ABSTRACT
The immune modulatory effect of tivozanib, a tyrosine kinase inhibitor, and the underlying immune mechanisms impacting survival of HCC patients have not been investigated. Pre-clinical studies have shown that tivozanib reduces Tregs and MDSCs accumulation through inhibition of c-Kit/SCF axis. We rationalized that c-Kit/SCF axis antagonism by tivozanib may reverse tumor-induced immune suppression in HCC patients. The frequency of circulating Tregs, MDSCs, CTLA-4+Tregs, PD-1+T cells, c-Kit+pERK-2+Tregs, and c-Kit+pERK-2+MDSCs were quantified in HCC patients at baseline and two time points during tivozanib treatment. We report for the first time that reduction in Tregs after tivozanib treatment and increased levels of baseline CD4+PD-1+T cells correlated with significant improvement in overall survival (OS) of the patients and these signatures may be potential biomarkers of prognostic significance. This immune modulation resulted from tivozanib-mediated blockade of c-Kit/SCF signaling, impacting ERK2 phosphorylation on Tregs and MDSCs. Low pre-treatment CD4+T cells: Treg ratio and reduction in the frequencies of Foxp3+c-Kit+pERK+Tregs after tivozanib treatment correlated significantly with progression free survival. In a comparative analysis of tivozanib vs sorafenib treatment in HCC patients, we demonstrate that decrease in the baseline numbers or frequencies of Foxp3+Tregs, MDSCs and exhausted T cells was significantly greater following tivozanib treatment. Additionally, greater increase in CD4+T cell: Treg ratio after tivozanib treatment was associated with significant improvement in OS compared to sorafenib treatment, highlighting the greater efficacy of tivozanib. These insights may help identify patients who likely would benefit from c-Kit/SCF antagonism and inform efforts to improve the efficacy of tivozanib in combination with immunotherapy.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most prevalent cancer globally and is the second leading cause of cancer related death worldwide.Citation1,Citation2 The risk of primary liver cancer is significantly enhanced by underlying liver cirrhosis, with chronic infection by hepatitis B or C viruses as the leading cause, followed by other etiologies of cirrhosis such as alcoholic hepatitis and nonalcoholic fatty liver disease with metabolic defect. There are limited treatment options for patients with advanced HCC making treatment for this disease a serious challenge. Angiogenesis is one of the characteristic features of HCC and multiple studies have shown the vital role of neovascularization in the development and progression of HCC. Endothelial cell migration, growth and differentiation are triggered by signaling molecules on rapidly growing tumor cells. Thus, development and progression of HCC are strongly associated with activity of receptor tyrosine kinases (RTKs) and their intracellular signal transduction pathways which regulate several cellular functions including proliferation, adhesion and angiogenesis.Citation3,Citation4 HCC is a malignancy driven by VEGF which initiates a signaling cascade that promotes new blood vessel formation. Targeting the proangiogenic signaling pathway is therefore a promising treatment for HCC.
Tivozanib is a potent and highly specific kinase inhibitor that targets vascular endothelial growth factor receptor-1 VEGFR1, VEGFR2, and VEGFR3 at very low concentrations and with a long half-life.Citation5 Tivozanib also inhibits other kinases such as c-Kit and PDGFR-β involved in the signaling cascade.Citation6,Citation7 The potential of VEGF/VEGFR pathway as a therapeutic target has been validated in solid tumors including drug resistant epithelial ovarian cancer where blockade of VEGF receptors by tivozanib reduced proliferative and invasive characteristics of epithelial ovarian cancer cells in vitro.Citation8Tivozanib treated patients demonstrated better response rate and PFS with favorable safety profile in advanced renal cell carcinoma.Citation6,Citation7 In metastatic colorectal cancer, treatment with tivozanib/FOLFOX6 resulted in PFS and overall response rate comparable to bevacizumab.Citation9 Tivozanib specific progression-free survival in metastatic colorectal patients was associated with low-serum neuropilin-1 (NRP-1) levels and NRP-1 seemed to be a biomarker of tivozanib response in clinical trials.Citation9,Citation10 Additionally, antitumor efficacy of tivozanib has been evaluated in solid tumors such as gastrointestinal cancers and breast cancer.Citation8,Citation10 Safety and tolerability profiles of tivozanib have been shown to be acceptable when used in combination therapy.Citation11
c-Kit signaling promotes cell proliferation and survival and this pathway has been shown to be aberrantly activated in cancer. c-Kit mutations are associated with several human malignancies, such as gastrointestinal stromal tumors, acute myeloid leukemia, mast cell leukemia, and melanoma.Citation12,Citation13 Recently, efforts have been made to develop approaches that efficiently inhibit this novel therapeutic target. c-Kit is one of the targets of tivozanib and functions as a receptor for stem cell factor (SCF), a well-studied tumor-derived factor expressed by various human and murine tumor cell lines.Citation14,Citation15 In mouse tumor models, abrogation of tumor-expressed SCF by RNA interference has been shown to reduce MDSC expansion significantly and restore the effector function of tumor-infiltrating T cells.Citation16 Furthermore, blockade of c-Kit by anti-c-Kit monoclonal antibody prevented the development of Tregs, tumor-specific T cell anergy and tumor angiogenesis in a mouse tumor model of MCA26 colon.Citation16 Targeting of c-Kit to prevent MDSC and Treg accumulation in murine tumor models has been demonstrated with sunitinib or tivozanib.Citation17–Citation19 In view of this role of c-Kit, we rationalized that the modulation of signaling via c-Kit/SCF axis by tivozanib may have a novel role in reversing tumor-induced immune suppression in HCC patients.
