ABSTRACT
The comprehensive analysis of patients with a complete resection of all metastases reveals the heterogeneity of the colorectal metastatic disease and its clinical impact. Complex tumor immune interrelations shape the metastatic landscape, not only in terms of number and size of lesions, or mutational pattern, but also in terms of immune cell infiltrate. Significantly higher densities of T-cells and lower density of B-cells were quantified in the tumor microenvironment of metastases compared with primary tumors. A high T cell infiltration and Immunoscore measured in the least-infiltrated metastasis were associated with a significantly lower number of metastases, larger metastasis, and prolonged survival while patients with increased metastatic burden had a lower Immunoscore. Immunoscore was evaluated on a biopsy, in a random metastasis or as the mean value of all metastases significantly predicting outcome. However, the most immune-infiltrated metastasis was not significantly predicting outcome, whereas the least immune-infiltrated metastasis was best in predicting clinical outcome. A good likelihood of concordance of Immunoscore was observed between one biopsy and complete metastasis, but the overall intra-metastatic immune infiltrate might be better estimated with multiple biopsies or sampling of larger tumor areas. This intra-metastatic adaptive immune reaction increases following aneoadjuvant treatment containing anti-EGFR monoclonal antibody, an effect that is currently therapeutically evaluated in clinical trials to improve the survival of metastatic patients.
Metastases and primary tumors have a heterogeneous immune environment
Over 90% of colorectal cancer (CRC) patients with synchronous metastases die within 5 y of diagnosis and therefore a better understanding of metastatic development is needed. Accumulating evidence suggests that tumor progression and metastasis are shaped by the intratumoral immune landscape. Metastatic cells must successfully negotiate complex steps leading to their establishment in a foreign tissue microenvironment. The acquisition of genomic alterations or behavioral changes does not completely explain this process, which is likely to be influenced not only by the tumor cells, but also by the tumor microenvironment. We previously reported the major role of cytotoxic and memory T lymphocytes in predicting survival of cancer patients within primary tumorsCitation1–7 and distant metastases.Citation8–10 A scoring system designated “Immunoscore” based on the quantification of two lymphocyte populations in the core (CT) and at the invasive margin (IM) of primary tumors has a prognostic value superior to the AJCC/UICC TNM-classification.Citation1,Citation3,Citation4
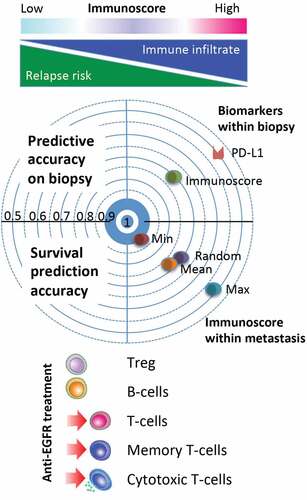
The characterization of intrametastatic immune infiltration was revealed in a comprehensive analysis including all resected metastases (n = 603) for 222 stage IV patients undergoing complete curative metastatic resection.Citation10 Whole slide automatic quantification assembled detailed information about the spatial immune cell distribution within metastases and primary tumors. Several parameters such as the area, the number of the metastases, and the variation of the infiltration level, considering mainly CD3+, CD8+, CD45RO+, CD20+, and FOXP3+ lymphocytes, in each metastasis were studied. Like primary tumors, metastases were infiltrated with adaptive immune cells in a non-uniform manner. Densely infiltrated areas can be observed in both tumor regions (CT and IM) and overall immune densities were higher at the IM of the metastases. Significantly higher densities of T-cells and lower density of B-cells were quantified in metastases compared with primary tumors. The most striking reported feature in patients bearing more than one metastatic lesion is the heterogeneity of such lesions in both lymphocyte infiltrates and the pattern of analyzed hotspot somatic mutations.Citation8,Citation10 A high T cell infiltration and Immunoscore measured in the least-infiltrated metastasis were associated with a significantly lower number of metastases, larger metastasis, and prolonged survival (disease-free and overall survival), while patients with increased metastatic burden had a lower Immunoscore (P < .001). Co-evolution of the cytotoxic immune response and cancer probably explains much of the phenomenology conducive to the number, size, and rate of progression of distant metastases. The intensity of T cell infiltration in primary and metastatic lesions probably speaks of a history of immunosurveillance, equilibrium, and escape in the relationship of anticancer immunity and the malignant cells, which ultimately results in heterogeneity of lesions and cancer patients.Citation8Figure 1
Figure 1. Immune heterogeneity of metatastic colorectal cancer. A high T cell infiltration and Immunoscore measured in the least-infiltrated metastasis were associated with a significantly lower number of metastases, larger metastasis, and prolonged survival while patients with increased metastatic burden had a lower Immunoscore. Immunoscore evaluated in a random biopsy or in a random metastasis or as the mean value of all metastases significantly predicting outcome. The most immune infiltrated metastasis (Max) was not significantly predicting outcome, whereas the least immune infiltrated metastasis (Min) was best in predicting clinical outcome. Receiver operating characteristics likelihood of concordance of biomarkers (Immunoscore and PD-L1) between biopsy and complete metastasis evaluation, shows better performance for Immunoscore. Preoperative treatment containing anti-EGFR monoclonal antibody is associated with increase T cell densities in the core of the metastases.
