ABSTRACT
The consensus Immunoscore is a routine assay quantifying the adaptive immune response within the tumor microenvironment. Its evaluation in the primary tumor of patients with stages I/II/III colorectal cancer (CRC) has prognostic value that has been confirmed in multiple studies. For metastatic patients, the evaluation of the consensus Immunoscore within resected metastases also significantly predicts the recurrence and survival of Stage IV patients. Since recurrence rates post-surgery are still very high, it is important to best evaluate risk parameters using the main patho-molecular and immune parameters. After preoperative treatment and curative resection of 582 metastases from 221 patients, clinico-pathological parameters, RAS mutation, and Immunoscore within metastases were assessed. Immunoscore and clinico-pathological parameters (number of metastases, surgical margin, histopathological growth pattern, and steatohepatitis) were associated with relapse in multivariable analysis. A Pathological Score (PS) that combines relevant clinico-pathological factors for relapse and Immunoscore was significantly (P < .0001) associated with Time to recurrence. In multivariable analysis, only Immunoscore (P < .001) and RAS mutations (P= .03) were prognostic and significantly associated with overall survival. Thus, among all parameters clinically relevant in the metastatic settings, PS and Immunoscore allow the stratification of stage IV CRC patients and identify patients with higher risk of recurrence. Immunoscore remained the major prognostic factor for overall survival (OS). In its latest edition, the WHO classification of Digestive System Tumors introduced for the first time the immune response as an essential and desirable diagnostic criterion for CRC. These novel results highlight the clinical utility of Immunoscore in Stage IV patients.
The prognostic impact of patho-molecular features and Immunoscore on stage IV metastatic patients
Colorectal cancer (CRC) is a major leading cause of cancer death worldwide, particularly for stage IV metastatic patients. Most metastases occur in the liver, lungs, and peritoneum. Surgical resection of colorectal liver metastases provides a 5-year survival rate of 50–60%, however, recurrence of the disease occurs in up to 70% of patients who have undergone such curative resection. Analysis of patho-molecular findings as well as immune parameters in samples of resected metastases could provide significant information on the aggressiveness of the tumor and on the efficacy of preoperative treatment. For liver metastases, the current clinico-pathological parameters relevant for the prognostic survival after metastatic resection are: (1) size and number of lesions, (2) surgical margin status, (3) pathological tumor response assessed by tumor regression grading (TRG), (4) histopathological growth pattern (HGP) of liver metastases, (5) molecular status assessed by the presence of RAS and BRAF mutations, and (6)chemotherapy-related liver injury (CALI) evaluated in distant parenchyma, which include sinusoidal obstructive syndrome, nodular regenerative hyperplasia, and steatohepatitis.Citation1
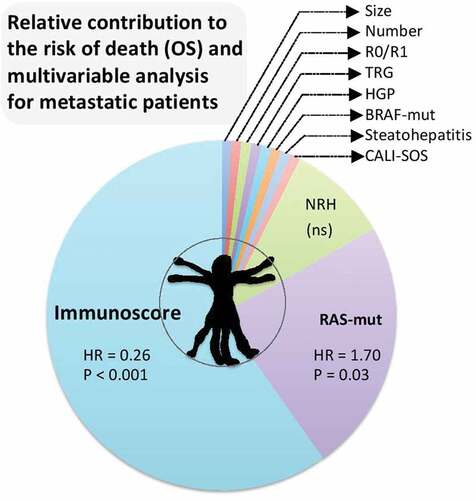
Figure 1. Clinical utility of immunoscore in metastasis. Relative contribution to the risk of death (OS) and Cox multivariateriable analysis for metastatic patients, including the following parameters, size of lesions, number of lesions, surgical margin status (R0/R1), pathological tumor response assessed by tumor regression grading (TRG), histopathological growth pattern (HGP) of liver metastases, molecular status assessed by the presence of BRAF or RAS mutations, chemotherapy-related liver injury (CALI), steatohepatitis, CALI with sinusoidal obstructive syndrome (SOS), CALI with nodular regenerative hyperplasia (NRH), and the consensus immunoscore.
The oncogenic process and tumor clone dissemination from pre-cancer lesionCitation2 to metastasisCitation3–Citation5 is deeply impacted by the immunity within the microenvironment. The consensus Immunoscore, assessing the tumor immune infiltration of T- and cytotoxic T-cells, plays a major role for patient’s relapse and survival in primaryCitation6–Citation8 and metastatic settings.Citation3–Citation5 To improve the predictive accuracy of patho-molecular examination and immune assessment of metastases, we investigated all these recognized prognostic factors to study comprehensively their prognostic impact in stratifying patient prognosis.
