ABSTRACT
The prognostic potential of anti-tumor immune responses is becoming increasingly important in adenocarcinoma of the gastroesophageal junction and stomach (AGE/S) especially regarding the use of immune checkpoint inhibitors. This study analyzes for the first time the prognostic impact of tumor-infiltrating lymphocytes (TILs) and checkpoint inhibitors in a large Caucasian cohort in patients with AGE/S. We screened tissue samples from 438 therapy-naïve patients with AGE/S undergoing surgery between 1992 and 2005, examined in a tissue microarray (TMA) and stained against human CD3, CD4, CD8, PD-1, and PD-L1. Out of 438 tissue samples, 210 were eligible for multivariate analysis. This revealed that high infiltration with CD3+, CD4+, or CD8+ TILs was associated with an increased overall survival in AGE/S patients, which could only be confirmed in multivariate analysis for CD3 (HR: 0.326; p = .023). Independent improved survival was limited to gastric cancer patients and to early tumor stages as long as TILs did not express PD-1 (HR: 1.522; p = .021). Subgroup analyses indicate that TIL-dependent anti-tumor immune response is only effective in gastric cancer patients in early stages of disease in PD-1 negative TILs. Combined analysis of PD-1 and CD3 could serve as a prognostic marker for the clinical outcome of gastric cancer patients and could also be of interest for immunotherapy.
1. Introduction
Adenocarcinoma of the gastroesophageal junction and stomach (AGE/S) represents the third most frequent tumor leading to death worldwide.Citation1 While the incidence of adenocarcinoma of the stomach has been constantly decreasing, mostly due to reclining Helicobacter pylori (HP) infections, the frequency of AGE is increasing in the Western world. This is thought to be driven by a rising incidence of obesity and consecutive reflux disease.Citation2–Citation4 The role of the immune system in tumor development and its role as a prognostic factor of the clinical course of gastric cancer and AGE/S are still controversially discussed.Citation5 On one hand, chronic inflammation due to HP-infection plays a significant role in gastric cancer development. On the other hand, lymphocytic infiltration is associated with clinical outcome in various tumor entities.Citation6 Expression of lymphocytes in tumor tissue correlates with an improved prognosis in different types of tumors such as breast cancer, esophageal squamous cell carcinoma, and colorectal cancer.Citation7–Citation9 A study by Galon et al. in 2006 found a significant improvement in survival in colorectal cancer patients with high lymphocyte infiltration in the tumor tissue.Citation10 They especially differentiated between tumor margin and tumor center, which lead to an improved accuracy in prediction of survival and ultimately to the “Immunoscore ®”; however, prediction of benefit from adjuvant chemotherapy in stage II patients is missing.Citation11
Tumor-infiltrating lymphocytes (TILs) have been investigated in several studies for AGE and gastric cancer patients. Thus, a study from 2001 indicated, that a high infiltration with CD8+ lymphocytes is associated with a longer overall survival in a cohort of 70 patients with squamous cell and adenocarcinoma of the esophagus.Citation12
A meta-analysis from 2017 including 31 studies and 4185 patients concerning the prognostic role of tumor-infiltrating lymphocytes (TILs) in gastric cancer identified a positive prognostic value of a high infiltration with CD3+ and CD8+ lymphocytes, but not for CD4+ lymphocytes.Citation13 The minority of patients with gastric cancer in this analysis was of Caucasian origin (13.0%) with a maximum sample size of 110 patients.
