ABSTRACT
The consensus Immunoscore has a prognostic value that has been confirmed in two randomized phase 3 clinical trials, and it provides a reliable estimate of the recurrence risk in colon cancer. The latest edition of the WHO classification of the Digestive System Tumors introduced for the first time the immune response as an essential and desirable diagnostic criteria for digestive cancers. Therefore, the immune response and Immunoscore evaluation within the tumor microenvironment is clinically relevant. In addition, the evaluation of the Immunoscore in stage III colon cancer patients from the IDEA France clinical trial evaluating 3 versus 6 months of oxaliplatin-based adjuvant chemotherapy demonstrated the predictive value of Immunoscore for treatment duration. Immunoscore predicted response to 6 months FOLFOX chemotherapy both in low- and high-risk Stage III patients. Low-risk patients (T1-3, N1) with High-Immunoscore had the 3-year DFS of 91.4% when treated with the 6-month FOLFOX, and only 80.8% with the 3-month regimen. The international validation of the prognostic value of the consensus Immunoscore together with its predictive value to guide treatment provides important information for the personalized management of colon cancer patients.
Impact of immunity on colon cancer patients
The IDEA-France prospective study, together with another phase 3 randomized clinical trial (N0147)Citation1 validated the value of Immunoscore in prognostication of relapse and death in stage III CC patients treated with a standard adjuvant treatment combining fluoropyrimidine and oxaliplatin. Similar Immunoscore prognostic results were found in N0147 and IDEA phase 3 trials. The Immunoscore-N0147 study was conducted in collaboration with clinicians and researchers from the Mayo Clinic.Citation1 The Immunoscore-IDEA France study was conducted in collaboration with PRODIGE, a digestive oncology intergroup gathering the GERCOR, the FFCD and UNICANCER organizations.Citation2,Citation3 The two studies were performed, using the pre-defined consensus Immunoscore, and included 559 patient samples from the FOLFOX alone arm of the NCCTG-N0147 trial, and 1062 patient samples from both arms (3 vs 6 months) of the IDEA France trial.
The American-Joint-Committee-on-Cancer/Union-for-International-Cancer-Control (AJCC/UICC) TNM staging-system is the standard tool to guide patient treatment strategies and predict colon cancer (CC) prognosis. Adjuvant therapy using the fluorouracil, leucovorin, and oxaliplatin (FOLFOX) regimen or capecitabine and oxaliplatin (CAPOX) regimen is the standard of care for patients with stage III CC. The International Duration Evaluation of Adjuvant Chemotherapy (IDEA) collaboration study prospectively evaluated the noninferiority of 3 versus 6 months of adjuvant therapy with either FOLFOX or CAPOX in patients with resected stage III CC, in phase 3 randomized trials. The primary objective of the study was not reached, and it was not possible to conclude to overall noninferiority of the 3 months arm in this trial. However, the IDEA study showed that shorter duration of adjuvant therapy was associated with a significantly lower incidence and severity of adverse events, especially for the long-lasting oxaliplatin-induced peripheral sensory neuropathy. Subgroup analyses showed that CAPOX met the criteria for non-inferiority, but those for FOLFOX did not. Since differences in DFS by adjuvant treatment duration were relatively modest, the data suggested that risk categories based on T and N stage grouping could be used to inform the duration of adjuvant chemotherapy. Specifically, low-risk (T1-3N1) patients were shown to have similar outcomes after 3 vs 6 months of CAPOX, but 3 months was statistically inferior to 6 months of FOLFOX in the high-risk (T4 and/or N2) group.Citation4 This study underlines the need for additional biomarkers for the efficacy of adjuvant chemotherapy in CC patients. Following these results on DFS, OS results were very recently communicated and showed very modest differences between 3 and 6 months of adjuvant therapy altogether, but still with a possible interest of 6 months FOLFOX in high-risk patients. Altogether, though not statistically significant, the non-inferiority of 3 vs 6 months of chemotherapy is well accepted for low-risk stage III patients but the consensus is more debated for high-risk stage III patients. Thus, tools to better classify patients needing 6 months of adjuvant therapy would be of major interest.
Multiple ways to classify CC have been proposed. These ways rely on tumor cell characteristics including molecular pathways, mutational status, and tumor gene expression-based methods. The major importance of the preexisting adaptive immune reaction within human tumors (i.e. Immunoscore) for prognostic purpose was demonstrated in 2006,Citation5 leading to paradigm shift in oncology.Citation6-Citation8 The latest edition of the WHO classification of the Digestive System Tumors introduced for the first time the immune response as essential and desirable diagnostic criteria for digestive cancer. The immune status of the tumor might further influence the magnitude of the response to chemotherapy since these agents are impacting the immunity of the patients.