The global burden of HCC is rising and there is an overwhelming need for combination therapies, targeted therapies with TKIs combined with checkpoint inhibitors/immunomodulators. Even though the recent immunotherapeutic strategies aimed at immune checkpoint receptor blockade resulted in a favorable outcome in HCC patients,Citation20 the relatively low response rates emphasizes the underlying strong immunosuppressive barriers that need to be circumvented by complementary immune-stimulatory approaches.Citation20,Citation21 Importantly, in our previous studies in patients with advanced HCC we have demonstrated that a multitude of redundant immunosuppressive mechanisms are acting in concert to facilitate the escape of tumor immune surveillance and targeted depletion of suppressor cells resulted in the restoration of T effector cell function.Citation22,Citation23 We have also previously demonstrated that sorafenib treatment of HCC patients beneficially reduces the extent of immune suppression caused by regulatory T cells (Tregs) and checkpoint molecules inducing T cell exhaustion, and this was associated with increased overall survival of patients.Citation24 In this study, we demonstrate the feasibility and consistency of tivozanib-mediated therapeutic disruption of the SCF/c-Kit signaling pathway to reverse immune suppression mediated by Tregs and MDSCs in patients with advanced HCC. Blockade of MDSC expansion can potentially lead to other beneficial antitumor effects, such as decreased tumor angiogenesis, decreased number of Foxp3+ Tregs, and possibly suppressed Th2 responses with concomitantly enhanced Th1 responses. Furthermore, we compared the efficacy of tivozanib (a potent and selective VEGFR inhibitor) with that of sorafenib from a broad perspective of its impact on immune suppressive cell types and concomittant survival benefit in HCC patients.
Results
Patient characteristics
20 patients with advanced HCC were enrolled for the study between April 2013 and December 2016 at Roswell Park Comprehensive Cancer Center, Buffalo of which 17 patients were evaluable for all biomarker endpoints. Clinical characteristics of patients are summarized in .
Table 1. Baseline characteristics of patients.
Clinical outcome
The Median age was 65.6 (23.3–81.8) years, all patients had Child Pugh Class A liver function and ECOG was either 0 (65%) or 1 (35%) and macrovascular invasion was present in 7 (41%) (). After a brief Phase 1 run in, the patients were treated at 1 mg orally daily 3 weeks on and one week off until progression or withdrawal of consent. Median progression-free and overall survival were 24 weeks, 95% CI (8.0- not reached) and 8.7 months, 95% CI: (5.3–22.6), respectively for patients treated at the phase 2 1 mg dose.The overall response rate (CR+PR) was 18% using RECIST 1.1, and 7 (41%) had stable disease while 7 (41%) had progressive disease as their best response. For those who had clinical benefit (PR+SD) the median duration of benefit was 24 95% CI (16.7–24) weeks. Treatment was well tolerated and along with pharmacokinetic studies confirming drug exposure, a decrease in soluble plasma VEGFR-2 was noted, assuring adequate target engagement. The clinical findings including safety and efficacy of the phase 1b/2 trial of tivozanib have been previously published, ClinicalTrials.gov NCT01835223.Citation25 For patients enrolled in this biomarker report there were 14 grade 1, 6 grade 2, and 4 grade 3 events; 12 patients had treatment interruptions and 4 permanently discontinued treatment due to toxicity; however, no treatment-related deaths were seen. The 4 grade 3 adverse effects were fatigue, anorexia, palmar-plantar dysaesthesia, and pulmonary embolism.
Reduction in the frequency and number of Tregs and MDSCs following Tivozanib treatment is associated with downregulation of c-Kit/ERK2 signaling pathway
In order to mechanistically evaluate the impact of tivozanib on immune modulation, we measured the extent of intracellular ERK2 phosphorylation in c-Kit+ MDSC and c-Kit+ Tregs in patients with advanced, inoperable hepatocellular carcinoma receiving tivozanib as part of a Phase II clinical trial. The levels of circulating CD4+Foxp3+ T cells and CD14−HLADR−CD11b+CD33+ MDSC in HCC patients were measured by flow cytometry before Tivozanib treatment as well as at early (28–35 days) and late treatment (160 days) stages (, , supplemental Figure 1A, 2A). Statistically significant reduction in the frequency and absolute numbers of CD4+Foxp3+Tregs (, ) and CD14−HLADR−CD11b+CD33+ MDSCs (, ) was observed in blood samples (n=17) collected on 28–35 days of tivozanib treatment as compared to pretreatment levels. Decrease in Tregs and MDSC were sustained during and up to 160 days of treatment and were also statistically significant compared to baseline values. The frequencies of Tregs and MDSCs were further decreased significantly on day 160 when compared to samples collected at 28–35 days (supplemental Figures 1A, 2A). Further decrease in absolute numbers did not occur beyond that measured at 28–35 days of treatment (supplemental Figures 1B, 2B)
Figure 1. Reduction in Tregs and dynamics of ERK phosphorylation on c-Kit+Tregs during tivozanib therapy (A) Representative histogram offset showing frequency of CD4+Foxp3+ Tregs measured at pre, 28–35d and 160d of tivozanib treatment. (B) Frequency and (C) absolute numbers of CD4+Foxp3+ Tregs pre vs 28–35d (D) Representative histogram offset showing frequency of Foxp3+c-Kit+ Tregs measured at pre, 28–35d and 160d of tivozanib treatment. (E) Frequency and (F) absolute numbers of Foxp3+c-Kit+ Tregs pre vs 28–35d (G) Representative histogram offset showing frequency of c-Kit+pPERK+ Tregs measured at pre, 28–35d and 160d of tivozanib treatment. (H) Frequency and (I) absolute numbers of c-Kit+pPERK+ Tregs pre vs 28–35d (J) Ratio of CD4+CD127+ T cells to CD4+Foxp3+ T cells (CD4+CD127+ T cells/CD4+Foxp3+ T cells) pre vs 28–35d. (K) Ratio of CD8+CD127+ T cells to CD4+Foxp3+ T cells pre vs 28–35d. Each symbol represents an individual HCC patient. Frequencies of Tregs and T effector cells were calculated based on CD3+CD4+ T/CD3+CD8+ T cell population. **** P < 0.0001, *** P < 0. 001, ** P < 0.01, * P < 0.05, paired t-test, Pre vs 28–35d n = 17.