Microsatellite instability (MSI), as a marker for a deficient mismatch-repair (MMR) system, is a histopathological parameter with the promising association to clinical parameters.Citation14 An MSI-high status correlates significantly with improved survival as well as lower risk of relapse in colorectal cancer patients, as stated in two meta-analyses including 71 studies in total.Citation15,Citation16 MSI status also seems to predict clinical benefit of immune checkpoint inhibition in this entity.Citation17,Citation18 The reason for this effect is the higher mutational burden caused by a deficient mismatch-repair system.Citation18 A high mutational burden leads to higher immunogenicity and therefore to a better response of immunotherapy.Citation19 Microsatellite instability has also been found to be of prognostic importance in gastric cancer patients.Citation20 A meta-analysis including 48 studies with a total of 18612 patients found a correlation between higher overall survival as well as lower tumor stages and MSI in gastric cancer.Citation21 An exploratory analysis of the MAGIC trial showed that patients with MSI-high gastroesophageal or gastric cancer treated by surgery only had a significantly better prognosis, whereas MSI-high AGE/S patients who received perioperative chemotherapy showed worse overall survival.Citation22
Recently, the T-cell inhibitory receptor PD-1 and its ligand PD-L1 came into the focus of research, as they play a central role in the functional suppression of T cell-dependent immune responses and the subsequent immune evasion of cancer cells.Citation23 In different mice models by Lau et al., the absence of PD-L1 positive tumor cells improved the anti-tumor response of the host. PD-L1 deficiency in host immune cells led to a significantly higher rate of tumor regression with almost complete prevention of immunoevasion of the tumor.Citation24 The inhibition of PD-1 is therefore not only realized by tumor cells expressing PD-L1 but also by TILs. The inhibition of PD-1 targeting PD-1 and PD-L1 has evolved as a therapeutic option for the treatment of various tumor entities.Citation25–Citation27 However, the prognostic value of PD-1 and PD-L1 expression in TILs appears to vary between tumor entities. Thus, Darb-Esfahani et al. observed a positive correlation between the overall survival of patients with ovarian cancer and a high expression of PD-1 and PD-L1 in TILs, whereas Thompson et al. described the opposite effect in renal cell carcinoma.Citation28,Citation29 In Caucasian gastric cancer patients, PD-L1 expression in TILs has been correlated with an increased survival in univariate, but only partly in multivariate analyses.Citation30
Due to these contradictory data, we examined the prognostic value of CD3+, CD4,+, and CD8+ TILs and PD-1 and PD-L1 expression in tumor cells and TILs in a large, thoroughly characterized, therapy-naïve, Caucasian AGE/S cohort with respect to well-known clinical prognostic parameters including histology subtypes and clinical tumor stages.Citation31
2. Materials and methods
2.1. Patients
Clinical data from patients with AGE/S of all tumor stages, with and without distant and lymph node metastasis and venous and lymphatic infiltration, primarily treated by surgery between 1992 and 2004 at the Charité – Universitätsmedizin Berlin, Charité Campus Buch, were collected retrospectively.
Overall survival was defined as time from diagnosis to death or last follow-up. Tumor-related survival was defined as time from diagnosis to tumor-related death or last follow-up. The data regarding patient characteristics and follow-up information were retrieved from the patient management software (SAP®) and the regional population-based cancer registry (“Gemeinsames Krebsregister”). The study was approved by the Institutional Review Board of the Charité (EA4/115/10) and conducted accordingly.
2.2. Tissue samples
Tissue samples were collected from the archive of the Institute of Pathology, Charité- – Universitätsmedizin Berlin. Four hundred thirty-eight samples of formalin-fixed and paraffin-embedded (FFPE) tissue were available of chemotherapy-naïve patients undergoing surgery. Tissue samples were reevaluated for postoperative histological diagnosis, tumor stage, grading, and were morphologically classified using the Laurén and Ming classification by a pathologist with a special focus on gastrointestinal pathology (M.W.). Additional data concerning tumor size, depth of invasion, and tumor invasion of veins or lymphatic vessels were obtained from the Charité – Universitätsmedizin Berlin patient management software.
2.3. Tissue microarray and immunohistochemistry
Tissue samples were screened using Hematoxylin- and Eosin-stained sections for representative areas of solid tumors. Two 1 mm-diameter tissue cores were punched out of the central tumor region from each of the 438 available samples and transferred to a recipient paraffin block. After re-melting, sections (4 µm thick) were consecutively cut from each tissue microarray (TMA) block. Hematoxylin and Eosin staining was performed on TMA sections for reconfirmation of tumor and non-tumor tissue.
Only TMA cores that contained representative tumor sections were considered for subsequent analyses ().
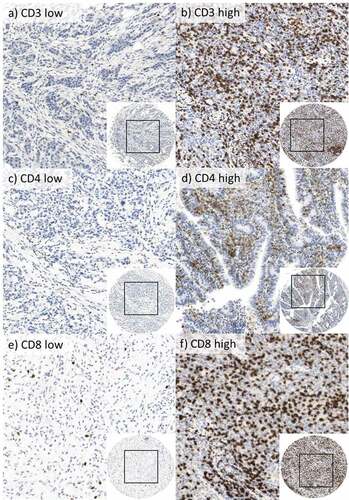
Figure 1. Immunohistological staining for CD3+, CD4+, and CD8+ cells in 400-fold magnification.
For checkpoint proteins PD-1 and PD-L1, automated immunohistochemistry was performed on 2 µm thick slides of paraffin-embedded tissue with Leica BOND-maxTM immunostainer (Leica Microsystems) using the Bond Polymer Refine (a peroxidase-based detection reagent). The slides were counterstained with Hematoxylin (). For mismatch-repair proteins, immunohistochemistry was performed on an automated staining system (BenchMark Ultra, Roche Ventana, Germany) using prediluted antibodies. For a detailed version of the visualizing agents, see Table S1 in the supplement.
Figure 2. Immunohistochemical staining for PD-1 and PD-L1 expression in tumor cells and TILs in 400fold (b,d,e) and 20fold (a,c,f) magnification.
