Neutrophils infiltration is common in most cancer types. In the recent years, accumulating evidence has shown that tumor-infiltrating neutrophils (TINs) are critical for tumor initiation and progression.Citation6–Citation11 However, the exact role of TINs in the tumor microenvironment remains controversial, which could acquire either anti-tumor (N1) or pro-tumor (N2) function.Citation9,Citation10,Citation12-Citation15 Our previous work revealed that MIBC patients with high CD66b+TINs infiltration had a poorer overall survival (OS) than those with low CD66b+TINs infiltration.Citation16 These results indicated that certain subtypes of CD66b+TINs might possess distinctive functions during tumor progression. Recent studies have shown CD66b+TINs could stimulate anti-tumor T cell responses in early-stage cancer.Citation14,Citation15 Notably, TINs were reported to express high level of CCR5 expression in early-stage cancer,Citation15 indicating CCR5 might regulate chemotaxis and function of TINs. However, previous studies on CCR5+CD66b+TINs are still obscure, which highlights the need for further research on CCR5+CD66b+TINs in MIBC.
Consequently, we analyzed the presence and function of CCR5+CD66b+TINs in MIBC and evaluated their correlation with clinical outcomes, especially in patients receiving ACT after radical cystectomy (RC) in the present study. Moreover, we characterized the immune-feature of CCR5+CD66b+TINs in MIBC, as well as their role in prediction of therapeutic response to ICIs treatment in MIBC.
Materials and methods
Study cohort
Two independent sets of patients with MIBC were enrolled in the study. After approved by the Clinical Research Ethics Committee of Zhongshan Hospital, Fudan University (FUZH) and the Ethics Committee of Fudan University Shanghai Cancer Center (FUSCC), we retrospectively evaluated 393 UC patients (FUZH: 215; FUSCC: 178) who underwent RC initially. The exclusion criteria were: 1) pathological diagnosed not as MIBC or combined with other pathological types; 2) with distant metastatic disease; 3) tumor tissues and follow-up information unavailable; 4) preoperative chemotherapy or radiotherapy. Finally, 259 eligible MIBC patients were included in this study. After RC, 119 patients received ACT (at least one cycle). The end of follow-up was July 2016. Endpoints included OS and recurrence-free survival (RFS), defined as time from the date of RC to death from all causes or recurrence, respectively, or to the date of the last visit. During immunohistochemistry, two patients’ dots were missing and the final patient number was 257. The detailed clinic-pathological features of 257 patients are listed in Supplementary Table 1.
Fresh samples
Fresh MIBC tumor (n = 95) and peritumor normal bladder samples (n = 10), were collected from four independent clinical centers in Shanghai, China (FUZH, FUSCC, Ruijin Hospital Affiliated to Shanghai Jiaotong Unversity School of Medicine and Shanghai General Hospital). The exclusion criterions were described above. EDTA-anticoagulated peripheral blood was collected from the same MIBC patients during surgery. Each patient signed an informed consent.
Immunohistochemistry and immunofluorescence
Immunohistochemistry (IHC) and immunofluorescence (IF) staining were performed on tissue microarray (TMA), which was established with formalin-fixed, paraffin-embedded MIBC surgical specimens. The details of antibodies were listed in the Supplementary Table 2.
The IHC performed according to the methods has been described previously.Citation16 For single IHC staining, the sections were incubated with the primary antibody at 4°C overnight. The staining was performed with DAB stain system. For double IHC staining, the first primary antibody staining was conducted as the same of single IHC DAB staining firstly. Then, the sections were exposed to the second primary antibody at 37°C for 2 h. After the second primary antibody was washed off and the stain was performed with Vector Blue AP Substrate Kit (Vector Laboratories). Finally, the slides were evaluated by two urologic pathologists from two different medical centers who were blind to the clinical data reviewed the slides independently with the assistance of Image-Pro Plus (Media Cybernetics Inc.). F tests in the reliability analyzes would be used for analyzing the reliability of these two counting results. If P value of F tests in the reliability analyses was≤0.05, second assessment would be underwent by the same two urological pathologists. The mean value of the two counting results was adopted to conduct further analyses. Cutoff value was determined by X-tile 3.6.1 (Yale University).
For IF, the sections were incubated with the primary antibodies overnight at 4°C. Then samples were incubated with species-appropriate rabbit/mouse secondary antibodies coupled to Alexa Fluor dyes (488, 555, Invitrogen) for 2 hours at room temperature. DAPI (ab104139) was used to mount cover slips. The slides were detected through Leica DMi8 microsystems.