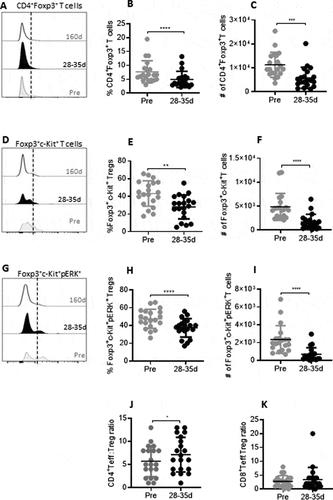
Figure 2. Reduction in MDSC and dynamics of ERK phosphorylation on c-Kit+ MDSCs during tivozanib therapy. (A) Representative histogram offset showing frequency of CD11b+CD33+ MDSCs measured at pre, 28–35d and 160d of tivozanib treatment. (B) Frequency and (C) absolute numbers of MDSCs pre vs 28–35. (D) Representative histogram offset showing frequency of c-Kit+ MDSCs measured at pre, 28–35d and 160d of tivozanib treatment. (E) Frequency and (F) absolute numbers of c-Kit+ MDSCs pre vs 28–35. (G) Representative histogram offset showing frequency of c-Kit+pERK+ MDSCs measured at pre, 28–35d and 160d of tivozanib treatment. (H) Frequency and (I) absolute numbers of c-Kit+pERK+ MDSCs pre vs 28–35. Each symbol represents an individual HCC patient. Frequencies of MDSCs were calculated based on CD14−HLA-DR− population **** P < 0.0001, *** P < 0. 001, ** P < 0.01, * P < 0.05, paired t-test, n = 17.
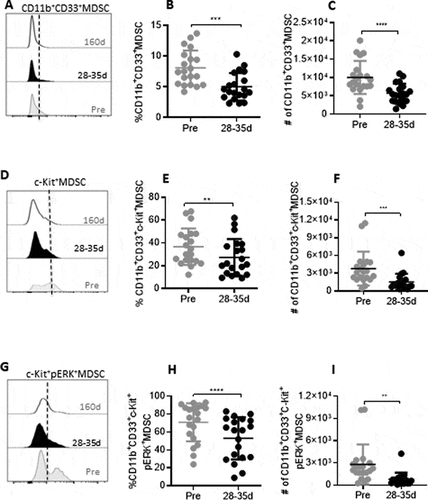
In addition to the decline in Tregs and MDSC frequencies, there was a significant reduction in the frequency and number of c-Kit+ Tregs (–1) and MDSCs (-) that were sustained for upto 160 days after treatment initiation (supplemental Figures 1C, 1D, 2C, 2D). The observed reduction in the levels of c-Kit+ immunosuppressive cells, attenuating antitumor immune responses in HCC may be partially attributed to susceptibility of these cell popualtions to tivozanib-mediated c-Kit signaling antagonism. Indeed, we found that the frequencies and absolute numbers of c-Kit+ERK+ Tregs (-1) and c-Kit+ ERK+MDSCs (-2) were significantly decreased in samples collected 28–35 days after initiation of tivozanib treatment. Decreased ERK phosphorylation levels on Tregs and MDSCs were also monitored at the second follow-up measurement after 160 days of tivozanib treatment. The decreased levels were sustained, however no statistically significant additional decrease occurred beyond that measured at 28–35 days of treatment, with the exception for the frequency of c-Kit+ERK+ Tregs (supplemental Figure 1E, 1F, 2E, 2F). Our studies reveal that signaling via c-Kit receptor/SCF axis on Tregs and MDSCs shows sustained inhibition by tivozanib resulting in the downregulation of ERK phosphorylation, implicating this to be a potential mechanism by which tivozanib impedes expansion of Tregs and MDSC in HCC patients.
The decline in the frequencies and absolute numbers of Tregs was reflected by a significant increase in the ratio of CD4+ T effector cells: Tregs after tivozanib treatment () that were sustained for a long duration (supplemental Figure 1G). Even though, the ratio of CD8+ T effector cells: Treg did not change significantly at the early time point after 28–35 days of treatment (), a significant increase was detected on day 160 (supplemental Figure 1H). Reduction of Tregs and MDSC and subsequent augmentation of Teff:Treg ratio following tivozanib therapy that were long lasting could potentially impact antitumor immune responses in these patients.
Data files generated from the flow cytometry analysis for individual samples (n =8) were subjected to concatenation and then to a dimension reduction process based on the Barnes–Hut SNE algorithm, followed by cell clustering based on the k-means clustering algorithm. Following this step, the cells formed distinct clusters (representing immune subsets) based on similarities in immune-marker expression. T-SNE plots for markers such as CD3, CD4, Foxp3, c-Kit, CD8, PD-1, CD14, HLA-DR, CD11b, CD33, and pERK-2 are shown in supplemental Figure 4A and 4B. Differences in node frequencies can be appreciated across two different compartments, baseline vs 28–35 days of tivozanib treatment. Importantly, the phenotypic signatures of immunosuppression and T cell exhaustion such as Foxp3+Tregs, CD11b+CD33+ MDSCs, c-Kit expression levels on Tregs/MDSCs and CD4+PD-1+ were differentially expressed across pre and 28–35 days of tivozanib treatment (supplemental Figure 4A and 4B), which corroborates with observed reduction in the frequencies or absolute numbers of suppressive phenotypes mesured after tivozanib therapy. These data imply that as a result of therapeutic intervention by tivozanib, there is an alteration in the dynamic immune landscapes of the PBMC of HCC patients.