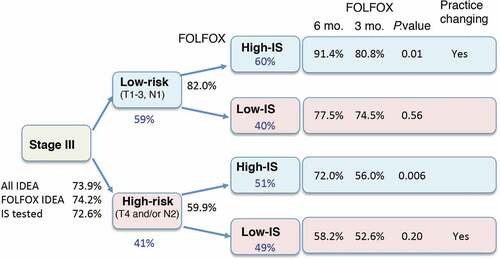
The consensus Immunoscore is the first internationally validated standardized digital-pathology-based assay to quantify the immune infiltrate. The evaluation of Immunoscore in the IDEA France trial allowed us to test the prognostic and predictive values of Immunoscore. The primary objective of this prospective ancillary study was to validate the prognostic performance of Immunoscore to predict DFS in stage III CC patients included in the IDEA France cohort study. The secondary objective was to investigate the predictive value of Immunoscore in terms of DFS in patients receiving 3 versus 6 months of oxaliplatin-based adjuvant chemotherapy. Densities of CD3+ and CD8 + T cells in the tumor and invasive margin of each patient were determined by immunohistochemistry, quantified by digital pathology, and converted into the pre-defined consensus Immunoscore for the 1062 available patients of the study.Citation3 Immunoscore Low and High were observed in 43.6%, 56.4% of patients, respectively. Immunoscore-Low identified patients at higher risk of relapse or death compared to Immunoscore-High (HR = 1.54; 95% CI, 1.24–1.93, P = .0001). The 3-year DFS was 66.8% (95% CI, 62.2–70.9) for Immunoscore-Low and 77.1% (95% CI, 73.0–80.4) for Immunoscore-High. In multivariable analysis, Immunoscore remained significantly independently associated with DFS (P = .0031) when adjusted for gender, histological grade, T-stage, N-stage, and MSI. For FOLFOX treated patients (91.6% of the cohort), a statistically significant interaction was observed for the predictive value of Immunoscore for treatment duration (3 vs 6 months) in terms of DFS. High-Immunoscore significantly predicted benefit of 6 months treatment (HR = 0.53; 95% CI, 0.37–0.75; Log-rank P = .0004), including clinical low- and high-risk stage III CC (all P < .001). Conversely, patients with Low-Immunoscore (46.4%) did not derive significant benefit from the 6-month FOLFOX versus 3-month. Thus, we report in the IDEA France trial a statistically significant interaction between Immunoscore and treatment duration suggesting the predictive value of Immunoscore in terms of DFS. Patients with stage III CC had a better outcome when treated for 6 months with FOLFOX as compared to 3 months if they had a high-Immunoscore but this was not observed anymore in patients with a low-Immunoscore. This was true independently of the clinical high- (T4 and/or N2) or low-risk (T1-3, N1) group, demonstrating the predictive value of Immunoscore. Patients with Low-Immunoscore (44.6%) appeared to be doubly penalized by an increased risk of recurrence and the lack of benefit from longer duration of treatment. Importantly, clinically low-risk patients (T1-3, N1) with High-Immunoscore had a 3-year DFS of 91.4% when treated with the 6-month FOLFOX, and only 80.8% with the 3-month regimen. Thus, even low-risk (T1-3, N1) patients could effectively benefit from 6 months of FOLFOX, depending on their Immunoscore status, thereby changing clinical practice (). Conversely, high-risk patients may not benefit from 6 months of adjuvant FOLFOX if they do have a low-Immunoscore (), and considering the poor outcome in these patients, new therapeutic options have to be developed. Component drugs in the FOLFOX regimen include 5-fluorouracil that may partially deplete or transiently inactivate inhibitory immune cells, and oxaliplatin that may increase cytotoxic T-cell infiltration and induce immunogenic cell death (ICD).Citation9 We can hypothesize that ICD-driven immunity can no longer operate in tumors classified as Low-Immunoscore, reflecting in situ immunological defects such as a weak immunogenicity of the tumor and/or immunosuppressive environment. Furthermore, Immunoscore within metastasis also predicted the risk of relapse and death in Stage IV colorectal cancer resected from all their metastatic lesions.Citation10,Citation11
Figure 1. Treatment decision-tree using low pathological risk (T1-3, N1) high pathological risk (T4 and/or N2) categories, and the consensus Immunoscore (IS) with pre-defined categories (high >70%, and low <70%). The 3 years DFS rates (%) (black), and proportion of patients (%) (blue) are illustrated. The predictive value of Immunoscore is calculated comparing 3 to 6 months FOLFOX treatment using Log rank statistical test.
The feasibility, robustness, and reproducibility of Immunoscore are essential steps for its integration in clinical practice. In a routine practice for prospective cases, the Immunoscore success rate exceeds 90% without retesting and 95% after retesting. Indeed, the analytical validity of Immunoscore was recently published.Citation12,Citation13 A limitation of our study is that 90% of patients in the IDEA France study were treated with the FOLFOX-regimen, precluding from any conclusion for patients receiving CAPOX. Furthermore, these important predictive results should be further validated in additional IDEA studies. Finally, ctDNA assessment after surgery seems to be a relevant marker of minimal residual disease and to stratify patients for their relapse risk and may bear complementary information to help guiding clinical decision.