Flow cytometry
Single cell suspensions of fresh samples were isolated by using collagenase IV and dealt with protein transport inhibitor (monensin, BD GolgiSTOP) and erythrocytes were depleted by RBC Lysis Buffer. Peripheral blood neutrophils (PBNs) were processed in a similar manner as described.Citation15 Cells were incubated with Fc Block (BD Biosciences) before staining with conjugated antibodies. Then single cell suspensions were stained with antibodies for 30 min at 4°C. Fluorochrome-conjugated antibodies are listed in Supplementary Table 3. For intracellular protein staining, samples were pre-incubated with the Fixation/Permeabilization Solution Kit (BD Biosciences) according to the manufacturer’s instructions. Fixable Viability Stain 510 (BD Biosciences) was used to identify and eliminate dead cells. The compensation was performed with BD CompBeads set (BD Biosciences). Flow cytometry was evaluated by FlowJo software (Tree Star). Gating strategy for TINs identification is listed in Supplementary Figure 1. The details of antibodies are listed in Supplementary Table 3.
In vitro intervention studies
The in vitro intervention studies were performed according to the methods described previously.Citation17 Single cell suspensions of fresh MIBC tumor samples including tumor cells, immune cells and other cells were co-cultured in medium, under 37°C and 5% CO2. Then the cells were cultured with maraviroc (10uM, Selleck) or dimethyl sulfoxide (DMSO)/Pembrolizumab (5 µg/mL. Selleck) or IgG4B κ isotype control (5 µg/ml, A1101-200, Bio Vision), for 12 h. After overnight culture, the cells were subjected to phenotypic analysis by flow cytometry as above.
Statistical analysis
Results are expressed as mean ± SEM. Two unpaired group samples were analyzed with unpaired Student’s t-test or Mann-Whiney U test. Matched samples or paired samples were analyzed with paired Student’s t-test or Wilcoxon signed-rank test. CCR5+CD66+TINs infiltrations in different sites or TMN stages were analyzed with One-way ANOVA followed by Tukey’s multiple comparisons test. Analysis of the correlation was made by Spearman’s correlation. Associations between CCR5+CD66+TINs and clinic-pathological characteristics of patients were analyzed with chi-square or Fisher’s exact test. OS and RFS were determined by Kaplan-Meier method. Multivariate analysis was performed with Cox proportional hazards regression model. A two-tailed P value of less than 0.05 was defined as statistical significance. Statistical analyses were conducted using SPSS, version 22.0, Graph Pad Prism Software 8.0 and Medcalc 15.
Results
CCR5+CD66b+TINs are accumulated and negatively correlate with tumor progression in MIBC
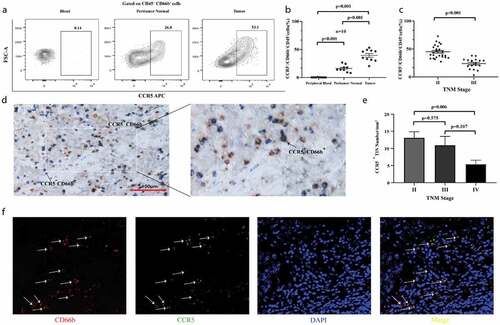
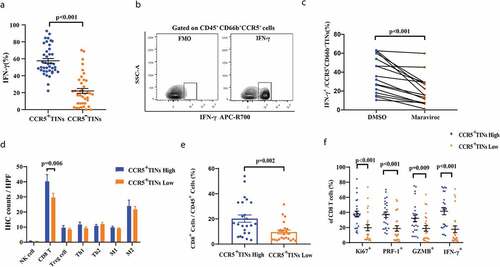
We analyzed the frequencies of CCR5+cells among total neutrophils in peripheral blood, peritumor normal bladder tissues and bladder cancer samples of MIBC patients, respectively. There was no difference between numbers of total CD45 in peritumor normal and tumor (P = .258, Supplementary Figure 2). Notably, we found that CCR5+CD66b+TINs densely infiltrated in tumor area (P < .001, ,b) and the infiltration of CCR5+CD66b+TINs was negatively correlated with TNM stage (P < .001, ). The intratumoral infiltration of CCR5+CD66b+TINs was further validated by IHC and IF staining, respectively (,f) and the infiltration level of CCR5+CD66b+TINs was higher in Stage II tumors than that in Stage IV tumors (P = .006, ).