Reduction in immune checkpoint receptors CTLA-4 and PD-1 on T cells following treatment with tivozanib
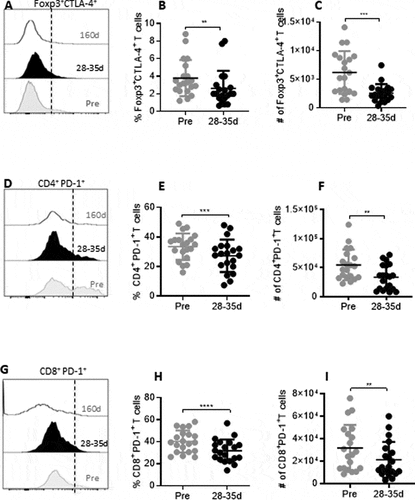
We rationalized that beneficial immune modulation mediated by tivozanib therapy might involve a downregulation of checkpoint receptors on Tregs and T effector cells. Our characterization of the pre- and post-treatment HCC patient samples demonstrated that both the frequency and number of Foxp3+CTLA-4+ Tregs were significantly reduced after tivozanib therapy (). In addition to a reduction in the frequency and number of Tregs coexpressing CTLA-4, tivozanib treatment also decreased checkpoint receptor PD-1, a molecular signature of T cell exhaustion on CD4+ and CD8+ T cells. A significant reduction in the frequency as well as absolute number of CD4+ and CD8+ T cells expressing PD-1 were observed on 28–35 days of tivozanib treatment as compared to pretreatment levels (). The frequencies and absolute numbers of Foxp3+CTLA-4+ Tregs, CD4+PD1+ T cells and CD8+PD1+ T cells remained low in the follow up blood samples collected on 160 days of treatment, indicating that tivozanib-mediated downregulation of this inhibitory checkpoints on T cells was sustained for a prolonged duration (supplemental Figure 3A–3F). However, no further significant decreases either in the frequencies or in the absolute numbers occurred beyond that measured at 28–35 days of treatment. Our results highlight that checkpoint inhibitor therapy combined with tivozanib could potentially generate a synergistic effect and may favor better clinical outcome in HCC patients.
Figure 3. Reduction in immune checkpoint receptors during and after tivozanib therapy. (A) Representative histogram offset showing frequency of Foxp3+CTLA-4+ Tregs measured at pre, 28–35d and 160d of tivozanib treatment. (B) Frequency and (C) absolute numbers of Foxp3+CTLA-4+ Tregs pre vs 28–35d. (D) Representative histogram offset showing frequency of CD4+PD-1+ T cells measured at pre, 28–35d and 160d of tivozanib treatment. (E) Frequency of CD4+PD-1+ T cells pre vs 28–35d (F) Absolute number of CD4+ PD-1+ T cells pre vs 28–35d (G) Representative histogram offset showing frequency of CD8+PD-1+ T cells measured at pre, 28–35d and 160d of tivozanib treatment. (H) Frequency of CD8+PD-1+ T cells pre vs 28–35d (I) Absolute number of CD8+PD-1+ T cells pre vs 28–35d. Each symbol represents an individual HCC patient. Frequencies of CTLA-4+Tregs and PD-1+ T cells were calculated based on CD3+CD4+ T cell/CD3+CD8+ T cell population and. **** P < 0.0001, *** P < 0. 001, ** P < 0.01, * P < 0.05, paired t-test, n = 17.
Predictive and prognostic immune correlates of survival in HCC patients
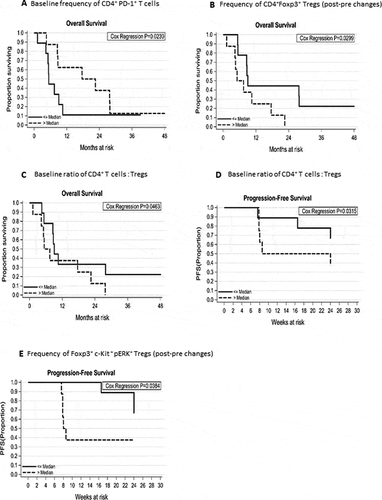
OS of the patients was significantly impacted by the increased frequencies of CD4+PD-1+ T cells before treatment initiation (Hazard ratio = 0.92, 95% Confidence interval: 0.9–1.0, P = 0.02, ), suggesting that patients with high baseline frequencies of CD4+PD-1+ T cells are more responsive to tivozanib therapy. Therefore, high prevalence of this phenotype in blood before the treatment initiation represents a potentially powerful biomarker of prognostic significance. Patients were stratified into 2 groups based on the changes in the before and after measurements of Foxp3 expression levels on CD4+ T cells following tivozanib treatment. Patients with greater decline in the frequencies of CD4+Foxp3+ Tregs (Median reduction: −2.5, range: −7.6 to −0.8), achieved significant improvement in OS as compared with patients with lesser decline, supporting the notion that tivozanib treatment reduces frequency of Tregs in a subset of patients who respond to therapy (Hazard ratio = 1.6, 95% Confidence interval: 1.0–2.3, P = 0.03, ). Furthermore, low ratio of CD4+T effector cells: Foxp3+ Tregs in the pre-treatment blood samples had significant association with better OS (Hazard ratio = 1.2, 95% Confidence interval: 1.0–1.5, P = 0.046, )) and PFS of patients (Hazard ratio = 1.4, 95% Confidence interval: 1.0–1.9, P = 0.03, ). Patients with greater decrease (changes in the before and after measurements) in the frequencies of Foxp3+c-Kit+pERK+Tregs measured after tivozanib treatment showed significant correlation with improved PFS at 24 weeks following commencement of treatment (Hazard ratio = 1.2, 95% Confidence interval: 1.0–1.5, P = 0.03, ).
Figure 4. Kaplan-Meier plots showing the predictive immune correlates of responses to tivozanib treatment in HCC patient. Association between immunophenotypic signatures and OS or PFS of patients was calculated as described in methods. (A) OS of the patients and increased frequencies of baseline CD4+PD-1+ T cells (HR = 0.92, 95% CI: 0.9–1.0, P = 0.02). (B) Reduction in the frequencies of CD4+Foxp3+ Tregs (post- pre changes) (median reduction: −2.5, range: −7.6 to −0.8), quantified after tivozanib therapy and OS of the patients (HR = 1.6, 95% CI: 1.0–2.3, P = 0.03). (C) Low baseline ratio of CD4+T effector cells to Foxp3+ Tregs and OS of the patients (HR = 1.2, 95% CI: 1.0–1.5, P = 0.046) as well as (D) Low baseline ratio of CD4+T effector cells to Foxp3+ Tregs and PFS of the patients at 24 weeks (HR = 1.4, 95% CI: 1.0–1.9, P = 0.03). (E). Decrease in the frequencies of Foxp3+c-Kit+pERK+Tregs (post-pre changes) and PFS of the patients at 24 weeks (HR = 1.2, 95% CI: 1.0–1.5, P = 0.03).
