ABSTRACT
This brief report details the measurement and identification of IgA antibodies to tropomyosin in a case of presumed ocular myositis with paraspinal myositis in a patient with metastatic uveal melanoma treated with checkpoint inhibitors. High-throughput functional protein microarray analysis and pathway analysis was conducted to identify IgG and IgA antibodies of interest. Antibody levels were compared to generic antibody screening results and levels of the antibodies in a cohort of melanoma patients without myositis (n = 100) at baseline prior to undergoing immunotherapy. The finding of specific muscle antibodies in this clinical case indicates the pathogenic potential of anti-tropomyosin IgA in the development of checkpoint inhibitor associated myositis and requires further investigation.
Brief report
We recently reported a case of presumed ocular myositis with paraspinal myositis in a patient with metastatic uveal melanoma treated with checkpoint inhibitors Pembrolizumab and Ipilimumab/Nivolumab.Citation1 Conventional IgG autoantibody screening in this case was negative (anti-AChR, anti-ganglioside, myositis specific antibodies and anti-MuSK). The patient’s level of creatine kinase (2452 U/l) and troponin I (0.36 micrograms/l) was elevated and an MRI of the cervical muscle was consistent with myositis.
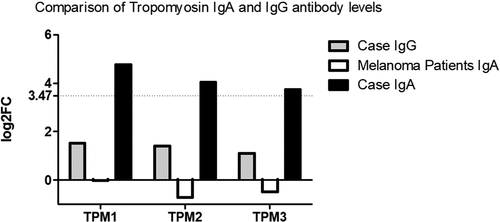
Serum was taken, two days after hospital admission and prior to steroid and intravenous immunoglobulin infusion that achieved complete clinical response. Unfortunately, the patient passed away at 12 months post immunotherapy commencement due to cerebral metastases and a further trial of radiotherapy and other checkpoint inhibitors. Retrospective informed consent was obtained from the legal representative of the deceased and is available upon request. The serum was analyzed by microarray for IgG and IgA binding efficiencies (Supplementary Tables 1 and 2, respectively) to greater than 21000 antigens (>81% of the human proteome) using the HuProtTM microarray (CDI Laboratories, USA). The sample was probed on the array at a 1:1000 dilution and analyzed for Z scores, which identified 84 and 172 positive hits (Z score > 3) for IgG and IgA respectively. Log2 fold changes (log2FC) were calculated by dividing the case signal intensity for each antigen by the mean case signal intensity of all antigens or the median signal intensity of all antigens across the cohort of other metastatic melanoma samples. In particular, the microarray results demonstrated high levels of IgA antibodies to tropomyosin (TPM) isoforms 1, 2 and 3 while IgG antibodies to these antigens were found to be negative. Tropomyosin 3 is essential for melanoma metastasis, enabling pseudopodium and invadopodium formation.Citation2 Sera from a group of metastatic melanoma patients without myositis (n = 100) at baseline prior to undergoing immunotherapy who were also screened on the array (unpublished, Edith Cowan University Ethics Committee application number 18957) were negative to these antigens ().
Figure 1. Comparison of IgA and IgG levels to tropomyosin isoforms 1, 2 and 3 in the reported clinical case study. IgA levels from the sera of a group of metastatic melanoma patients without myositis (n = 100) at baseline prior to undergoing immunotherapy are also illustrated. Levels are presented as Log2 fold changes (log2FC) and were calculated by dividing the case signal intensity for each antigen by the mean case signal intensity of all antigens or the median signal intensity of all antigens across the cohort of other metastatic melanoma samples. The horizontal line at log2FC = 3.47 represents the cutoff where the case Z score > 3, indicating a positive signal intensity.
The log2 IgA and IgG data for all antigens in this case study was then uploaded to Advaita Bio’s iPathwayGuide (http://www.advaitabio.com/ipathwayguide) software to determine major pathways enriched by the differentially elevated antibodies (p-value < 0.05 and a |log2FC| > 3.47 (Z score > 3). A detailed description of the pathway analysis approach utilized is described elsewhere.Citation3 Using this software, a total of 97 elevated antibodies were identified and 19 biological pathways were found to be significantly impacted, with cardiac muscle contraction, carbon metabolism and hypertrophic cardiomyopathy pathways showing the most significant involvement in the case study’s IgA binding profile (p = 5.188 x 10−4, p = .004, p = .007, respectively). Similar to the microarray results, the most significant identified biological components associated with the case IgA binding profile, included muscle thin filament tropomyosin, striated muscle thin filament and myofilament (p = 7.4 x 10−7, p = 8.1 x 10−5, p = 9.9 x 10−5, respectively).