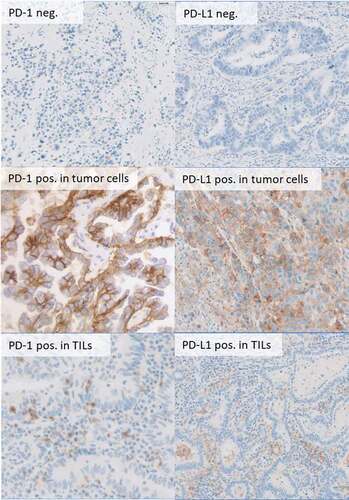
All cases were evaluated by two experts blinded to the patients’ clinical information visualized in one high power field in two cores per tumor sample. Representative areas from each core were chosen for TIL counting. Due to the procedure of cutting and staining, some samples could not be used for the evaluation. Samples with insufficiently representative tumor tissue were also excluded from the evaluation. The average of positive cells was calculated per square millimeter. The representativity of each core was jointly discussed and cases with diverging results were analyzed in detail. Detailed discussion was necessary in 62 cases for CD3, in 35 cases for CD4, and in 28 cases for CD8. PD-1 and PD-L1 were analyzed by two different experts using the CPS (combined positive score).
2.4. Statistical analyses
Statistical analyses were performed using the IBM SPSS software Version 24. Because TILs were not normally distributed, they were grouped in high and low by using the median, not the mean, as a cutoff. The examination of survival analysis for quartiles showed a gradual decrease in survival from a high TIL infiltration to a low TIL infiltration (see Figure S4). To simplify further analysis, we decided for binary categories by using the median for statistics. For PD-1 expression on immune cells, ≥1% was used as a cutoff. For the interpretation of the PD-L1 staining, the CPS (Combined Positive Score) with the cutoff ≥1 was chosen. Quantitative values were expressed as mean± standard deviation, median, range, and categorical values with absolute and relative frequencies (count and percent). Overall survival was evaluated using Kaplan–Meier plots. Associations of CD3+, CD8+, and CD4+ densities with tumor size, distant and lymph node metastasis, venous and lymphatic infiltration, Laurén and Ming classification, grading, and UICC classification were tested using the chi-square-test (X2-test). Univariate survival analyses were performed according to the Kaplan–Meier method using log-rank test for the assessment of statistical significance. Cox regression was performed for multivariate analyses in a stepwise forward/backward selection with the level of significance set to p < .05. Due to a high correlation between CD3+, CD4+, and CD8+, we analyzed each factor in single cox-regression models.
Low CD3+, CD4+, or CD8+ TILs states served as an analytical reference category in the multivariate analyses. For interaction analyses between CD3+, CD4+, and CD8+ TILs state and UICC stage, a low TILs state and UICC stage I and II were used as a reference category.
Reporting recommendations for tumor marker prognostic studies (REMARK) were applied for this study whenever applicable.Citation32
3. Results
3.1. Patient characteristics
Data of 438 patients were collected for this study (female = 178, median age = 62 y) (95% CI: 60.97–63.15 y).
The detailed clinicopathological characteristics of our patient cohort are summarized in the supplement (see Supplement Tables S2, S3, and S4). The mean follow-up was 121.7 months (95% CI: 113.9–129.5 months) and 291 patients (66.4%) died during follow-up time, 225 (51.4%) of those tumor-related. The 5-year overall survival was 38.1%, the 5-year tumor-related survival was 45.4%.
3.2. MMR status
Deficient mismatch repair (dMMR) was shown in 45 patients (10.3%) using immunohistochemical staining against MSH2 and MLH1, whereas 338 (77.2%) showed proficient mismatch repair (pMMR), in 55 patients (12.6%) MMR status was not evaluable. Mean survival was 47.22 months in dMMR patients and 79.78 months in pMMR patients (p = .025). In this AGE/S cohort, dMMR patients were diagnosed in higher tumor stages compared to pMMR patients (dMMR: T1/T2: 40% T3/T4: 60% vs pMMR: T1/T2: 62%, T3/T4: 38% (x2: p = .032); Figure S2). Stage-related overall survival analyses showed no significant differences in the survival of dMMR and pMMR patients.
3.3. CD3, CD4 and CD8 expression
The significance of TILs in AGE/S in Caucasian patients is yet to be examined in larger cohorts. In order to determine the prognostic value of TILs in AGE/S, the expression of CD3, CD4, and CD8 in TILs was determined in tumor tissue, but not in non-neoplastic gastric and esophageal tissue. Out of 438 samples, 349 CD3, 335 CD4, and 344 CD8 stained samples were evaluable for the analysis. The analysis of the remaining samples was not possible due to missing representative tumor tissue in the respecting cores. Exemplary sample images of the analyzed IHC staining are shown in . Examples for TIL count are shown in Figure S1 in the supplement.
The mean count of TILs/mm2 was 122 in the CD3 low group and 513 in the CD3 high group, 30 in the CD4 low group and 251 in the CD4 high group and 74 in the CD8 low group, and 360 in the CD8 high group. High/low status of CD3 correlated strongly with the state of CD4 (p < .0001) and CD8 (p < .0001).