Tivozanib and sorafenib treatment differentially impact immunosuppressive phenotypes in HCC patients
Percentage change or decrease from the baseline numbers of both Foxp3+Tregs and Foxp3+CTLA-4+ Tregs were significantly greater in HCC patients treated with tivozanib as compared to sorafenib (P =0.001, , P =0.001, ). Percentage change from the baseline numbers of CD4+PD-1+T cells was also significantly greater in patients treated with tivozanib than in patients treated with sorafenib (P =0.02, ). While the mean of the percentage change from baseline numbers of CD8+PD-1+T cell was greater in the tivozanib group, however the change was not statistically significant when compared to the sorafenib group (P =0.2, ). Importantly, percentage change from baseline frequencies as well as numbers of MDSCs were significantly greater in patients treated with tivozanib in comparison with sorafenib treatment (P =0.006, , P =0.05 ).
Figure 5. Differential effect of tivozanib vs sorafenib treatment on immune suppressive cell subsets in HCC patients and impact of post-treatment changes in the ratio of CD4+T effector cell: Foxp3+ Tregs on overall survival of HCC patients after tivozanib vs sorafenib treatment. Box plots represent mean/median percentage changes from baseline number or frequency of different immune cell subsets after 28–25 days of sorafenib vs. tivozanib treatment (A) Foxp3+ Tregs (B) Foxp3+CTLA-4+ Tregs (C) CD4+PD-1+ T cells (D) CD8+PD-1+ T cells (E) % CD11b+CD33+ MDSC (F) # CD11b+CD33+ MDSC (G) Kaplan-Meir plots showing the association of greater percentage change or increase in the ratio of CD4+T cells: Tregs from baseline and survival probability of HCC patients after sorafenib or tivozanib treatment. P values are shown inside the respective plots; sorafenib n = 49, tivozanib n = 17.
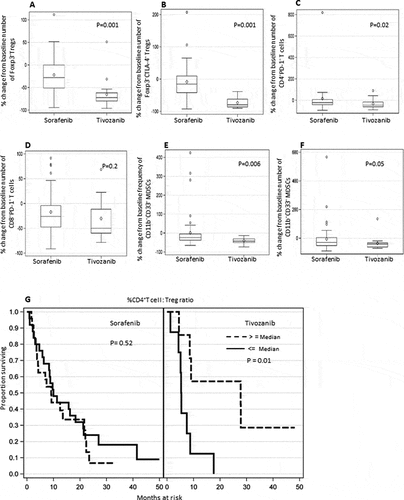
Comparison of survival probability of HCC patients based on the biomarker effect after tivozanib or sorafenib treatment
OS of the patients was significantly impacted by percentage changes from baseline ratio of CD4+ T effector cells to Tregs after tivozanib treatment as compared to sorafenib treatment. Greater survival rate is significantly associated with greater percentage change (i.e. increase) in the ratio of CD4+T cells: Tregs from baseline, nonetheless an opposite of this trend is in seen sorafenib treated patients (P =0.01, P =0.52 respectively, ). Tivozanib-mediated c-Kit signalling antagonism impedes expansion and development of Tregs as reflected by enhanced CD4+ T effector cells to Tregs ratio with potential impact on antitumor immunity and survival. Our observation that patients with low baseline CD4+T cells to Treg ratio having improvement in OS and PFS () corroborates with the above finding.
Discussion
HCC develops multiple strategies to evade the host’s immune defense mechanisms which includes the induction of Tregs, MDSCs, immune checkpoint receptors and inhibitory cytokines.Citation22,Citation23 These redundant immunosuppressive factors are responsible for the subversion of tumor specific immunity in HCC, facilitating cancer progression and metastasis. Tregs and MDSCs exploit the intrinsic tolerogenic properties of the liver to accumulate and exert various immune-suppressive and tumor-promoting mechanisms. This wide spectrum of suppressive mechanisms makes the development of efficacious immunotherapeutic approaches challenging. The new generation VEGF TKI tivozanib that potently and selectively inhibits all three VEGFRs (VEGF-R1, R2, and R3) demonstrated prolonged half-life, improved antitumor activity and tolerability in RCC and HCC with a distinct therapeutic profile from other TKIs.Citation25–Citation27 Recent studies showed that tivozanib had a low incidence of class-related off-target adverse events typically associated with less selective VEGFR tyrosine kinase inhibitors (i.e. fatigue, palmar-plantar erythrodysesthesia syndrome and diarrhea) .Citation28 The antineoplastic efficacy of tivozanib compared to sorafenib as first-line treatment has an overall response rate (ORR, RECIST 1.1) of 21% vs 6.5%.Citation25 It has been reported that treatment with ramucirumab, which selectively inhibits VEGFR-2 failed to produce any objective responses in HCC Citation29 and therefore potential inhibition of other tivozanib targets, rather than VEGFR-2, might be involved in the tumor reduction accomplished by tivozanib treatment. C-Kit, one of the therapeutic targets of tivozanib plays an important role in the proliferation of Tregs and MDSCs, and is aberrantly mutated in several malignancies.Citation12 Furthermore, blockade of c-Kit signaling using anti-c-Kit monoclonal antibody or targeting c-Kit using sunitinib prevented the accumulation of Tregs and MDSCs in preclinical models.Citation16–Citation18 We hypothesized that tivozanib-mediated therapeutic disruption of signaling via SCF-c-Kit axis may impede the relentless accumulation of Tregs and MDSCs in HCC patients. From this perspective, we rationalized measuring c-Kit expression on Tregs, MDSC and the downstream target of tivozanib, pERK2 on c-Kit+ Tregs and c-Kit+ MDSCs before the initiation of tivozanib treatment and at different time points during treatment of HCC patients.