Biopsy simulations accurately estimate the intra-metastatic immune infiltrate
The densities of adaptive immune cells were predicting recurrence and overall survival when evaluated in a random metastasis or as the mean value of all metastases. However, the most immune-infiltrated metastasis was not significantly predicting outcome, whereas the least immune-infiltrated metastasis was best predicting clinical outcome.Citation10 The accuracy of biopsies in estimating the metastatic immune infiltrate was investigated. The biopsy of a metastasis was representative for tumors with homogeneous infiltrate but it over- or under-estimated the total immune infiltrate in heterogeneous tumors and was less accurate in patients with inter-metastatic heterogeneity. The simulation of a metastasis biopsy on the whole cohort (computed assisted, one small region in the CT selected randomly for each patient and quantified for CD8 density, study repeated 100 times per patient) showed a very good specificity, low-infiltrated patients being correctly identified in more than 90% of the cases, even if a single CT region of 0.64 mm2 (equivalent of a single little biopsy) was analyzed. In more than 30%, this simulated biopsy was log rank significant for DFS and OS. The sensitivity increased with the number of investigated CT regions. At 10 simulated biopsies, more than 80% of high-infiltrated patients were correctly classified and the great majority of these repetitions of biopsy correlated with patient survival (DFS and OS). Similar results were obtained for CD3 and Immunoscore (biopsy adapted). In contrast, PDL1 was less sensitive and specific (32%, 94%, respectively) than CD3, CD8, or Immunoscore (65% and 99%, respectively) in estimating the reality of the positivity across the whole metastatic slide, especially for low numbers of CT region.Citation10 In rectal cancer, biopsy-adapted Immunocore provides a strong prognostic factor for DFS and clinical and pathological response to preoperative treatment, possibly identifying group of patients that should benefit from less invasive therapeutic strategies (i.e. Watch-and-Wait or minimally invasive surgery)Citation11
Biopsies represent only a static snapshot of a metastasis. A single biopsy accurately identifies low-infiltrated metastases, but the overall intra-metastatic immune infiltrate might be better estimated with multiple biopsies or sampling of larger tumor areas. The use of a single, randomly selected tumor-biopsy could thus limit and bias the discovery of new biomarkers for targeted treatments, especially in patients with increased metastatic burden.
Preoperative treatment impacts the tumor immune microenvironment
Current preoperative treatment of CRC metastases frequently involves chemotherapy with anti-VEGF or anti-EGFR monoclonal antibodies.Citation12–15 This permitted to explore differences in the immune infiltrate of metastases depending on the type of preoperative regimen received. While chemotherapy together with an anti-EGFR IgG1 monoclonal antibody (cetuximab) leads to increased T cell infiltration specifically in the CT of the metastasis, anti-VEGF combined with chemotherapy did not increase T lymphocyte infiltrates but increased the expression of B-cell and NK-cell genes. These differences could be attributed to the mechanism of action of cetuximab that coats tumor cells and activates Fc-gamma receptors on NK and myeloid cells, whereas anti-VEGF neutralizes a soluble factor. Of interest, RAS mutation status does not seem to be a driver of baseline Immunoscore but determines lower T cell infiltration in response to anti-EGFR plus chemotherapy. Over the recent years, it has been established that almost all cancer treatments affect antitumor immunity.Citation3,Citation4 This includes anti-VEGF, anti-EGFR, and conventional chemotherapy that reshapes the composition of the microenvironment and may induce immunogenic cell death.
This intra-metastatic adaptive immune reaction increasing following a neoadjuvant treatment is currently therapeutically exploited and evaluated in many clinical trials to improve the survival of metastatic patients.
Immune heterogeneity and immunosuppression of CRC make it as a formidable enemy for immunotherapy, even if the immune system has inherent abilities to adapt. These observations are probably explained by the coevolution of the immune response and the malignant cells fitting the “three E model” (elimination, equilibrium, and escape). When studying primary tumors, and metastases, we are more likely dealing with both an equilibrium and a partial escape.Citation8 Even at the metastatic stage, adaptive immune cells are still infiltrating metastases and are strongly influencing the clinical outcome and survival of patients. However, the immune escape is likely to be the heterogeneous Darwinian result of serial successful escape variants explaining immunodivergence of the metastases in the snapshots that we see upon surgery or necropsy.
Disclosure of Potential Conflicts of Interest
JG and BM have patents associated with the immune prognostic biomarkers. JG is co-founder of HalioDx biotech company. Immunoscore® a registered trademark from the National Institute of Health and Medical Research (INSERM) licensed to HalioDx.
Acknowledgments
The work was supported by INSERM, the LabEx Immuno-oncology, the Transcan ERAnet European project, The Qatar National Research Fund (QNRF), grant number NPRP11S-0121-180351, the Society for Immunotherapy of Cancer (SITC), Association pour la Recherche contre le Cancer (ARC), CARPEM, AP-HP, HalioDx, and Institut National du Cancer, France (INCa).