Figure 1. CCR5+CD66b+TINs densely populate in MIBC and negatively correlate with tumor progression. (a-b) The representative flow cytometric figures (a) and cumulative results (b) showing expression of CCR5 on gated peripheral blood neutrophils (PBNs), peritumor normal bladder neutrophils and bladder neutrophils CD45+CD66b+tumor-infiltrating-neutrophils (TINs) of MIBC patients (n = 10) by flow cytometry. Bars represent mean ± SEM. Data were analyzed using Tukey’s multiple comparisons test. (c) Scatterplots representing the CCR5+ cells frequencies of CD66b+CD45+ cells in tumors based on TNM stage (n = 50). Bars represent mean ± SEM. Data were analyzed using Mann-Whiney U test. (d) CCR5+CD66b−cell (Left, brown arrow), CCR5−CDbb+cell (Left, blue arrow), CCR5+CD66b+cell (Right, black arrow). (e) CCR5+CD66+ cells number of IHC in MIBC tissue microarray (TMA) according to the TNM stage (n = 257). Data were analyzed using Tukey’s multiple comparisons test. Bars represent SEM. (f) Representative immunofluorescence staining for CD66b (red) and CCR5 (green) in MIBC TMA. Nuclei counterstained blue with DAPI. The lower right panel was merged by the previous three images (CCR5+CD66b+TINs, white arrow).
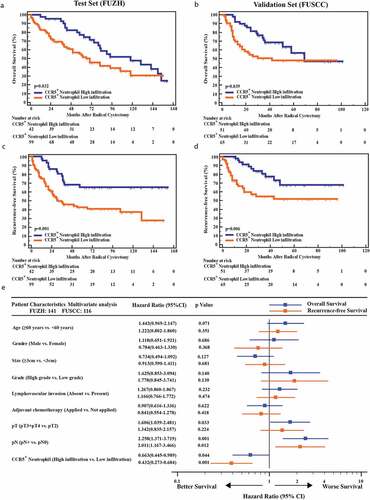
Furthermore, we compared the OS and RFS between CCR5+CD66b+TINs high/low infiltration patients. In FUZH and FUSCC cohort, the cutoff value was identified at 9 cells/mm.Citation2 Patients with CCR5+CD66b+TINs high infiltration showed better OS (P= .032 and 0.039, respectively, ,b) and RFS (P= .001 and 0.006, respectively, ,d) in both cohorts. However, it should be pointed out that both groups met an almost identical OS eventually in test set and validation set. Multivariate analysis revealed that infiltration level of CCR5+CD66b+TINs in MIBC was an independent prognosticator regarding OS and RFS (P = .044 and 0.001; Hazard Ratio [HR] = 0.663 and 0.432; respectively, ).
Figure 2. Kaplan–Meier and Cox analysis of OS and RFS in MIBC patients from the test set (FUZH) and the validation set (FUSCC). (a-d) Kaplan-Meier curve of OS (overall survival, a, b) and RFS (recurrence-free survival, c, d) in test cohort (FUZH, left, 141 patients) and validation cohort (FUSCC, right, 116 patients) according to high/low CCR5+CD66b+neutrophils infiltration subgroups. Data were analyzed using log-rank test. (e) Multivariate Cox analysis of OS and RFS for CCR5+CD66b+neutrophils infiltration high/low subgroups and clinical-pathologic factors in total cohort including training cohort and validation cohort (257 patients).
CCR5+CD66b+TINs high infiltration could be associated with better ACT effectiveness in pT2N0 MIBC patients
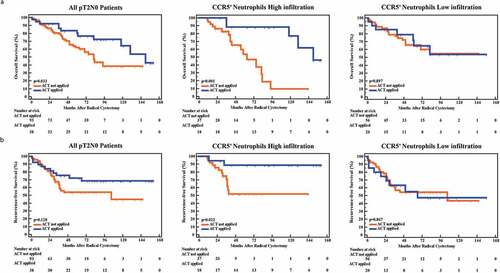
Interesting, our previous studies discovered opposite roles of CD66b+TINs in predicting therapeutic response postoperative ACT in gastric cancer and MIBC.Citation16,Citation18 Consequently, we sought to evaluate whether ACT effectiveness would be different in patients with CCR5+CD66b+TINs high/low infiltration. Notably, ACT application did not show statistical significant survival benefit in all patients (P = .622, HR = 0.907, 95% Confidence Interval [CI] = 0.616–1.336 for OS, and P = .418; HR = 0.841, 95% CI = 0.554–1.278 for RFS; ). MIBC patients of pT3/T4 or pN+ usually receive ACT after RC based on high level clinical evidences.Citation2 However, in pT3/T4 or pN+ MIBC patients, there was no association between CCR5+CD66b+TINs infiltration and survival benefits from ACT (P> .05, Supplementary Figure 3). As to pT2N0 MIBC, patients treated with ACT showed better OS (P= .033, left panel). Notably, CCR5+CD66b+TINs high infiltration subgroup showed superior therapeutic response to ACT in pT2N0 MIBC (P< .001 for OS and P = .022 for RFS, respectively, middle panel). Contrarily, CCR5+CD66b+TINs low infiltration subgroup did not show significant survival benefit from ACT (P = .897 for OS, P= .867 for PRS, right panel). Conclusively, CCR5+CD66b+TINs high infiltration could be associated with superior therapeutic response to ACT in pT2N0 MIBC patients.