Our findings of the decrease in the frequencies and absolute numbers of Tregs and MDSC with concomitant enhancement in Teff: Treg ratio observed in HCC patients after tivozanib therapy, supports preclinical studies which demonstrated the reduction of Tregs and MDSC accumulation resulting from the disruption of signaling via the c-Kit-SCF axis or treatment with sunitinib or tivozanib.Citation16–Citation19 In our study, the serial monitoring of suppressive phenotypes, c-Kit+ Tregs and c-Kit+ MDSC revealed the dynamic changes of these phenotypes during tivozanib treatment. Levels of ERK2 phosphorylation on c-Kit+ Tregs and MDSCs were significantly decreased after tivozanib treatment. ERK2 is the downstream target of tivozanib and the decrease in ERK2 phosphorylation is accomplished by tivozanib-mediated blockade of signaling via c-Kit-SCF axis on Tregs and MDSC. c-Kit-SCF signaling pathway has been shown to be vital for the development and proliferation of Tregs and MDSCs.Citation16–Citation18 Previously, we had reported the significant correlation between increased baseline number of flt-3+pERK+MDSCs and PFS of HCC patients treated with sorafenib.Citation30 HCC patients with increased number of baseline flt-3+pERK+MDSC+ may be more responsive to antagonism of VEGF-R signaling. MDSCs are one of the key drivers of tumor-mediated immune evasion and therefore targeting MDSC appears to be a clinically promising strategy in HCC patients. Furthermore, blockade of MDSC accumulation can potentially lead to beneficial antitumor effects, such as decreased tumor angiogenesis, reduced number of Foxp3+Tregs with concomitant enhancement in Th1 responses. In a recent study in HCC patients, the frequency of MDSC was shown to be correlated with levels of alpha- fetoprotein (AFP) and tumor volume and surgical removal of tumors reduced the population of MDSC in these patients.Citation31
Additionally, by performing a comparative analysis, we report the differential impact of tivozanib vs sorafenib treatment on immunosuppressive phenotypes. We report significantly greater decreases in the absolute numbers of Foxp3+Tregs, CTLA-4+Tregs, CD4+PD-1+ T cells, and MDSCs observed in patients treated with tivozanib as compared to patients treated with sorafenib, highlighting the underappreciated anti-tumor efficacy of tivozanib. An earlier report on the antineoplastic efficacy of tivozanib compared to sorafenib with an overall response rate of 21% vs 6.5% corroborates with our findings.Citation25 Significantly higher impact of tivozanib versus sorafenib treatment on progression free survival reported for advanced renal cancer patients strongly support our finding in HCC patients.Citation28
The strong association between survival benefit and decrease in Treg frequencies of patients post tivozanib therapy is supported by our earlier studies which demonstrated the significant correlation between OS and decrease in the absolute number of Tregs in HCC patients after sorafenib treatment.Citation24 Our results suggest that tivozanib treatment reduces Tregs in a proportion of HCC patients who respond to therapy. HCC patients with low baseline ratio of CD4+T effector cells to Tregs achieved significant improvement in the OS after tivozanib therapy which corroborates with our previous report in HCC patients treated with sorafenib.Citation24 The decrease in the frequencies of Foxp3+c-Kit+pERK+Tregs measured after tivozanib treatment significantly impacted PFS of the patients. Signaling via c-Kit leading to phosphorylation of ERK-2 is critical for the development and proliferation Foxp3+Tregs and tivozanib targets the c-Kit signaling pathway resulting in an impairment in the phosphorylation of ERK2 on Foxp3+c-Kit+Tregs.
We report for the first time that higher frequencies of CD4+PD-1+ T cells prior to tivozanib treatment significantly impacted OS of the patients. These results implicate increased responsiveness of HCC patients with high baseline CD4+PD-1+ T cells to tivozanib therapy. Therefore, high prevalence of this phenotype in pretreatment blood samples may be a biomarker of prognostic significance. These findings were further validated by our previous report on elevated pre-treatment levels of CD4+PD-1+ T cells that were significantly associated with OS of HCC patients treated with sorafenib.Citation24 Additionally, recent clinical trials that reported improvement in OS and PFS of unresectable HCC patients treated with atezolizumab in combination with bevacizumab corroborates with our findings.Citation32,Citation33 Furthermore, by performing a comparative study of biomarker effect on survival benefit, we report for the first time the significant association of elevated CD4+T cells: Tregs ratio with OS of patients treated with tivozanib as compared to patients treated with sorafenib. Better antititumor efficacy, particularly significant improvement in progression-free survival reported in advanced renal cell carcinoma patients treated with tivozanib versus those patients treated with sorafenib, is supportive of our results in HCC patients.Citation28 Therefore, monitoring the kinetics of CD4+T cells: Treg ratio or CD4+PD-1+ T cells in HCC patients during tivozanib treatment represents a potentially powerful biomarker of prognostic significance. Our study has limitations, including the sample size and the resulting small size of the different immune cell subsets. Nevertheless, our results provide the scientific rationale for combination therapy; administration of immunotherapeutic approaches such as checkpoint inhibitors/anti-PD-1 prior to tivozanib in order to activate antitumor immune responses in HCC patients.
PD-1 and CTLA-4 are critical players in maintaining immune tolerance during tumor growth. By simultaneously inhibiting these immune checkpoints, tivozanib provides a greater opportunity to establish an immune reactive microenvironment that recognizes and eliminates cancer cells rapidly. Clinical trials involving combinatorial approaches using tivozanib and anti-PD-1 antibody might yield superior tumor growth inhibition in a larger proportion of subjects, simultaneously exhibiting a variety of immunostimulatory changes associated with enhanced anti-tumor immune responses, as compared to anti-PD-1 treatment alone. Such a regimen should be capable of both targeting tumor angiogenesis and down regulating immune suppressor cell phenotypes, an ‘off target’ effect of tivozanib on the immune system. Tivozanib demonstrated synergy in combination with nivolumab (anti PD-1) in a Phase 2 study in RCC and is being investigated in several other tumor types, including renal cell, hepatocellular, colorectal, and breast cancers. A significant decrease in soluble plasma VEGFR-2 with an overall response rate of 21% was reported in this group of HCC patients treated with tivozanib.Citation25 Previous reports highlighting a favorable safety profile of tivozanib compared with those of other tyrosine kinase inhibitors of VEGFR, with a low incidence of class-related adverse events and related dose adjustments is encouraging. Recent reports on the therapeutic efficacy of several new generation second line TKIs (ramicirumab, cabozantinib, lenvatinib) in HCC patients as well as effective treatment for hepatitis C is rapidly changing the landscape of HCC treatment.Citation34 Given the high response rate, tolerability and durability of response in the trial of tivozanib, a trial of tivozanib and PD-L1 inhibitor durvalumab is now underway (NCT03970616).The proof of concept of anti-VEGF and checkpoint inhibition in HCC was recently confirmed in a large phase 3 study with atezolizumab and bevacizumab.Citation32,Citation33
In summary, our studies provide an important insight into ‘off-target’ effect of tivozanib in the targeted therapy of HCC as reflected by its beneficial effect on reduction of immunosuppressive phenotypes. Importantly of clinical relevance, reduction in Tregs after tivozanib therapy significantly improved OS of the patients. High baseline frequencies of CD4+PD-1+ T cells showed strong correlation with survival benefit of patients. In our comparative study based on biomarker effect, greater increase in CD4+T cell: Treg ratio after tivozanib treatment was significantly associated with improvement in OS of the patients versus sorafenib treated patients, which highlights better efficacy of tivozanib.