The study included 582 metastases from 221 patients.Citation1 Nearly all patients had a BRAF wild-type and microsatellite stable tumor. Most of the patients had several synchronous metastases and received preoperative chemotherapy (84,6%) mainly associated with a targeted therapy (anti-VEGF or anti-EGFR), followed by surgical resection of metastases. Component drugs in the FOLFOX regimen was shown to induce immunogenic cell death.Citation9 Patients with multiple metastases had heterogeneous TRG and HGP. Desmoplastic and mixed HGP metastases were the most frequent patterns. Better pathological response (TRG1-3) was significantly associated with desmoplastic and pushing HGP but also with higher Immunoscore.
In univariate analysis, several parameters were associated with shorter time to relapse (TTR), including the presence of involved lymph-node (pN+) during primary tumor resection (P = .0172), the presence of more than three metastases (P= .0001), R1 positive margin (P= .0017), presence of replacement and mixed HGP (P= .0002), and low Immunoscore (P< .00001). On the overhand, significant prognostic parameters for overall survival (OS) were RAS mutation (P= .0109), TRG (P= .0471), the presence of more than three metastases (P= .0229), and Immunoscore (P= .0001). Thus, only two parameters were significantly associated with both recurrence and overall survival, presence of more than three metastases, and Immunoscore.Citation1
In Cox multivariate analyses, replacement and mixed HGP, presence of steatohepatitis, number of resected metastases, resection margin status, and Immunoscore were significantly associated with TTR. In contrast, only RAS mutation and Immunoscore were significant for OS. The relative contribution of each parameter included in multivariate analysis for TTR and OS revealed that Immunoscore had the highest contribution for TRR (30%) and OS (64%).
After the assessment of the individual impact of clinico-pathological parameters for patient outcome, their combined power on the patient prognosis was investigated using an optimized Pathological Score (PS, PathoScore). PS was significantly associated with TTR in univariate analysis. Patients with a worse PS have more than two times higher-risk to relapse compared with patients with a favorable PS (P < .00001). In multivariable analysis for TTR, PathoScore and Immunoscore were significant (both P < .001). In contrast, multivariable analysis for OS revealed that RAS-mutation status (P= .03) and Immunoscore (P= .0009) were the only significant parameters (). Hence, only Immunoscore was significant in multivariate analysis for both TTR and OS.Citation1
Patients combining a favorable PathoScore and a high Immunoscore had the lowest risk of relapse. The worst OS was observed for patients with poor PathoScore and low Immunoscore. Regardless of the PathoScore, patients with a high-Immunoscore had a prolonged survival compared to patients with a low-Immunoscore.Citation1
Immunoscore remained a major prognostic factor for TTR and OS confirming that within metastatic CRC the adaptive immune response plays a central role in preventing tumor recurrence.Citation10–Citation12 The role of the natural immunity and long-lasting capacity of memory T-cells, as well as immunoediting, could play a central role for patients’ survival.
The limit of our study is its retrospective design. Additional studies to validate the importance of the PS and the other parameters should be further considered. Strengths of our study include a patient population of uniform tumor stage with molecular annotation and rigorously collected patient outcome data. Furthermore, a centralized Immunoscore evaluation on all resected metastases was performed assuring uniformity in its determination and analysis.
Another studyCitation4 revealed that a strong infiltrate with adaptive immune cells and Immunoscore were prolonging the survival (disease-free survival and overall survival) of the patients, even if they were classified as nonresponders (no tumor regression following neo-adjuvant chemotherapy-based treatment). The immune parameters remained the only statistically significant prognostic factor associated with DFS and OS in multivariable analysis, while response to treatment was not.Citation4 These findings underline the importance of immunological markers and Immunoscore in determining the prognosis and response to therapy.
Immunoscore for guiding patient management decisions
These results confirm the limited impact of the preoperative treatment and highlight the strongest prognostic factors in Stage IV patients. No survival benefit for preoperative treatment has ever been demonstrated for patients with resectable liver colorectal metastases. The prognostic value of Immunoscore has been demonstrated in Stage I/II/III, Stage II, Stage III,Citation6–Citation8 and its predictive value of response to chemotherapy demonstrated for randomized phase 3 trial of Stage III patients.Citation13 These results further reinforce the clinical utility of Immunoscore in Stage IV patients.Citation1
Tumor and immunological markers are shaping of an efficient immune reaction and can serve as targets for novel therapeutic approaches. Thus, the strength of the immune reaction could advance our understanding of cancer evolution and have important consequences in clinical practice.Citation14 The combination of Pathoscore and Immunoscore, both important markers to assess the risk of patient tumor relapse, helps clinicians in the decision-making process, and for the best post-metastasectomy clinical approach. Independently of relapse, Immunoscore remains the major determinant of patient overall survival. The 2020 ESMO Clinical Practice Guidelines for colon cancer included Immunoscore to refine the prognosis and thus adjust the chemotherapy decision-making process. Furthermore, the introduction into the latest (fifth) edition of the WHO Digestive System Tumors of the immune response, as measured by Immunoscore, as essential and desirable diagnostic criteria for colorectal cancer, further supports the introduction of a new TNM-Immune classification system.