Figure 3. High CCR5+CD66b+TINs infiltration could be associated with better adjuvant chemotherapy effectiveness in pT2N0 MIBC patients. (a-b) Kaplan-Meier curve of OS (overall survival, a) and RFS (recurrence-free survival, b) in all pT2N0 patients (left), CCR5+ neutrophils high infiltration of pT2N0 patients (middle) and CCR5+ neutrophils low infiltration of pT2N0 patients (right) according to the adjuvant chemotherapy (ACT) application. Data were analyzed using log-rank test.
CCR5+CD66b+TINs in MIBC could secret IFN-γ, which could be reduced by CCR5 inhibitor, maraviroc partially
Since CCR5+CD66b+TINs high infiltration in MIBC predicted favorable prognostic, we attempted to investigate the cellular mechanism. Flow cytometry analysis indicated that CCR5+CD66b+TINs secreted significantly more IFN-γ than CCR5−CD66b+TINs (), and was validated by fluorescence minus one (FMO, ). There was no difference in apoptotic state between CCR5+TINs and CCR5−TINs (P = .773, Supplementary Figure 4). Notably, the secretion of IFN-γ from CCR5+CD66b+TINs could be partly reduced by CCR5 inhibitor, maraviroc (P < .773, ). Consequently, these results indicated that TINs might secrete IFN-γ partially via CCR5 pathway in MIBC.
Figure 4. CCR5+CD66b+TINs could illustrate anti-tumor immunity contexture in MIBC patients through IFN-γ secretion, which could be reduced by CCR5 inhibitor maraviroc partially. (a) Flow cytometry analysis of IFN-γ+ cells frequencies of CCR5+TIN cells and CCR5−TIN cells in MIBC fresh samples (n = 40). Data were analyzed using Wilcoxon signed-rank test. (b) The contour plot representative gating figures for IFN-γ+ cells by flow cytometry of IFN-γ mAB stained CCR5+CD66b+TINs compared with IFN-γ mAB unstained CCR5+CD66b+TINs in one MIBC patient fresh sample. FMO, fluorescence minus one. (c) Quantification of IFN-γ+CCR5+CD66b+TINs frequencies of CCR5+CD66b+TINs in MIBC (n = 16) tissue samples after treated with CCR5 inhibitor maraviroc (10uM) or DMSO for 12 hours. Data were analyzed using Wilcoxon signed-rank test. (d) Quantification analysis of immune cells between CCR5+CD66b+TINs high/low subgroups in MIBC tissue microarray (TMA) by immunohistochemistry (IHC) (n = 141). Data were analyzed using Mann-Whiney U test, and showed as mean ± SEM. (e) Flow cytometric analysis of the CD8+ cells frequencies/CD45+ cells between CCR5+CD66b+TINs high/low subgroups in fresh samples of MIBC (n = 48). (f) Flow cytometric analysis of the Ki67+, PRF-1+, IFN-γ+, GZMB+ cells frequencies of CD8 T cells (n = 48) between CCR5+CD66b+TINs high/low subgroups in fresh samples of MIBC. (e-f) CCR5+CD66b+TINs High/low were grouped by median. (d-f) Data were analyzed using Mann-Whiney U test, and showed as mean ± SEM. Treg cell = T regulatory cells: foxp3; Th1 = type 1 helper cells; Th2 = type 2 helper cells; M1 = type 1 macrophages; M2 = type 2 macrophages; IFN = interferon; GZMB = granzyme B; PRF-1 = perforin.
CCR5+CD66b+TINs could orchestrate anti-tumor immunity contexture in MIBC patients
To better characterize the specific role of CCR5+CD66b+TINs play in MIBC immune microenvironment, predominant immune cells were evaluated by IHC. The representative IHC images are illustrated in Supplementary Figure 5. Notably, the quantity of CD8+T cells seemed to be significant different between high/low CCR5+CD66b+TINs subgroups (P = .006, ). Further flow cytometry analysis illustrated that the more CD8 T cells infiltrated in CCR5+CD66b+TINs high subgroup (P= .002, ). Furthermore, we investigated the functional phenotype of CD8+T cells, NK cells and CD4+Foxp3−T cells between CCR5+CD66b+TINs high/low subgroups. Interestingly, the expression effector molecules expression within CD8+T cells, NK cells and CD4+Foxp3−T cells were marginally or significantly different between the two subgroups ( and Supplementary Figure 6A-C). In the meanwhile, CCR5+CD66b+TINs high subgroup showed enhanced CD8 infiltration and cytotoxicity, which was independent of tumor progression (Supplementary Figure 6D-E). These findings suggest CCR5+CD66b+TINs could possibly orchestrate anti-tumor immunity contexture in MIBC.