Conclusion
These results strengthen Immunoscore Level of Evidence and position Immunoscore as an essential diagnostic tool to enable a personalized management of stage III CC patients. Indeed, in the latest (5th) edition of the WHO Digestive System Tumors classification, was introduced “the immune response as essential and desirable diagnostic criteria for colorectal cancer”, and citing the consensus Immunoscore as best clinical evidence in colon cancer. Furthermore, the 2020 ESMO Clinical Practice Guidelines for colon cancer included Immunoscore to refine the prognosis and thus adjust the chemotherapy decision-making process in stage II and even in low-risk stage III patients. These results and recent guidelines argue for the benefit of implementing the Immunoscore in clinical practice and for its introduction in a new TNM-Immune (TNMI) classification system. The Immunoscore assay has been developed as an in vitro diagnostic test (CE-IVD) and is available in FDA CLIA-certified laboratories for routine use. Thus, personalized colon cancer evaluation with Immunoscore should be done for better patient care management.
Disclosure of potential conflicts of interest
JG and FP have patents associated with the immune prognostic biomarkers. JG is co-founder of HalioDx biotech company. Immunoscore® a registered trademark from the National Institute of Health and Medical Research (INSERM).
Acknowledgments
The authors thank the patients, their caregivers, the GERCOR team, and the PRODIGE investigators.
Additional information
Funding
References
- Sinicrope FA, Shi Q, Hermitte F, Zemla TJ, Mlecnik B, Benson AB, Gill S, Goldberg RM, Kahlenberg MS, Nair SG, et al. Contribution of immunoscore and molecular features to survival prediction in stage III colon cancer. JNCI Cancer Spectr. 2020;4:pkaa023. doi:10.1093/jncics/pkaa023.
- Andre T, Vernerey D, Mineur L, Bennouna J, Desrame J, Faroux R, Fratte S, Hug de Larauze M, Paget-Bailly S, Chibaudel B, et al. Three versus 6 months of oxaliplatin-based adjuvant chemotherapy for patients with stage III colon cancer: disease-free survival results from a randomized, open-label, international duration evaluation of adjuvant (IDEA) France, phase III trial. J Clin Oncol. 2018;36:1469–3. doi:10.1200/JCO.2017.76.0355.
- Pagès F, André T, Taieb J, Vernerey D, Henriques J, Borg C, Marliot F, Ben Jannet R, Louvet C, Mineur L, et al. Prognostic and predictive value of the Immunoscore in stage III colon cancer patients treated with oxaliplatin in the prospective IDEA France PRODIGE-GERCOR cohort study. Ann Oncol. 2020;31:921–929. doi:10.1016/j.annonc.2020.03.310.
- Grothey A, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, Souglakos J, Shi Q, Kerr R, Labianca R, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378:1177–1188. doi:10.1056/NEJMoa1713709.
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi:10.1126/science.1129139. PMID: 17008531.
- Galon J, Fox BA, Bifulco CB, Masucci G, Rau T, Botti G, Marincola FM, Ciliberto G, Pages F, Ascierto PA, et al. Immunoscore and Immunoprofiling in cancer: an update from the melanoma and immunotherapy bridge 2015. J Transl Med. 2016;14:273. doi:10.1186/s12967-016-1029-z.
- Kirilovsky A, Marliot F, El Sissy C, Haicheur N, Galon J, Pages F. Rational bases for the use of the Immunoscore in routine clinical settings as a prognostic and predictive biomarker in cancer patients. Int Immunol. 2016;28:373–382. doi:10.1093/intimm/dxw021. PMID: 27121213.
- Pages F, Galon J, Fridman WH. The essential role of the in situ immune reaction in human colorectal cancer. J Leukoc Biol. 2008;84:981–987. doi:10.1189/jlb.1107773. PMID: 18559950.
- Vacchelli E, Galluzzi L, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Kroemer G. Trial watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2012;1:179–188. doi:10.4161/onci.1.2.190262011ONCOIMM0105. PMID: 22720239; [pii].
- Angelova M, Mlecnik B, Vasaturo A, Bindea G, Fredriksen T, Lafontaine L, Buttard B, Morgand E, Bruni D, Jouret-Mourin A, et al. Evolution of metastases in space and time under immune selection. Cell. 2018;175:751–765 e716. doi:10.1016/j.cell.2018.09.018.
- Mlecnik B, Van den Eynde M, Bindea G, Church SE, Vasaturo A, Fredriksen T, Lafontaine L, Haicheur N, Marliot F, Debetancourt D, et al. Comprehensive intrametastatic immune quantification and major impact of immunoscore on survival. J Natl Cancer Inst. 2018;110:97–108. doi:10.1093/jnci/djx123.
- Marliot F, Chen X, Kirilovsky A, Sbarrato T, El Sissy C, Batista L, Van den Eynde M, Haicheur-Adjouri N, Anitei MG, Musina AM, et al. Analytical validation of the Immunoscore and its associated prognostic value in patients with colon cancer. J Immunother Cancer. 2020;8:e000272. doi:10.1136/jitc-2019-000272.
- Pages F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–2139. doi:10.1016/S0140-6736(18)30789-X.