Patients with a high CD3+ state showed a significantly increased mean overall survival in comparison to those with a low CD3 count (CD3: 106.04 months vs. 64.38 months; p = .002). The same effect could be detected for CD4+ and CD8+ (CD4: 116.91 months vs. 56.36 months; p < .001; CD8: 101.53 months vs. 67.34 months; p = .016) (; Supplementary Figure S3(a,b)).
Figure 3. (a) Overall survival depending on high CD3 positive lymphocyte infiltration state. (b) Overall survival depending on PD-1 expression on tumor-infiltrating lymphocytes. (c) Overall survival depending on high CD3 positive lymphocyte infiltration state and UICC stage. (d) Overall survival depending on PD-1 expression on TILs and on CD3 positive lymphocyte infiltration state. (e) Overall survival depending on PD-1 expression in tumor-infiltrating lymphocytes and tumor localization. Tumor samples were grouped for low versus high infiltration with CD3 positive TILs by using the median. For grouping in PD-1 and PD-L1 positive/negative, an expression ≥1% was used as a cutoff.
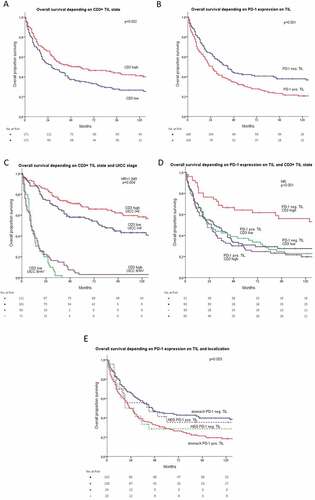
Subgroup analyses showed that the positive prognostic effects of TIL infiltration were limited to UICC stage I. In higher tumor stages, these effects were no longer detectable or even opposed in UICC stage III (, ); Supplementary Figure S3(c,d)).
Table 1. Subgroup analyses: overall survival in months related to TIL infiltration state and UICC stage.
Table 2. Univariate and multivariate survival analyses including CD3, CD4, CD8 positive infiltration state and PD-1 and PD-L1 positive infiltration state.
To recognize confounding and interacting parameters, a multivariate and interaction analysis was performed with all parameters that reached statistical significance in the univariate analysis. For multivariate analysis, only cases with evaluable staining for each included marker were used (n = 210). Due to the high correlation of CD3, CD4, and CD8 TIL state, these parameters were analyzed in separate cox-regression models. The multivariate analysis showed that a high CD3 state (CD3: HR: 0.326; p = .023) as well as the interaction between CD3+ state and UICC stage are independent prognostic factors, but only in the backward analysis (CD3*UICC: HR: 1.949; p = .004). The corresponding cox-regression models for high CD4 and CD8 infiltration showed neither significance for CD4+ or CD8+ cells nor significant interaction with UICC. Interaction between CD4+ lymphocytes and UICC stage did only reach statistical significance in backward, but not in the forward analysis (CD4*UICC: HR: 1.607; p = .044). All results of univariate and multivariate analysis are depicted in Table 2 as well as in the supplements (Tables S4, S5).
3.4. PD-L1 and PD-1 expression
PD-L1 and PD-1 expression was analyzed in tumor cells and in TILs. In 328 cases (74.9%) staining could be evaluated.
Depending on the presence or absence of PD-L1 and PD-1, we separated the findings in lymphocytes and tumor cells into two groups: PD-L1 and PD-1 positive and negative.
3.4.1. PD-1
A significantly increased mean overall survival in univariate analysis was shown in the group with no PD-1 expression in TILs: neg: 95.07 months vs. pos: 56.51 months; p = .001 (). PD-1 expression was strongly correlated to the state of CD3+ TILs. Among all tumors, 30.9% (n = 92) had a high CD3 state and were PD-1 positive in TILs and 17.4% (n = 52) of those were PD-1 negative (x2: p < .001). The interaction between CD3 state and PD-1 expression in TILs also reached statistical significance in multivariate analysis, which makes it an independent prognostic marker (). The protective effect on overall survival of high CD3 state described above (see 3.2) was not detectable in patients with PD-1 expression in TILs (PD-1 pos: CD3 high vs low: 53.7 vs. 59.8 months p = .714; PD-1 neg: 132.6 vs 65.9 months, p = .001). The interaction between PD-1 expression and CD4 and CD8 state did not reach statistical significance (see Supplement Tables S4, S5). PD-1 expression in TILs was not significantly disbalanced over the UICC tumor stages (I: 41.1%, II 47.3%, III 64.4%, IV 51.2%; p = .066).