High infiltration of CCR5+CD66b+TINs could be associated with superior therapeutic response to pembrolizumab in MIBC
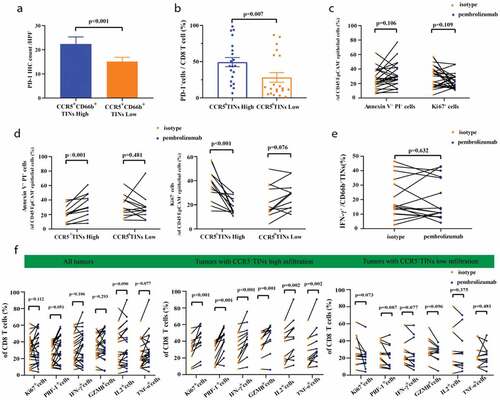
Furthermore, we compared the OS and RFS between patients with both CCR5+CD66b+TINs high/low and PD-1-PD-L1 high/low strata in FUZH cohort (Supplementary Figure 7A-B). In CCR5+TINs low group, patients with PD-L1 low subgroup showed better OS than patients with PD-L1 high subgroup (P= .022, Supplementary Figure 7B). The PD-1+cells frequencies of CD8 was highest among PD-1+CD8 T, PD-1+NK cells and PD-1+TINs (P < .001, 0.006, respectively, Supplementary Figure 7C-D). In addition, the infiltration of CD8 T cells in tumor area was associated with PD-1 expression level (r = 0.265, P = .002, Supplementary Figure 7E). We further observed higher PD-1 expression and higher percentage of PD-1+cells among CD8+T cells in CCR5+CD66b+TINs high subgroup (P < .001, 0.007, respectively, –b). Therefore, we assumed that CCR5+CD66b+TINs might predict therapeutic response to pembrolizumab in MIBC. When treated with pembrolizumab for 12 hours, the Annexin V+PI+ % and Ki67+ % of tumor cells showed no difference between pembrolizumab group and control group (P = .106 and 0.109, respectively, ). Interestingly, the Annexin V+PI+ % and Ki67+ % of tumor cells exhibited significant differences between pembrolizumab group and control group in CCR5+CD66b+TINs high subgroup (P < .001, <0.001, respectively, ), as well as effector molecules levels within CD8 T cells ( middle panel). In CCR5+CD66b+TINs low subgroup, however, no significant differences were observed between control group and pembrolizumab group, regarding Annexin V+PI+ % and Ki67+ % of tumor cells (P = .481,0.076, respectively, ) and effector molecules of CD8+ T cells (, right panel). In addition, pembrolizumab treatment could not restore IFN-γ production by neutrophils (P = .632, ). Besides, when divided into Stage II and III tumors, significant differences between pembrolizumab group and control group of CCR5+CD66b+TINs high subgroup were still observed on whether in Stage II or in Stage III tumors (Supplementary Figure 8A-D). In conclusion, high infiltration of CCR5+CD66b+TINs could be associated with superior therapeutic response to pembrolizumab in MIBC, which was independent on tumor progression.
Figure 5. High infiltration of CCR5+CD66b+TINs in MIBC could be associated with superior pembrolizumab response. (a) Quantification analysis (left) of PD-1 expression level between CCR5+TIN high/low subgroups in MIBC tissue microarray (TMA) by immunohistochemistry (IHC) (n = 141). (b) Flow cytometric analysis (right) of the PD-1+CD8+T cell frequencies of CD8 T cells (n = 40) between CCR5+CD66b+TINs high/low subgroups in fresh samples of MIBC. CCR5+CD66b+TINs High/low were grouped by median. (a-b) Data were analyzed using Mann-Whiney U test, and presented as mean ± SEM. (c) Flow cytometric analysis (left) of the Annexin V+ PI+ cells frequencies and Ki67+ cells frequencies of CD45−EpCAM+epithelial cells (n = 24) in fresh samples treated with isotype or Pembrolizumab for 12 hours. (d) Flow cytometric analysis of the Annexin V+PI+ cells frequencies and Ki67+ cells frequencies of CD45−EpCAM+epithelial cells (n = 24) in CCR5+CD66b+TINs high/low subgroups. (e) Flow cytometric analysis of the IFN-γ+ cells frequencies of CD66b+TINs cells (n = 16) in fresh samples treated with isotype or pembrolizumab for 12 hours. (f) Flow cytometric analysis of the Ki67+, PRF-1+, IFN-γ+, GZMB+, IL2+, TNF-α+ cells frequencies of CD8 T cells (n = 24) in fresh samples treated with isotype or pembrolizumab for 12 hours. (d, f) CCR5+ CD66b+TINs High/low were grouped by median. (c-f) Data were analyzed using Wilcoxon signed-rank test, and presented as mean ± SEM. IFN = interferon; GZMB = granzyme B; PRF-1 = perforin; TNF = Tumor Necrosis Factor.
