ABSTRACT
Cancer immunotherapy based on anti-PD-1/PD-L1 blockade is particularly effective in responding to patients with hot tumors. These tumors are characterized by the accumulation of proinflammatory cytokines and T cell infiltration. In our recent report published in Science Advances, we demonstrate that targeting the autophagy-related protein Vps34 switched cold immune desert tumors into hot inflamed immune-infiltrated tumors and enhanced the efficacy of anti-PD-1/PD-L1. Our study provides the preclinical rationale to set up combination immunotherapy clinical trials using selective Vps34 inhibitors and immune checkpoint blockers in melanoma and CRC.
Immune checkpoint blockade (ICB)-based cancer immunotherapy revolution has just started and it has transformed the field of immuno-oncology and the way we used to treat cancer. During the past 10 years, treatment with ICB as single agents or in combination has shown impressive clinical benefits in diverse cancers including melanoma and colorectal carcinoma (CRC). Despite the exciting and encouraging clinical responses obtained, the majority of patients treated with anti-CTLA-4, anti-PD-1, or anti-PD-L1 monotherapy still show none or partial objective responses.Citation1 Therefore, to achieve durable tumor regression in melanoma and CRC patients, there is a strong need to design new combination immunotherapies in order to bring the benefit of ICB to a larger number of cancer patients.
In cold immune desert tumors, one of the most important reasons of non-responsiveness to ICB is the complete absence or the limited presence of cytotoxic immune effectors notably T cells. It is now well established that driving cytotoxic T cells into immune cold tumors significantly improves their response to ICB. Undeniably, durable clinical responses to ICB have been observed in melanoma patients with hot immune infiltrated tumors harboring preexisting cytotoxic effector T cells. Therefore, many ongoing clinical trials in immuno-oncology are focusing on strategies making cold tumors hot, so patients can better benefit from ICB.Citation2
We have previously reported that hypoxia-induced autophagy in tumor cells was associated with tumor resistance to cytotoxic T lymphocyte-Citation3 and natural killer cells (NK)-mediated killing.Citation4 Additionally, we showed that targeting autophagy improved the efficacy of cancer vaccination and promoted tumor regression in melanoma.Citation3 We further revealed that silencing the autophagy-related protein Beclin1 decreased tumor growth by inducing a massive infiltration of NK cells through the release of CCL5/RANTES by melanoma cells.Citation5 However, the effect of targeting autophagy on the tumor immune landscape and its impact on the therapeutic benefit of ICB is still poorly explored.Citation6,Citation7
Vacuolar protein sorting 34 (Vps34), also known as class III phosphoinositide 3-kinase (PIK3C3) is involved in the initiation of autophagy and in the process of endocytosis.Citation8 We first assessed the impact of targeting Vps34 (both genetically and pharmacologically) on tumor growth and tumor weight in different cancer models. Genetic targeting or pharmacological inhibition of Vps34 kinase activity using two Vps34 inhibitors (Vps34i) SB02024 and SAR405 significantly decreased tumor growth, tumor weight, and improved mice survival in B16-F10 melanoma, CT26 colorectal cancer, Renca renal cell carcinoma, and genetically engineered melanoma mouse (GEMM) tumors-bearing mice. We next investigated whether Vps34-dependent inhibition of tumor growth was associated with a modulation of the tumor immune landscape. We observed an increased infiltration of major immune effector cells (NK, CD8+ and CD4+ T cells, DC and M1 macrophages) in Vps34i-treated B16-F10 and CT26 tumors as compared to vehicle-treated control tumors. By using immunocompromised NOD SCID Gamma (NSG) mice or immunocompetent mice depleted for NK or CD8+ cells, we demonstrated that both NK and CD8+ T effector cells are the major immune cells controlling the growth of Vps34-targeted B16-F10 tumors.Citation9 We next evaluated the expression of several proinflammatory chemokines/cytokines involved in the recruitment of CD8+ T cells into human melanomas.Citation10 Our data indicated that Vps34 inhibition induced the secretion of proinflammatory CCL5, CXCL10, and IFNγ within the tumor microenvironment, most likely generated by melanoma and CRC tumor cells. In addition, antibodies blocking CCL5 in Vps34i-treated B16-F10 and CT26 tumor-bearing mice significantly rescued the inhibition of tumor growth and weight and prevented infiltration of NK and CD8+ into the tumor bed. Based on these data, we concluded that targeting Vps34 induces CCL5 and CXCL10 in tumor cells, which subsequently attract more NK and CD8+ T cells and reprogram cold immune desert into hot inflamed immune infiltrated melanoma and CRC tumors. Mechanistically, we reported that pharmacological inhibition of Vps34 by SB02024 or SAR405 induced both STAT1 and IRF7 which independently upregulate the proinflammatory cytokines CCL5 and CXCL10 (). We next evaluated whether Vps34i could improve the response to anti-PD-L1 or anti-PD-1 in terms of tumor growth, tumor weight, and mice survival. Interestingly, combining Vps34i with either anti-PD-L1 or anti-PD-1 significantly improved their therapeutic benefit as compared to anti-PD-L1 or PD-1 monotherapy, in both B16-F10 and CT26 tumor models.Citation9