PD-1 expression in tumor cells was only measurable in UICC I tumor samples; high significant differences in overall survival between PD-1 positive and negative tumors were therefore caused by this disbalanced expression pattern over the tumor stages: PD-1: neg. 58.96 months vs. pos. 175.79 months; p < .001. The positive prognostic effect of CD3 was not influenced by PD-L1 expression in tumor cells.
Due to the strong correlation to UICC stages, PD-1 expression in tumor cells was excluded from multivariate analyses.
3.4.2. PD-L1
The expression of PD-L1 in TILs showed no effect on overall survival (p = .428). PD-L1 expression in tumor cells was associated with a significantly higher mean survival in univariate analysis: 119.56 months vs. 68.23 months, p = .003. This effect was not confirmed in multivariate analysis (HR: 0.848; p = .580). This might have been caused by the disbalanced distribution of PD-L1 positive tumor cells over the UICC stages in favor of early tumor stages (I: 25.7%, II: 6.3%, III: 6.5%, IV: 4.8%; p < .0001). In the stage-dependent subgroup analysis, PD-L1 expression in tumor cells had no effect on overall survival in any UICC stage. PD-L1 positive tumor cells were detectable in 6.3% (n = 10) of tumors with low CD3 state and in 17.2% (n = 25) with high CD3 state (x2 p < .003). The prognostic effect of CD3 was not associated with PD-L1 state in tumor cells. All results of univariate and multivariate analysis are depicted in the supplement (Tables S4, S5).
3.5. Localization
Tumors were localized in 84.9% (n = 372) in the stomach and in 15.1% at the gastroesophageal junction (n = 66). Distribution over tumor stages was well balanced (AGE: UICC I/II: 24.9% and III/V 22.9% p = .107). Localization did not influence median overall survival (AGE: 80.1 vs. stomach: 84.0 month; p = .799). Furthermore, the localization did not influence the positive effect of CD3+ TILs on overall survival (AGE: CD3 low 74.1 vs. 109.1 month (p = .276); stomach: CD3 low 63.3 vs high 104.8 months (p = .004)). The negative prognostic effect of PD-1 expression in TILs could only be shown in the stomach subgroup (AGE: PD-1 TILs neg 65.5 vs 70.0 months; p = .636); stomach: PD-1 TILs neg 98.9 vs 54.3 months (p < .001) ().
4. Discussion
The impact of TILs concerning Caucasian patients with AGE/S still lacks a solid analysis, since only small cohorts have been investigated so far.Citation12,Citation33 Most existing data concern patients of Asian origin, which underlines the importance of our data in Caucasian patients.Citation34,Citation35 Our data showed that increased infiltration with CD3+ lymphocytes is an independent prognostic factor for increased overall survival in AGE/S patients in a large cohort with long follow-up data. With insight into subgroup analyses, this effect is however limited to early tumor stages. Thus, the positive prognostic effect of high TIL infiltration is restricted to tumors with PD-1 negative TILs. While the positive prognostic effect of CD3+ cell infiltration was independent from tumor localization, the negative prognostic influence of PD-1 expression in TILs could only be shown in gastric cancer patients. Our findings suggest that immune defense exerts a significant role for the overall survival in early stages of AGE/S and the expression of PD-1 in TILs may inhibit the anti-tumorous immune response.
The reason why the positive prognostic effect of TILs on prognosis is limited to early tumor stages in our study remains unclear. The concept of acquired resistance and tumor immune evasion via mechanisms like direct interaction through T-regulatory cells (Tregs) or T-cell exhaustion might be of importance.Citation6,Citation36 It has been stated in the past that the alteration of tumor reactivity toward TILs during tumor progression could be caused by an immunosuppressive network in the tumor microenvironment.Citation37
As already stated, a deficient MMR system improves prognosis in gastric cancer patients.Citation21 The reported higher response to immunotherapy caused by a higher immunogenicity in MSI-high tumors would be of high interest in this analysis. As opposed to existing data, patients in our study showed a significantly decreased overall survival with dMMR in univariate analyses. This effect was caused by significantly lower tumor stages of pMMR patients. Because of this imbalance in our cohort with MMR status being strongly dependent on tumor stage, independent interaction between MMR status and prognostic relevance of TILs could not be examined.
Immune therapeutic approaches have successfully been integrated into therapeutic routine in several disease entities but results in AGE/S are still unsatisfactory.Citation38–Citation40 Studies concerning the treatment of AGE/S tumors with checkpoint inhibitor therapeutics such as Pembrolizumab are using PD-L1 as a single identification marker to evaluate the predictive value.Citation40,Citation41 These unsatisfactory results may occur, because patient selection and predictive markers are yet to be improved.Citation42 This study indicates that PD-1 expression in correlation to CD3+ TILs may influence the predictive value in a favorable way. Whilst overall survival significantly improved when CD3+ lymphocyte infiltration was high and TILs did not express PD-1, this effect on survival was not found when PD-1 was expressed. This could indicate that patients with a high TIL state as well as a PD-1 expression in TILs in gastric cancer could significantly profit from checkpoint inhibitor therapy. This would concern 30.9% of patients in our study. This effect remains to be examined in further studies or could also be retrospectively evaluated in past studies.