The graphical summary of this study is shown in .
Discussion
Previous studies based on murine or human TINs demonstrated that neutrophils could execute a dual influence on carcinogenesis, promotion to metastasis and response to therapy.Citation1,Citation3,Citation6,Citation10,Citation12,Citation18-Citation20 In recent years, CD66b+TINs have been introduced as a significant prognostic factor of survival in various cancer types.Citation16,Citation18,Citation20-Citation22 Interestingly, the role played by TINs might be opposite even in the same cancer type.Citation18,Citation22 These findings suggest an urgency to identify the multiple subsets of neutrophil in the tumor microenvironment. In this study, we described a subset of CD66b+TINs, CCR5+CD66b+TINs, were densely populate in MIBC, especially in early-stage human MIBC, which was consistent with previous study of CD66b+TINs in early-stage human lung cancer.Citation15 Apart from these, we found out that CCR5+CD66b+TINs were negatively associated with MIBC progression. Similarly, early research on murine models reported that TINs in early tumors were more cytotoxic to tumor cells than TINs in larger, established tumors.Citation23 Additionally, the analysis of the clinically annotated TMAs from two clinical centers reveals that CCR5+CD66b+TINs high infiltration could be associated with favorable prognosis including OS and RFS, which is an independent significant factor for prognosis, especially RFS. Meanwhile, we assessed the correlation between CCR5+CD66b+TINs infiltration and clinical benefits from ACT. Our previous studies revealed the heterogeneity of TINs in terms of their controversial role in predicting therapeutic response to ACT.Citation16,Citation18 Gastric cancer patients with high TINs would benefit from ACT,Citation18 while MIBC patients with low TINs were easier to benefit from ACT than those with high TINs.Citation16 The current study demonstrated that, only in pT2N0 patients, patients with CCR5+CD66b+TINs high infiltration subgroup showed better survival benefit from ACT application. In clinical guideline of MIBC, ACT was recommended for patients with pT3/T4 or pN+.Citation2 CCR5+CD66b+TINs high infiltration subgroup of those patients could not have significant therapeutic response to ACT. Consequently, these CCR5+CD66b+ TINs high, pT3/T4 or pN+ MIBC patients should be recommended for more stringent follow-up and other postoperative adjuvant therapy combined with conventional ACT.
Potential mechanisms behind the favorable clinical outcomes of CCR5+CD66b+ TINs infiltration in MIBC were investigated in detail. Previous studies revealed that CCR5 might be expressed on several kinds of cancer cells, including breast cancer,Citation24 and melanoma,Citation25 and exert an important influence on tumor progression. However, other studies exhibited CCR5 could mediate Th1 cells homingCitation26 and IFN-γ secretion within CCR5+CD8+T cellsCitation27 and CCR5+effector T cells.Citation26 CCR5+CD66b+TINs could secret IFN-γ, which could be partly reduced by maraviroc as the same as CCR5+CD8+T cells.Citation27 However, IFN-γ production of CCR5+CD66b+TINs isolated from three MIBC samples did not reduce after maraviroc treatment, which suggests that the IFN-γ secretion of CCR5+CD66b+TINs in MIBC is not only in a CCR5 dependent way and remains to be further investigated. CCR5+CD66b+TINs could possibly orchestrate prime anti-tumor immunity, which could be proved by the analysis on immune cells and effector molecules of CD8 T cells, NK cells and CD4+Foxp3−T cells between CCR5+CD66b+TINs high/low subgroups in MIBC. IFN-γ could suppress tumor by acting directly on tumor cells, strengthening the function of tumor-infiltrating immune cells (Th1 cells, CD8 T cells and other cells) and modulating stromal cell function to alter metabolism and suppress angiogenesis.Citation28–Citation30 In addition, IFN-γ could improve the efficacy of chemotherapy with cisplatin and doxorubicin by targeting stromal cell functions.Citation31 Furthermore, IFN-γ could contribute to immunotherapy by acting on endothelial cells to promote blood vessel normalization and regression.Citation28,Citation32,Citation33 Nevertheless, the underlying mechanism behind CCR5-mediated and IFN-γ secretion of CD66b+TINs still remains to be further explored.