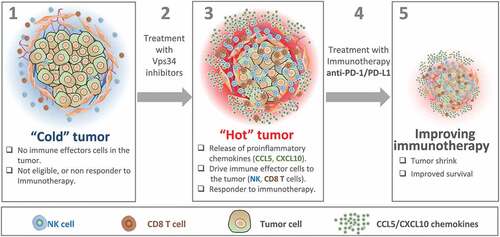
Figure 1. Vps34 inhibition improves anti-PD-1/PD-L1 immunotherapy by switching cold into hot tumors. Cold tumors are characterized by the absence of immune cells or the limited number of cytotoxic immune cells in the tumor microenvironment. Therefore, cold tumors are not eligible or most likely not responding to immunotherapy (1). Treatment of cold tumors with Vps34 inhibitors (2) induces the release by tumor cells of proinflammatory chemokines such as CCL5 and CXCL10. These chemokines drives more NK and CD8 T cells to the tumor microenvironment. Vps34i-treated tumors become hot and therefore eligible to anti-PD-1/PD-L1 based immunotherapy (3). Combined Vps34i with anti-PD-1/PD-L1 (4) improves the therapeutic benefit of immunotherapy and significantly decreases the tumor growth.Citation5
Solid tumor microenvironment consists of not only tumor cells but also endothelial cells, fibroblasts, and diverse subsets of innate and adaptive immune cells. The influence of Vps34i on the composition, proportion, activation, or functional states of various immune populations within the tumor bed remains largely uninvestigated. Therefore, it would be interesting to carry out a deep phenotypic, functional, and metabolic characterization of different immune cells infiltrating Vps34i tumors, notably NK and CD8+ T cells.
Our current study provides an innovative therapeutic approach for designing novel clinical trials using Vps34i in combination with several immune checkpoint blockers to extend their use to non-responder cancer patients.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author’s view
Noman MZ, Parpal S, Van Moer K, Xiao M, Yu Y, Viklund J, De Milito A, Hasmim M, Andersson M, Amaravadi RK, Martinsson J, Berchem G, Janji B. Inhibition of Vps34 reprograms cold into hot inflamed tumors and improves anti-PD-1/PD-L1 immunotherapy. Sci Adv. 2020 April 29;6(18):eaax7881. doi: 10.1126/sciadv.aax7881.
Additional information
Funding
References
- Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016;13(3):143–2. doi:10.1038/nrclinonc.2015.209.
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi:10.1038/nature13954.
- Noman MZ, Janji B, Kaminska B, Van Moer K, Pierson S, Przanowski P, Buart S, Berchem G, Romero P, Mami-Chouaib F, et al. Blocking hypoxia-induced autophagy in tumors restores cytotoxic T-cell activity and promotes regression. Cancer Res. 2011;71(18):5976–5986. doi:10.1158/0008-5472.CAN-11-1094.
- Baginska J, Viry E, Berchem G, Poli A, Noman MZ, van Moer K, Medves S, Zimmer J, Oudin A, Niclou SP, et al. Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer-mediated lysis under hypoxia. Proc Natl Acad Sci U S A. 2013;110(43):17450–17455. doi:10.1073/pnas.1304790110.
- Mgrditchian T, Arakelian T, Paggetti J, Noman MZ, Viry E, Moussay E, Van Moer K, Kreis S, Guerin C, Buart S, et al. Targeting autophagy inhibits melanoma growth by enhancing NK cells infiltration in a CCL5-dependent manner. Proc Natl Acad Sci U S A. 2017;114(44):E9271–E9. doi:10.1073/pnas.1703921114.
- Poillet-Perez L, Xie X, Zhan L, Yang Y, Sharp DW, Hu ZS, Su X, Maganti A, Jiang C, Lu W, et al. Autophagy maintains tumour growth through circulating arginine. Nature. 2018;563(7732):569–573. doi:10.1038/s41586-018-0697-7.
- Yang A, Herter-Sprie G, Zhang H, Lin EY, Biancur D, Wang X, Deng J, Hai J, Yang S, Wong -K-K, et al. Autophagy sustains pancreatic cancer growth through both cell-autonomous and nonautonomous mechanisms. Cancer Discov. 2018;8(3):276–287. doi:10.1158/2159-8290.CD-17-0952.
- Marsh T, Debnath J. Ironing out VPS34 inhibition. Nat Cell Biol. 2015;17(1):1–3. doi:10.1038/ncb3089.
- Noman MZ, Parpal S, Van Moer K, Xiao M, Yu Y, Viklund J, De Milito A, Hasmim M, Andersson M, Amaravadi RK, et al. Inhibition of Vps34 reprograms cold into hot inflamed tumors and improves anti-PD-1/PD-L1 immunotherapy. Sci Adv. 2020;6(18):eaax7881. doi:10.1126/sciadv.aax7881.
- Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69(7):3077–3085. doi:10.1158/0008-5472.CAN-08-2281.