Myositis is an autoimmune/antibody-mediated condition and several myositis-related and -associated autoantibodies have been identified to date.Citation4,Citation5 The regionalization of the myositis (paraspinal, ocular and myocardial) in this case is interesting as in a mouse model of muscular dystrophy, deletion of a tropomyosin 3 isoform (Tpm3.1) caused muscle disease in a similar distribution.Citation6 Garaud et al.Citation7 performed microarray antibody analysis in breast cancer, with sera and breast tissue displaying high levels of tumor specific IgA to tumor antigens, including cancer/testis antigen 1B (CTAG1B) and ankyrin repeat domain 30B like protein (ANKRD30BL). These were not related to tumor progression or survival. There was however, a correlation with the development of tertiary lymphoid structures within the tumor suggesting local IgA production. B cell infiltration in tumors is rare but B cell activation does occur in primary, secondary and tertiary lymphoid structures and antibodies may play a role in tumor destruction or progression. Whilst IgA is unable to directly activate the complement pathway, it can do so via the mannose lectin pathway. It is now recognized that monomeric IgA opsonised on cell membranes is able to cause apoptosis and necrosis by binding to the FcαR1 receptor (CD89) on neutrophils.Citation8 The exact mechanism of subsequent tissue damage is under debate and a new novel process called trogoptosis has been suggested.Citation9
In summary, the finding of specific muscle antibodies in this case may have played a role in the checkpoint induced myositis and further studies are required to elucidate the pathogenic potential of anti-tropomyosin IgA antibodies.
Author contributions
Acknowledgments
We would like to thank the patient of this report and his family for their permission to study of these clinical findings. We would also like to thank Professor Merrilee Needham, Consultant Neurologist at the Perron Institute for Neurological and Translational Science, for her help with reviewing this manuscript.
Disclosure statement
The authors of the manuscript declare no conflict of interest.
Supplemental Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Ozarczuk TR, Prentice DA, Kho LK, vanHeerden J. Checkpoint inhibitor myasthenia-like syndrome and myositis associated with extraocular muscle atrophy. J Clin Neurosci. 2020;71:271–2. doi:10.1016/j.jocn.2019.11.038.
- Miyado K, Kimura M, Taniguchi S. Decreased expression of a single tropomyosin isoform, TM5/TM30nm, results in reduction in motility of highly metastatic B16-F10 mouse melanoma cells. Biochem Biophys Res Commun. 1996;225:427–435. doi:10.1006/bbrc.1996.1190.
- Draghici S, Khatri P, Tarca AL, Amin K, Done A, Voichita C, Georgescu C, Romero R. A systems biology approach for pathway level analysis. Genome Res. 2007;17(10):1537–1545. doi:10.1101/gr.6202607.
- Milone M. Diagnosis and management of immune-mediated myopathies. Mayo Clin Proc. 2017;92:826–837. doi:10.1016/j.mayocp.2016.12.025.
- Palterer B, Vitiello G, Carraresi A, Giudizi MG, Cammelli D, Parronchi P. Bench to bedside review of myositis autoantibodies. Clin Mol Allergy. 2018;16:5. doi:10.1186/s12948-018-0084-9.
- Kee AJ, Schevzov G, Nair-Shalliker V, Robinson CS, Vrhovski B, Ghoddusi M, Qiu MR, Lin JJC, Weinberger R, Gunning PW, et al. Sorting of a nonmuscle tropomyosin to a novel cytoskeletal compartment in skeletal muscle results in muscular dystrophy. J Cell Biol. 2004;166(5):685–696. doi:10.1083/jcb.200406181.
- Garaud S, Zayakin P, Buisseret L, Rulle U, Silina K, de Wind A, Van den Eyden G, Larsimont D, Willard-Gallo K, Linē, A. Antigen specificity and clinical significance of IgG and IgA autoantibodies produced in situ by tumor-infiltrating b cells in breast cancer. Front Immunol. 2018;9. doi:10.3389/fimmu.2018.02660.
- Brandsma AM, Bondza S, Evers M, Koutstaal R, Nederend M, Jansen JHM, Rösner T, Valerius T, Leusen JHW, Ten Broeke, T. Potent Fc receptor signaling by IgA leads to superior killing of cancer cells by neutrophils compared to IgG. Front Immunol. 2019;10:704. doi:10.3389/fimmu.2019.00704.
- Matlung HL, Babes L, Zhao XW, van Houdt M, Treffers LW, van Rees DJ, Franke K, Schornagel K, Verkuijlen P, Janssen H, et al. Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep. 2018;23(13):3946–3959.e3946. doi:10.1016/j.celrep.2018.05.082.