Additional information
Funding
References
- Angell HK, Bruni D, Barrett JC, Herbst R, Galon J. The immunoscore: colon cancer and beyond. Clin Cancer Res. 2020;26:332–3. PMID: 31413009. doi:10.1158/1078-0432.ccr-18-1851.
- Bindea G, Mlecnik B, Fridman WH, Galon J. The prognostic impact of anti-cancer immune response: a novel classification of cancer patients. Semin Immunopathol. 2011;33:335–340. PMID: 21461991. doi:https://dx.doi.org/10.1007/s00281-011-0264-x.
- Bruni D, Angell HK, Galon J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. 2020. PMID: 32753728. doi::https://dx.doi.org/10.1038/s41568-020-0285-7.
- Galon J, Bruni D. Tumor immunology and tumor evolution: intertwined histories. Immunity. 2020;52:55–81. PMID: 31940273. doi::https://dx.doi.org/10.1016/j.immuni.2019.12.018.
- Galon J, Fox BA, Bifulco CB, Masucci G, Rau T, Botti G, Marincola FM, Ciliberto G, Pages F, Ascierto PA, et al. Immunoscore and Immunoprofiling in cancer: an update from the melanoma and immunotherapy bridge 2015. J Transl Med. 2016;14:273. PMID: 27650038 doi::https://dx.doi.org/10.1186/s12967-016-1029-z.
- Kirilovsky A, Marliot F, El Sissy C, Haicheur N, Galon J, Pages F. Rational bases for the use of the Immunoscore in routine clinical settings as a prognostic and predictive biomarker in cancer patients. Int Immunol. 2016;28:373–382. PMID: 27121213. doi::https://dx.doi.org/10.1093/intimm/dxw021.
- Pages F, Galon J, Fridman WH. The essential role of the in situ immune reaction in human colorectal cancer. J Leukoc Biol. 2008;84:981–987. PMID: 18559950. doi:https://dx.doi.org/10.110777310.1189/jlb.1107773.
- Angelova M, Mlecnik B, Vasaturo A, Bindea G, Fredriksen T, Lafontaine L, Buttard B, Morgand E, Bruni D, Jouret-Mourin A, et al. Evolution of metastases in space and time under immune selection. Cell. 2018;175:751–765 e716. PMID: 30318143 doi::https://dx.doi.org/10.1016/j.cell.2018.09.018.
- Mlecnik B, Van den Eynde M, Bindea G, Church SE, Vasaturo A, Fredriksen T, Lafontaine L, Haicheur N, Marliot F, Debetancourt D, et al. Comprehensive intrametastatic immune quantification and major impact of immunoscore on survival. J Natl Cancer Inst. 2018;110:97–108. PMID: 28922789 doi::https://dx.doi.org/10.1093/jnci/djx123.
- Van den Eynde M, Mlecnik B, Bindea G, Fredriksen T, Church SE, Lafontaine L, Haicheur N, Marliot F, Angelova M, Vasaturo A, et al. The link between the multiverse of immune microenvironments in metastases and the survival of colorectal cancer patients. Cancer Cell. 2018;34:1012–1026 e1013. PMID: 30537506 doi::https://dx.doi.org/10.1016/j.ccell.2018.11.003.
- El Sissy C, Kirilovsky A, Van den Eynde M, Musina AM, Anitei MG, Romero A, Marliot F, Junca A, Doyen J, Mlecnik B, et al. A diagnostic biopsy-adapted immunoscore predicts response to neoadjuvant treatment and selects patients with rectal cancer eligible for a watch-and-wait strategy. Clin Cancer Res. 2020. PMID: 32669377. doi:10.1158/1078-0432.CCR-20-0337
- Buque A, Bloy N, Aranda F, Castoldi F, Eggermont A, Cremer I, Fridman WH, Fucikova J, Galon J, Marabelle A, et al. Trial watch: immunomodulatory monoclonal antibodies for oncological indications. Oncoimmunology. 2015;4:e1008814. PMID: 26137403 doi:10.1080/2162402X.2015.1008814.
- Galluzzi L, Vacchelli E, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Zucman-Rossi J, Zitvogel L, Kroemer G. Trial watch: monoclonal antibodies in cancer therapy. Oncoimmunology. 2012;1:28–37. PMID: 22720209. doi::https://dx.doi.org/10.4161/onci.1.1.179382011ONCOIMM0018.
- Vacchelli E, Eggermont A, Galon J, Sautes-Fridman C, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: monoclonal antibodies in cancer therapy. Oncoimmunology. 2013;2:e22789. PMID: 23482847. doi::https://dx.doi.org/10.4161/onci.22789.
- Vacchelli E, Galluzzi L, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Kroemer G. Trial watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2012;1:179–188. PMID: 22720239. doi: :https://dx.doi.org/10.4161/onci.1.2.190262011ONCOIMM0105.