Disclosure of potential conflicts of interest
JG and BM have patents associated with the immune prognostic biomarkers. JG is co-founder of HalioDx biotech company. Immunoscore® a registered trademark from the National Institute of Health and Medical Research (INSERM) licensed to HalioDx.
Additional information
Funding
References
- Baldin P, Van den Eynde M, Mlecnik B, Bindea G, Beniuga G, Carrasco J, Haicheur N, Marliot F, Lafontaine L, Fredriksen T, et al. Prognostic assessment of resected colorectal liver metastases integrating pathological features, RAS mutation and Immunoscore. J Pathol Clin Res. 2020;e178. doi:10.1002/cjp2.178.
- Mascaux C, Angelova M, Vasaturo A, Beane J, Hijazi K, Anthoine G, Buttard B, Rothe F, Willard-Gallo K, Haller A, et al. Immune evasion before tumour invasion in early lung squamous carcinogenesis. Nature. 2019;571:570–3. doi:10.1038/s41586-019-1330-0.
- Angelova M, Mlecnik B, Vasaturo A, Bindea G, Fredriksen T, Lafontaine L, Buttard B, Morgand E, Bruni D, Jouret-Mourin A, et al. Evolution of metastases in space and time under immune selection. Cell. 2018;175:751–765 e716. doi:10.1016/j.cell.2018.09.018.
- Mlecnik B, Van den Eynde M, Bindea G, Church SE, Vasaturo A, Fredriksen T, Lafontaine L, Haicheur N, Marliot F, Debetancourt D, et al. Comprehensive intrametastatic immune quantification and major impact of immunoscore on survival. J Natl Cancer Inst. 2018;110:97–108. doi:10.1093/jnci/djx123.
- Van den Eynde M, Mlecnik B, Bindea G, Fredriksen T, Church SE, Lafontaine L, Haicheur N, Marliot F, Angelova M, Vasaturo A, et al. The link between the multiverse of immune microenvironments in metastases and the survival of colorectal cancer patients. Cancer Cell. 2018;34:1012–1026 e1013. doi:10.1016/j.ccell.2018.11.003.
- Galon J, Fox BA, Bifulco CB, Masucci G, Rau T, Botti G, Marincola FM, Ciliberto G, Pages F, Ascierto PA, et al. Immunoscore and immunoprofiling in cancer: an update from the melanoma and immunotherapy bridge 2015. J Transl Med. 2016;14:273. doi:10.1186/s12967-016-1029-z.
- Kirilovsky A, Marliot F, El Sissy C, Haicheur N, Galon J, Pages F. Rational bases for the use of the immunoscore in routine clinical settings as a prognostic and predictive biomarker in cancer patients. Int Immunol. 2016;28:373–382. doi:10.1093/intimm/dxw021.
- Pages F, Galon J, Fridman WH. The essential role of the in situ immune reaction in human colorectal cancer. J Leukoc Biol. 2008;84:981–987.
- Vacchelli E, Galluzzi L, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Kroemer G. Trial watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2012;1:179–188. doi:10.4161/onci.1.2.19026.
- Angell HK, Bruni D, Barrett JC, Herbst R, Galon J. The immunoscore: colon cancer and beyond. Clin Cancer Res. 2020;26:332–339. doi:10.1158/1078-0432.ccr-18-1851.
- Galon J, Bruni D. Tumor immunology and tumor evolution: intertwined histories. Immunity. 2020;52:55–81. doi:10.1016/j.immuni.2019.12.018.
- Marliot F, Chen X, Kirilovsky A, Sbarrato T, El Sissy C, Batista L, Van den Eynde M, Haicheur-Adjouri N, Anitei MG, Musina AM, et al. Analytical validation of the Immunoscore and its associated prognostic value in patients with colon cancer. J Immunother Cancer. 2020;8:e000272. doi:10.1136/jitc-2019-000272.
- Pagès F, André T, Taieb J, Vernerey D, Henriques J, Borg C, Marliot F, Ben Jannet R, Louvet C, Mineur L, et al. Prognostic and predictive value of the immunoscore in stage III colon cancer patients treated with oxaliplatin in the prospective IDEA France PRODIGE-GERCOR cohort study. Ann Oncol. 2020;31:921–929. doi:10.1016/j.annonc.2020.03.310.
- Bindea G, Mlecnik B, Fridman WH, Galon J. The prognostic impact of anti-cancer immune response: a novel classification of cancer patients. Semin Immunopathol. 2011;33:335–340. doi:10.1007/s00281-011-0264-x.