There exist contradicting data about the association of PD-1/PD-L1 expression in AGE/S concerning the prognostic value on overall survival: whilst in accordance with our data several studies demonstrated a negative effect on overall survival, Citation43 Böger et al. indicated a positive correlation with longer survival in patients with high expression of PD-L1 in tumor and in immune cells in a large Caucasian cohort.Citation30 A study by Thompson et al. indicated that the absence of PD-L1 positive tumor cells leads to improved overall survival in AGE/s patients.Citation44 Wu et al. detected a positive prognostic effect of PD-L1 expression in TILs in gastric cancer patients.Citation45 Because of an uneven distribution of PD-L1 expression throughout the UICC stages in our patient cohort, we could not reproduce these results.
The limitations of our study lay firstly in methodical issues concerning the Tissuemicroarray technique. By using this technique, the evaluation of tumor tissue focuses on a distinct area of the tumor, which was chosen as representative for the tumor in its entirety. However, intratumoral heterogeneity as well as an uneven distribution of TILs throughout the tumor is clearly neglected by using this method and should be taken into consideration when assessing these data.Citation46 We were also unable to differentiate between invasive margin and tumor center, which plays a significant prognostic role according to Galon et al.Citation10 A considerable number of patients had to be excluded for survival analysis for different reasons, such as palliative surgery or unevaluable staining. Overall, we performed multivariate analysis in 210 cases, which is still a large number for an AGE/S cohort. Lastly, we examined a cohort, which was treated between 1992 and 2005. These patients did not receive treatment by modern standards (e.g. perioperative chemotherapy), which makes it difficult to evaluate the data in these aspects. However, this gave us the opportunity to examine chemotherapy-naïve tumor tissue over all UICC stages, which allows better comparability.
Overall, we demonstrated a strong positive prognostic effect of CD3+ and PD-1 negative tumor-infiltrating lymphocytes in early tumor stages for gastric cancer patients in a large AGE/S patient cohort. Our study has strong advantages with a high patient number, positive statistics in univariate and multivariate analyses, and a homogenous study population. In our cohort, the number of patients with early tumor stages is increased compared to other studies with Caucasian cohorts. This is certainly due to the retrospective design of the study. However, it is worth speculating that this increased patient number reached statistical significance to underline the role of the immune system in these tumor stages. Subgroup analyses based on immune cell infiltration could explain disappointing effects of checkpoint inhibitors as seen, e.g. in the Keynote 028, 061, and 062 studies.Citation38,Citation40,Citation47 Our data may help to identify high-risk groups in early tumor stages. The negative predictive value of the PD-1 expression in TILs could also be an indication that it can be of prognostic significance for evaluating the effectiveness of checkpoint inhibition.
Abbreviations
TILs Tumor-infiltrating lymphocytes
AGE/S Adenocarcinoma of the gastroesophageal junction and stomach
PD-1 Programmed cell death protein 1
PD-L1 Programmed cell death 1 ligand 1
MMR mismatch repair
pMMR proficient mismatch repair
dMMR deficient mismatch repair
Supplemental Material
Download ()Acknowledgments
We are particularly indebted to Mrs. Andrea Stroux for the statistical assistance. This work was supported by the BIH Clinician scientist program. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure statement
The authors declare no conflicts of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–10. doi:10.3322/caac.21492.
- Roberts SE, Morrison-Rees S, Samuel DG, Thorne K, Akbari A, Williams JG. Review article: the prevalence of Helicobacter pylori and the incidence of gastric cancer across Europe. Aliment Pharmacol Ther. 2016;43:334–345. doi:10.1111/apt.13474.
- Abrams JA, Gonsalves L, Neugut AI. Diverging trends in the incidence of reflux-related and Helicobacter pylori-related gastric cardia cancer. J Clin Gastroenterol. 2013;47:322–327. doi:10.1097/MCG.0b013e318260177a.
- Lagergren J, Lagergren P. Recent developments in esophageal adenocarcinoma. CA Cancer J Clin. 2013;63:232–248. doi:10.3322/caac.21185.
- Parmiani G. Tumor-infiltrating T cells - Friend or foe of neoplastic cells? New Engl J Med. 2005;353:2640–2641. doi:10.1056/NEJMp058236.
- Pagès F, Galon J, Dieu-Nosjean MC, Tartour E, Sautès-Fridman C, Fridman WH. Immune infiltration in human tumors: A prognostic factor that should not be ignored. Oncogene. 2010;29:1093–1102. doi:10.1038/onc.2009.416.