Patients and methods
This report is based on a Multicenter Phase 1b/2 ClinicalTrials.gov (NCT01835223) of Tivozanib in Patients with Advanced Inoperable Hepatocellular Carcinoma conducted at Roswell Park Comprehensive Cancer Center (RPCCC).The study protocol (I 229,112) was approved by Roswell Park Institutional Review Board. Informed consent was obtained from eligible patients in a manner consistent with the World Medical Association Declaration of Helsinki and Intitute standards to be included in this prospective open label clinical trial with biomarker study endpoints.
Immune monitoring
Heparinized peripheral blood samples were obtained from HCC patients before the initiation of treatment, after 28-35 days and 160 days of tivozanib treatment, through the Data Bank and Biorepository at RPCCC. PBMCs were isolated by Ficoll-Paque density gradient centrifugation and cryopreserved.Citation22 The following antibodies were used for surface staining of specific immune cell types: APC-H7 anti-CD3, PerCP anti-CD4, V500 anti-CD8, PE-Cy7 PD-1, BV421 CD117 (c-Kit), FITC CD11b, V450 HLADR, APC CD14 and PECy5 CD33. Intracellular staining using Alexa-488 Foxp3 and PE ERK2 was carried out after fixation and permeabilization of cells and samples were acquired on LSRII flow cytometer.Citation35 Data were analyzed using Flowjo 10.03. Frequency and phenotype of Tregs, MDSC and PD-1+ T cells were determined by polychromic flow cytometry as described before. Citation22,Citation35
Statistics
This study was a non-randomized longitudinal study of biomarker changes from baseline to days 28–35 and day 160 following tivozanib therapy in 17 subjects. Furthermore, a combined retrospective analysis of biomarker changes from baseline to days 28–35 days following sorafenib treatment in 49Citation24,Citation30 subjects was performed for comparison. The primary endpoints were PFS and OS as a function of baseline biomarker levels and percentage changes from baseline to days 28–35. Biomarker values were dichotomized for baseline values as above or below the median and above or below the median change scores. Comparisons between the two strata were carried out using a log-rank test, tested at α = 0.05 (2 sided). P values of less than 0.05 were considered significant. Differences in mean biomarker changes over time were examined using a paired t-test at alpha =0.05 (two-sided). A Cox regression model was used to examine biomarker baseline values as well as changes over time in terms of relative risk of death. All analyses were performed use SAS version 9.4 (SAS Institute, Cary, NC).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions:
Conception and design: S. Kalathil, Y. Thanavala
Development of methodology: S. Kalathil, Y. Thanavala
Acquisition of data: S. Kalathil, Katy Wang, A. Hutson, Y. Thanavala
Analysis and interpretation of data: S. Kalathil, Katy Wang, A. Hutson, Y. Thanavala
Writing, reviewing, and or revision of the manuscript: S. Kalathil, A. Hutson,
R. Iyer, Y. Thanavala
Supplemental Material
Download ()Acknowledgments
We would like to thank Melissa Robins, Clinical research Coordinator for the coordination of protocol amendment and quarterly status reports submissions. We also thank, Andrea Frazer for work on this project in data assemblage and quality control. The authors would like to thank Paul Wallace and Orla Maguire for their help with flow cytometry experiments.
Supplementary material
Supplemental data for this article can be accessed on thehere
Additional information
Funding
References
- El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology. 2014;60(5):1767–11. doi:10.1002/hep.27222.
- Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018.
- Regad T. Targeting RTK Signaling Pathways in Cancer. Cancers (Basel). 2015;7(3):1758–1784. doi:10.3390/cancers7030860.
- Du Z, Lovly CM. Mechanisms of receptor tyrosine kinase activation in cancer. Mol Cancer. 2018;17(1):58. doi:10.1186/s12943-018-0782-4.
- Momeny M, Moghaddaskho F, Gortany NK, Yousefi H, Sabourinejad Z, Zarrinrad G, Mirshahvaladi S, Eyvani H, Barghi F, Ahmadinia L, et al... Blockade of vascular endothelial growth factor receptors by tivozanib has potential anti-tumour effects on human glioblastoma cells. Sci Rep. 2017;7(1):44075. doi:10.1038/srep44075.
- Hepgur M, Sadeghi S, Dorff TB, Quinn DI. Tivozanib in the treatment of renal cell carcinoma. Biologics. 2013;7:139–148.
- Motzer RJ, Nosov D, Eisen T, Bondarenko I, Lesovoy V, Lipatov O, Tomczak P, Lyulko O, Alyasova A, Harza M, et al.. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial. J Clin Oncol. 2013;31(30):3791–3799. doi:10.1200/JCO.2012.47.4940.
- Momeny M, Sabourinejad Z, Zarrinrad G, Moghaddaskho F, Eyvani H, Yousefi H, Mirshahvaladi S, Poursani EM, Barghi F, Poursheikhani A, et al.. Anti-tumour activity of tivozanib, a pan-inhibitor of VEGF receptors, in therapy-resistant ovarian carcinoma cells. Sci Rep. 2017;7(1):45954. doi:10.1038/srep45954.
- Benson AB, Kiss I, Bridgewater J, Eskens FALM, Sasse C, Vossen S, Chen J, Van Sant C, Ball HA, Keating A, et al.. BATON-CRC: A Phase II Randomized Trial Comparing Tivozanib Plus mFOLFOX6 with Bevacizumab Plus mFOLFOX6 in Stage IV Metastatic Colorectal Cancer. Clin Cancer Res. 2016;22(20):5058–5067. doi:10.1158/1078-0432.CCR-15-3117.