The abysmal prospect of MIBC has been changed as the intervention of ICIs. Still, quite a lot of patients do not experience benefit from ICIs.Citation4,Citation5 Potential biomarkers of ICIs’ clinical response have been observed, however, these biomarkers are not yet ready for clinical practice for lack of prospective clinical studies.Citation34 To simulate the clinical ICIs response in MIBC patients, fresh tumor samples were performed with pembrolizumab ex vivo in our study. Targeting PD-1 could restore anti-tumor immunity of CD8 T cells and induce tumor cells apoptosis.Citation35 However, increased apoptosis and decreased proliferation of tumor cells along with increased cytotoxicity of CD8 T cells was observed only in CCR5+CD66b+ high subgroup. Pembrolizumab treatment might not restore the IFN-γ production by neutrophils, while previous study showed that the phagocytic function of neutrophils in patients with sepsis could be restored by anti–PD-L1 or anti–PD-1 mAbs.Citation36 CCR5+CD66b+TINs might be a new potential biomarker for predicting pembrolizumab response in MIBC, while the mechanism should be established further.
Several limitations of this study should be mentioned. The major limitation is inherent to the retrospective nature of the study. In addition, the mechanism of high CCR5+CD66b+TINs infiltration associated with better ACT response still remains unknown. On the basis of our findings, we will further establish whether and how CCR5 ligands CCL3/4/5 is involved in IFN-γ production in MIBC through in vitro and in vivo experiments. Moreover, the exact biologic mechanism under this observation that CCR5+CD66b+TINs high infiltration might be associated with better response to anti-PD-1 treatment is still unclear.
In summary, CCR5+CD66b+TINs could be as an independent favorable prognosis factor for clinical outcomes. CCR5+CD66b+TINs high infiltration could also become a potential biomarker for both better ACT benefit in pT2N0 MIBC and more effective response to anti-PD-1 treatment. In addition, CCR5+CD66b+TINs could enhance CD8 T cells activation and cytokine production through autonomous IFN-γ release, which potentially orchestrate antitumor immune independently on tumor progression in MIBC tumor immune microenvironment at the same time. These findings might elevate the personalized prognosis prediction and ACT and ICIs application of MIBC patients after cystectomy.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Authors’ contributions
Z. X., Q. Z., H. Z. and Z. W. for acquisition of data, analysis and interpretation of data, statistical analysis and drafting of the manuscript; H. Z., Z. L., Q. H., Y. C., Q. B., Y. X., Y. W., L. L., Y. Z., L. X. and B. D. for technical and material support; J. W., J. G. and J. X. for study concept and design, analysis and interpretation of data, drafting of the manuscript, obtained funding and study supervision. All authors read and approved the final manuscript.
Abbreviations
ACT : adjuvant chemotherapy
CI: confidence interval
DMSO: dimethyl sulfoxide
FMO: fluorescence minus one
FUSCC: Fudan University Shanghai Cancer Center
FUZH: Zhongshan Hospital, Fudan University
HR: Hazard Ratio
ICIs: immune checkpoint inhibitors
IF: immunofluorescence
IFN-γ: interferon-γ
IHC: immunohistochemistry
MDSC: myeloid-derived suppressor cells
MIBC: muscle-invasive bladder cancer
ORR: sobjective response rates
OS: overall survival
RC: radical cystectomy
RFS: recurrence-free survival
TINs: tumor-infiltrating neutrophils
TMA: tissue microarray
TMB: tumor mutation burden
UC: urothelial cancer
Acknowledgments
We thank Dr. Lingli Chen (Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai, China) and Dr. Peipei Zhang (Department of Pathology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China) for their excellent pathological technology help.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7.
- Alfred WJ, Lebret T, Comperat EM, Cowan NC, De Santis M, Bruins HM, Hernandez V, Espinós EL, Dunn J, Rouanne M, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71:462.
- Rodriguez-Vida A, Perez-Gracia JL, Bellmunt J. Immunotherapy combinations and sequences in urothelial cancer: facts and hopes. Clin Cancer Res. 2018;24:6115.
- Felsenstein KM, Theodorescu D. Precision medicine for urothelial bladder cancer: update on tumour genomics and immunotherapy. Nat Rev Urol. 2018;15:92.
- Lattanzi M, Balar AV. Current status and future direction of immunotherapy in urothelial carcinoma. Curr Oncol Rep. 2019;21:24.
- Ng LG, Ostuni R, Hidalgo A. Heterogeneity of neutrophils. Nat Rev Immunol. 2019;19:255.
- Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol. 2019;16:601.
- Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:431.
- Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33:949.
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183.
- Veglia F, Tyurin VA, Blasi M, De Leo A, Kossenkov AV, Donthireddy L, To TK, Schug Z, Basu S, Wang F, Ricciotti E. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature. 2019;569:73.
- Ponzetta A, Carriero R, Carnevale S, Barbagallo M, Molgora M, Perucchini C, Magrini E, Gianni F, Kunderfranco P, Polentarutti N, et al. Neutrophils driving unconventional T cells mediate resistance against murine sarcomas and selected human tumors. Cell. 2019;178:346.