- Kong JC, Guerra GR, Pham T, Mitchell C, Lynch AC, Warrier SK, Ramsay RG, Heriot AG. Prognostic impact of tumor-infiltrating lymphocytes in primary and metastatic colorectal cancer: A systematic review and meta-analysis. Dis Colon Rectum. 2019;62:498–508. doi:10.1097/DCR.0000000000001332.
- Vihervuori H, Autere TA, Repo H, Kurki S, Kallio L, Lintunen MM, Talvinen K, Kronqvist P. Tumor-infiltrating lymphocytes and CD8+ T cells predict survival of triple-negative breast cancer. J Cancer Res Clin Oncol. 2019;145:3105–3114. doi:10.1007/s00432-019-03036-5.
- Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012:14. doi:10.1186/bcr3148.
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi:10.1126/science.1129139.
- Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, et al. Towards the introduction of the “Immunoscore” in the classification of malignant tumours. J Pathol. 2014;232:199–209. doi:10.1002/path.4287.
- Schumacher K, Haensch W, Röefzaad C, Schlag PM. Prognostic significance of activated CD8+ T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–3936.
- Zheng X, Song X, Shao Y, Xu B, Chen L, Zhou Q, Hu W, Zhang D, Wu C, Tao M, et al. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: a meta-analysis. Oncotarget. 2017;8:57386–57398. doi:10.18632/oncotarget.18065.
- Vilar E, Gruber SB. Microsatellite instability in colorectal cancer- the stable evidence. Nat Rev Clin Oncol. 2010;7:153–162. doi:10.1038/nrclinonc.2009.237.
- Petrelli F, Ghidini M, Cabiddu M, Pezzica E, Corti D, Turati L, Costanzo A, Varricchio A, Ghidini S, Barni S, et al. Microsatellite instability and survival in stage II colorectal cancer: A systematic review and meta-analysis. Anticancer Res. 2019;39:6431–6441. doi:10.21873/anticanres.13857.
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–618. doi:10.1200/JCO.2005.01.086.
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber, BS, Azad NS, Laheru D, et al. PD-1 blockade in tumors with mismatch-repair deficiency. New Engl J Med. 2015;372:2509–2520. doi:10.1056/NEJMoa1500596.
- Lee V, Murphy A, Le DT, Diaz LA. Mismatch repair deficiency and response to immune checkpoint blockade. Oncologist. 2016;21:1200–1211. doi:10.1634/theoncologist.2016-0046.
- Schrock AB, Ouyang C, Sandhu J, Sokol E, Jin D, Ross JS, Miller VA, Lim D, Amanam I, Chao J, et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Annal Oncol. 2019;30:1096–1103. doi:10.1093/annonc/mdz134.
- Fang WL, Chang SC, Lan YT, Huang KH, Chen JH, Lo SS, Hsieh M-C, Li AF-Y, Wu CW, Chiou SH, et al. Microsatellite instability is associated with a better prognosis for gastric cancer patients after curative surgery. World J Surg. 2012;36:2131–2138. doi:10.1007/s00268-012-1652-7.
- Polom K, Marano L, Marrelli D, De Luca R, Roviello G, Savelli V, Tan P, Roviello F, et al. Meta-analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer. Br J Surg. 2018;105:159–167. doi:10.1002/bjs.10663.
- Smyth EC, Wotherspoon A, Peckitt C, Gonzalez D, Hulkki-Wilson S, Eltahir Z, Fassan M, Rugge M, Valeri N, Okines A, et al. Mismatch repair deficiency, microsatellite instability, and survival: an exploratory analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial. JAMA Oncol. 2017;3:1197–1203. doi:10.1001/jamaoncol.2016.6762.
- Seliger B. Basis of PD1/PD-L1 Therapies. J Clin Med. 2019;8:2168. doi:10.3390/jcm8122168.
- Lau J, Cheung J, Navarro A, Lianoglou S, Haley B, Totpal K, Sanders L, Koeppen H, Caplazi P, McBride J, et al. Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat Commun. 2017;8:1–11. doi:10.1038/ncomms14572.
- Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi:10.1038/nri2326.
- Sharon E, Streicher H, Goncalves P, Chen HX. Immune checkpoints in cancer clinical trials. Chin J Cancer. 2014;33:434–444. doi:10.5732/cjc.014.10122.
- La-Beck NM, Jean GW, Huynh C, Alzghari SK, Lowe DB. Immune checkpoint inhibitors: new insights and current place in cancer therapy. Pharmacotherapy. 2015;35:963–976. doi:10.1002/phar.1643.
- Darb-Esfahani S, Kunze CA, Kulbe H, Sehouli J, Wienert S, Lindner J, Bockmayr M, Dietel M, Denkert C, Braicu L, et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget. 2015;7:1486–1499. doi:10.18632/oncotarget.6429.
- Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi:10.1158/0008-5472.CAN-05-4303.
- Böger C, Behrens HM, Mathiak M, Krüger S, Kalthoff H, Röcken C. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget. 2016;7:24269–24283. doi:10.18632/oncotarget.8169.