- Jamil MO, Hathaway A, Mehta A. Tivozanib: status of development. Curr Oncol Rep. 2015;17(6):24. doi:10.1007/s11912-015-0451-3.
- Escudier B, Porta C, Eisen T, Belsey J, Gibson D, Morgan J, Motzer R. The role of tivozanib in advanced renal cell carcinoma therapy. Expert Rev Anticancer Ther. 2018;11:1113–1114.
- Stankov K, Popovic S, Mikov M. C-KIT signaling in cancer treatment. Curr Pharm Des. 2014;20(17):2849–2880. doi:10.2174/13816128113199990593.
- Ali S, Ali S. Role of c-kit/SCF in cause and treatment of gastrointestinal stromal tumors (GIST). Gene. 2007;401(1–2):38–45. doi:10.1016/j.gene.2007.06.017.
- Foster BM, Zaidi D, Young TR, Mobley ME, Kerr BA. CD117/c-kit in Cancer Stem Cell-Mediated Progression and Therapeutic Resistance. Biomedicines. 2018;6.
- Abbaspour BM, Kamalidehghan B, Saleem M, Huri HZ, Ahmadipour F. Receptor tyrosine kinase (c-Kit) inhibitors: a potential therapeutic target in cancer cells. Drug Des Devel Ther. 2016;10:2443–2459. doi:10.2147/DDDT.S89114.
- Pan PY, Wang GX, Yin B, Ozao J, Ku T, Divino CM, Chen SH. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111(1):219–228. doi:10.1182/blood-2007-04-086835.
- Kao J, Ko EC, Eisenstein S, Sikora AG, Fu S, Chen SH. Targeting immune suppressing myeloid-derived suppressor cells in oncology. Crit Rev Oncol Hematol. 2011;77(1):12–19. doi:10.1016/j.critrevonc.2010.02.004.
- Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, Schwartz M, Divino CM, Pan PY, Chen SH. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69(6):2514–2522. doi:10.1158/0008-5472.CAN-08-4709.
- Pawlowski N, Hoerzer H, Singh HJ, Hilf N. Impact of various first- and second-generation tyrosine-kinase inhibitors on frequency and functionality of immune cells. Proc 104th Annu Meeting Am Assoc Cancer Res. 2013;73:8.
- Immune Checkpoint KM. Inhibition in Hepatocellular Carcinoma: Basics and Ongoing Clinical Trials. Oncology. 2017;92(Suppl 1):50–62. doi:10.1159/000451016.
- Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681–700.
- Kalathil S, Lugade AA, Miller A, Iyer R, Thanavala Y. Higher Frequencies of GARP(+)CTLA-4(+)Foxp3(+) T Regulatory Cells and Myeloid-Derived Suppressor Cells in Hepatocellular Carcinoma Patients Are Associated with Impaired T-Cell Functionality. Cancer Res. 2013;73(8):2435–2444. doi:10.1158/0008-5472.CAN-12-3381.
- Lugade AA, Kalathil S, Miller A, Iyer R, Thanavala Y. High immunosuppressive burden in advanced hepatocellular carcinoma patients: Can effector functions be restored? Oncoimmunology. 2013;2(7):e24679. doi:10.4161/onci.24679.
- Kalathil SG, Lugade AA, Miller A, Iyer R, Thanavala YPD. 1(+) and Foxp3(+) T cell reduction correlates with survival of HCC patients after sorafenib therapy. JCI Insight. 2016;1:11:e86182.
- Fountzilas C, Gupta M, Lee S, Krishnamurthi S, Estfan B, Wang K, Attwood K, Wilton J, Bies R, Bshara W, et al.. A multicentre phase 1b/2 study of tivozanib in patients with advanced inoperable hepatocellular carcinoma. Br J Cancer. 2020;122:963–970. doi:10.1038/s41416-020-0737-6.
- Bukowski RM. Third generation tyrosine kinase inhibitors and their development in advanced renal cell carcinoma. Front Oncol. 2012;2:13. doi:10.3389/fonc.2012.00013.
- Nosov DA, Esteves B, Lipatov ON, Lyulko AA, Anischenko AA, Chacko RT, Doval DC, Strahs A, Slichenmyer WJ, Bhargava P. Antitumor activity and safety of tivozanib (AV-951) in a phase II randomized discontinuation trial in patients with renal cell carcinoma. J Clin Oncol. 2012;30:1678–1685. doi:10.1200/JCO.2011.35.3524.
- Rini BI, Pal SK, Escudier BJ, Atkins MB, Hutson TE, Porta C, Verzoni E, Needle MN, McDermott DF. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol. 2020;21(1):95–104. doi:10.1016/S1470-2045(19)30735-1.
- Zhu AX, Kang Y-K, Yen C-J, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, et al.. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi:10.1016/S1470-2045(18)30937-9.
- Kalathil SG, Hutson A, Barbi J, Iyer R, Thanavala Y. Augmentation of IFN-γ+ CD8+ T cell responses correlates with survival of HCC patients on sorafenib therapy. JCI Insight. 2019;4(15):15:e130116. doi:10.1172/jci.insight.130116.
- Lee WC, Wang YC, Cheng CH, Wu TH, Lee CF, Wu TJ, Chou HS, Chan KM. Myeloid-derived suppressor cells in the patients with liver resection for hepatitis B virus-related hepatocellular carcinoma. Sci Rep. 2019;9:2269. doi:10.1038/s41598-019-38785-3.
- Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al.. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894–1905. doi:10.1056/NEJMoa1915745.
- Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, Hack SP, Spahn J, Liu B, Abdullah H, et al.. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21:808–820. doi:10.1016/S1470-2045(20)30156-X.
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2.
- Kalathil SG, Lugade AA, Pradhan V, Miller A, Parameswaran GI, Sethi S, Thanavala Y. T-Regulatory Cells and Programmed Death 1 + T Cells Contribute to Effector T-Cell Dysfunction in Patients with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2014;190(1):40–50. doi:10.1164/rccm.201312-2293OC.