- Shaul ME, Fridlender ZG. Cancer-related circulating and tumor-associated neutrophils - subtypes, sources and function. Febs J. 2018;285:4316.
- Singhal S, Bhojnagarwala PS, O’Brien S, Moon EK, Garfall AL, Rao AS, Quatromoni JG, Stephen TL, Litzky L, Deshpande C, et al. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell. 2016;30:120.
- Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, Akimova T, Vachani A, Litzky L, Hancock WW, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest. 2014;124:5466.
- Zhou L, Xu L, Chen L, Fu Q, Liu Z, Chang Y, Lin Z, Xu J. Tumor-infiltrating neutrophils predict benefit from adjuvant chemotherapy in patients with muscle invasive bladder cancer. Oncoimmunology. 2017;6:e1293211.
- Lin C, He H, Liu H, Li R, Chen Y, Qi Y, Jiang Q, Chen L, Zhang P, Zhang H, et al. Tumour-associated macrophages-derived CXCL8 determines immune evasion through autonomous PD-L1 expression in gastric cancer. Gut. 2019;68:1764.
- Zhang H, Liu H, Shen Z, Lin C, Wang X, Qin J, Qin X, Xu J, Sun Y. Tumor-infiltrating neutrophils is prognostic and predictive for postoperative adjuvant chemotherapy benefit in patients with gastric cancer. Ann Surg. 2018;267:311.
- Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, Scheidmann MC, Donato C, Scherrer R, Singer J, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566:553.
- Galdiero MR, Bianchi P, Grizzi F, Di Caro G, Basso G, Ponzetta A, Bonavita E, Barbagallo M, Tartari S, Polentarutti N, Malesci A. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. Int J Cancer. 2016;139:446.
- Governa V, Trella E, Mele V, Tornillo L, Amicarella F, Cremonesi E, Muraro MG, Xu H, Droeser R, Däster SR, et al. The interplay between neutrophils and CD8+ T cells improves survival in human colorectal cancer. Clin Cancer Res. 2017;23:3847.
- Wang TT, Zhao YL, Peng LS, Chen N, Chen W, Lv YP, Mao FY, Zhang JY, Cheng P, Teng YS, et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut. 2017;66:1900.
- Mishalian I, Bayuh R, Levy L, Zolotarov L, Michaeli J, Fridlender ZG. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol Immunother. 2013;62:1745.
- Velasco-Velazquez M, Jiao X, De La Fuente M, Fuente M, Pestell TG, Ertel A, Lisanti MP, Pestell RG, et al. CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer Res. 2012;72:3839.
- Umansky V, Blattner C, Gebhardt C, Utikal J. CCR5 in recruitment and activation of myeloid-derived suppressor cells in melanoma. Cancer Immunol Immunother. 2017;66:1015.
- Li J, McArdle S, Gholami A, Kimura T, Wolf D, Gerhardt T, Miller J, Weber C, Ley K. CCR5+T-bet+FoxP3+ effector CD4 T cells drive atherosclerosis. Circ Res. 2016;118:1540.
- Wang X, Russell-Lodrigue KE, Ratterree MS, Veazey RS, Xu H. Chemokine receptor CCR5 correlates with functional CD8+ T cells in SIV-infected macaques and the potential effects of maraviroc on T-cell activation. Faseb J. 2019;33:8905.
- Ivashkiv LB. IFNγ: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat Rev Immunol. 2018;18:545.
- Parker BS, Rautela J, Hertzog PJ. Antitumour actions of interferons: implications for cancer therapy. Nat Rev Cancer. 2016;16:131.
- Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836.
- Hannesdóttir L, Tymoszuk P, Parajuli N, Wasmer MH, Philipp S, Daschil N, Datta S, Koller JB, Tripp CH, Stoitzner P, et al. Lapatinib and doxorubicin enhance the Stat1-dependent antitumor immune response. Eur J Immunol. 2013;43:2718.
- Kammertoens T, Friese C, Arina A, Idel C, Briesemeister D, Rothe M, Ivanov A, Szymborska A, Patone G, Kunz S, et al. Tumour ischaemia by interferon-γ resembles physiological blood vessel regression. Nature. 2017;545:98.
- Tian L, Goldstein A, Wang H, Lo HC, Kim IS, Welte T, Sheng K, Dobrolecki LE, Zhang X, Putluri N et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017;544:250.
- Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, Peters S. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30:44.
- Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187.
- Patera AC, Drewry AM, Chang K, Beiter ER, Osborne D, Hotchkiss RS. Frontline science: defects in immune function in patients with sepsis are associated with PD-1 or PD-L1 expression and can be restored by antibodies targeting PD-1 or PD-L1. J Leukoc Biol. 2016;100:1239.