- Arnold A, Daum S, von Winterfeld M, Berg E, Hummel M, Horst D, Rau B, U Stein U, Treese C. Analysis of NTRK expression in gastric and esophageal adenocarcinoma (AGE) with pan-TRK immunohistochemistry. Pathol Res Pract. 2019;215:152662. doi:10.1016/j.prp.2019.152662.
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat. 2006;100:229–235. doi:10.1007/s10549-006-9242-8.
- Chiaravalli AM, Feltri M, Bertolini V, Bagnoli E, Furlan D, Cerutti R, Capella C. Intratumour T cells, their activation status and survival in gastric carcinomas characterised for microsatellite instability and Epstein-Barr virus infection. Virchows Arch. 2006;448:344–353. doi:10.1007/s00428-005-0066-4.
- Kono K, Kawaida H, Takahashi A, Sugai H, Mimura K, Miyagawa N, Omata H, Fujii H. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother. 2006;55:1064–1071. doi:10.1007/s00262-005-0092-8.
- Kim ST, Jeong H, Woo OH, Seo JH, Kim A, Lee ES, Shin SW, Kim YH, Kim JS, Park KH. Tumor-infiltrating lymphocytes, tumor characteristics, and recurrence in patients with early breast cancer. Am J Clin Oncol. 2013;36:224–231. doi:10.1097/COC.0b013e3182467d90.
- Pandya PH, Murray ME, Pollok KE, Renbarger JL. The immune system in cancer pathogenesis: potential therapeutic approaches. J Immunol Res. 2016:2016. doi:10.1155/2016/4273943.
- Saleh R, Elkord E. Acquired resistance to cancer immunotherapy: role of tumor-mediated immunosuppression. Semin Cancer Biol. 2019:1–15. doi:10.1016/j.semcancer.2019.07.017.
- Doi T, Piha-Paul SA, Jalal SI, Mai-Dang H, Saraf S, Koshiji M, Csiki I, Bennouna J. Updated results for the advanced esophageal carcinoma cohort of the phase 1b KEYNOTE-028 study of pembrolizumab. J Clin Oncol. 2016;34:4046. doi:10.1200/jco.2016.34.15_suppl.4046.
- Doi T, Piha-Paul SA, Jalal SI, Saraf S, Lunceford J, Koshiji M, Bennouna J. Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J Clin Oncol. 2018;36:61–67. doi:10.1200/JCO.2017.74.9846.
- Tabernero J, Van Cutsem E, Bang Y-J, Fuchs CS, Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Castro Salguero HR, et al. Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: the phase III KEYNOTE-062 study. J Clin Oncol. 2019;37:LBA4007–LBA4007. doi:10.1200/JCO.2019.37.18_suppl.LBA4007.
- Kawazoe A, Yamaguchi K, Yasui H, Negoro Y, Azuma M, Amagai K, Hara H, Baba H, Tsuda M, Hosaka H, et al. Safety and efficacy of pembrolizumab in combination with S-1 plus oxaliplatin as a first-line treatment in patients with advanced gastric/gastroesophageal junction cancer: cohort 1 data from the KEYNOTE-659 phase IIb study. Eur J Cancer. 2020;129:97–106. doi:10.1016/j.ejca.2020.02.002.
- Weinberg BA, Xiu J, Hwang JJ, Shields AF, Salem ME, Marshall JL. Immuno‐oncology biomarkers for gastric and gastroesophageal junction adenocarcinoma: why PD‐L1 testing may not be enough. Oncologist. 2018;23:1171–1177. doi:10.1634/theoncologist.2018-0034.
- Gu L, Chen M, Guo D, Zhu H, Zhang W, Pan J, Zhong X, Li X, Qian H, Wang X. PD-L1 and gastric cancer prognosis: A systematic review and meta-analysis. PLoS ONE. 2017;12:1–14. doi:10.1371/journal.pone.0182692.
- Thompson ED, Zahurak M, Murphy A, Cornish T, Cuka N, Abdelfatah E, Yang S, Duncan M, Ahuja N, Taube JM, et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. 2016;66:794–801. doi:10.1136/gutjnl-2015-310839.
- Wu Y, Cao D, Qu L, Cao X, Jia Z, Zhao T Zhao T, Wang Q, Jiang J. PD-1 and PD-L1 co-expression predicts favorable prognosis in gastric cancer. Lancet Oncol. 2008;9:279–287. doi:10.1016/S1470-2045(08)70072-X.
- Khouja MH, Baekelandt M, Sarab A, Nesland JM, Holm R. Limitations of tissue microarrays compared with whole tissue sections in survival analysis. Oncol Lett. 2010;1:827–831. doi:10.3892/ol_00000145.
- Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, Fornaro L, Olesiński T, Caglevic C , Chung HC, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123–133. doi:10.1016/S0140-6736(18)31257-1.
