ABSTRACT
This review details the analytical performance characteristics of the consensus Immunoscore, measuring the immune response to cancer, improving the estimation of risk of recurrence, and predicting response to treatment for patients with colon cancer. The analytical validation of Immunoscore has been documented. Immunoscore is a robust, reproducible, quantitative, and standardized immune assay, with a high prognostic performance, independent of all of the prognostic markers currently used in clinical practice. Immunoscore evaluation within the tumor microenvironment is clinically relevant, and Immunoscore was recently introduced into ESMO Clinical Practice Guidelines for colon cancer and into the WHO classification of the Digestive System Tumors. This paves the way for the use of Immunoscore in clinical practice in colorectal tumors and likely soon in many other solid tumors.
Usefulness of Immunoscore
The tumor microenvironment is composed of diverse and heterogeneous immune infiltrates of almost all immune cell types. The different subpopulations of immune cells are associated with variable prognostic significance.Citation1,Citation2 Multiple analyses and meta-analyses have highlighted the role T lymphocytes and cytotoxic T-cells having a major influence on patient survival. Citation1–7,Citation8 The positive impact of lymphocytic infiltration the tumor on survival was first reported in 1921.Citation2 Almost a century later, this parameter is just been introduced into daily routine clinical practice in colon cancer. Indeed, the immune response, as measured by Immunoscore, was introduced for the first time into the latest (5th) edition of the WHO Digestive System Tumors as “essential and desirable diagnostic criteria for colorectal cancer”. Furthermore, the 2020 ESMO Clinical Practice Guidelines for colon cancer included Immunoscore to refine the prognosis and thus adjust the chemotherapy decision-making process. This further supports the introduction of a new TNM-Immune (TNMI) classification system in clinical practice. Difficulties in the past for introducing immune markers into cancer prognostic evaluation was two-fold (i) it was long thought by oncologists, geneticists, and pathologists that the immune infiltration was irrelevant, or was the consequence of a chronic inflammation feeding the tumor, (ii) the infiltrating immune cells were difficult to report due to their complex phenotype and a lack of standardized methodology leading to inter-observer variability between pathologists.
We showed that the densities of T-cells and cytotoxic T-cells predicted clinical outcome at all stages of colorectal cancer. Furthermore, multivariable analyses revealed that tumor progression (T-stage) and invasion (N-stage) were statistically dependent upon these immune cells. The quantification of these cells within specific tumor regions (invasive margin and tumor core) has been standardized in an assay called Immunoscore. Recently, an international consortium implemented and validated a Consensus Immunoscore to predict the clinical outcome in stage I–III patients colon cancer.Citation9 Univariable and multivariable analyses showed the major prognostic value of Immunoscore to predict recurrence and overall survival. Not only a biomarker has to be powerful, but also importantly it has to be accurate and reproducible. Multiple studies and analysis were performed to validate the analytical performance of Immunoscore.
Robustness of Immunoscore
The biological variability of the tumor was evaluated. Several FFPE tumor tissues blocks of colon cancer are often eligible for Immunoscore testing and a wide range of slide-cutting levels can be achieved within the same tumor block. This raises the question of whether the Immunoscore is impacted or not by tumor heterogeneity.
A selected FFPE block from the tumor of each patient (n = 166) has been quantified and compared to a random FFPE block from the same tumor (). CD3+ and CD8+ immune densities were quantified. The Immunoscore percentile values remained remarkably constant between the blocks. The correlation coefficient were R = 0.94 and R = 0.97 for CD8 and CD3, respectively. The concordance between results obtained with the selected blocks and the random blocks were 93% (95% CI 88–96%).Citation10
Figure 1. Immunoscore analytical validation. (a) Inter-assay repeatability, block repeatability, image density reproducibility, inter-laboratories reproducibility and Immunoscore concordance. (b) Representative cases (n = 36) of the whole international SITC cohort were taken from centers in Belgium, Canada, China, France, and USA, and were re-analyzed by 8 independent pathologists from different centers, using the Immunoscore digital pathology software (left). The reproducibility of the results of the Immunoscore were compared with that of a visual assessment of the density of tumor-infiltrating immune cells in tumor tissue stained with hematoxylin and eosin (HE). HE-images from representative cases (n = 268) from the international SITC cohort were visually assessed by 11 observers the density of tumor-infiltrating immune cells (right). Concordance index is visualized for 25 representative cases (red dots)
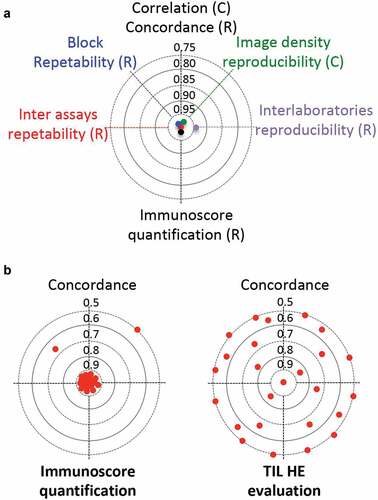
Furthermore, tumor block (n = 10) were cut into 100 adjacent slides, and a total of 13 slides per block were quantified at the cutting-levels 1, 3, 5, 7, 9, 20, 30, 40, 50, 60, 70, 80, 99.Citation10 Regardless of the cutting-levels, densities of immune cells and Immunoscore categories remained remarkably similar. Indeed, the repeatability of Immunoscore was evaluated on all cases and revealed excellent accuracy (95.7%), sensitivity (94.8%), specificity (100%), and an overall ROC Area of 0.99.
The technical variability of the method was evaluated with lot-to-lot reproducibility and Immunoscore assay precision measurements. Consecutive slides from three colon cancers were assessed for CD3+ and CD8 + T cells densities using three different antibody lots, three DAB revelation kit lots, two different Benchmark autostainers, three different runs, and three different operators. A concordance of 100% was observed between Immunoscore categories.Citation10
The analytical variability of the quantification by digital pathology was evaluated. Representative cases (n = 36) of the whole international SITC cohort were taken from centers in Belgium, Canada, China, France, and USA, and were re-analyzed by eight independent pathologists from different centers. Selected images of tissue stained for CD3 and CD8, with Immunoscores ranging from 2.5th to 90th percentiles were re-quantified. Determination of mean cell densities in each tumor region revealed a strong inter-observer reproducibility (r = 0.97 for tumor; r = 0.97 for invasive margin; p < .0001). Only 2.1% variation in CD3+ and CD8 + T-cell densities were detected between the 8 observers.Citation9 This shows the reproducibility of Immunoscore within the ranges of immune cell densities observed in colon tumors.
A full assessment of Immunoscore reproducibility was performed in two laboratories. Each laboratory had its own Immunoscore workflow including staining, scanning, and analysis. Nonconsecutive cutting slides from the same tumor block were used to assess Immunoscore of 100 representative cases. The inter-laboratory correlation for CD3+ and CD8+ cells were 0.94 (p < .001) and the overall categorical Immunoscore concordance between the two centers was 93%. This also included biological variability of the tumor.Citation10
The reproducibility of the results of the Immunoscore were compared with that of a visual assessment of the density of tumor-infiltrating immune cells in tumor tissue stained with hematoxylin and eosin (HE). HE-images from representative cases (n = 268) from the international SITC cohort were assessed by 11 observers. Only 4% of cases were concordant between all observers, 8% of cases were concordant between 90% of observers, 8% of cases were concordant between 80% of observers, 16% of cases were concordant between 70% of observers and discordant for 30% of observers, 19% of cases were concordant between 60% of observers and discordant for 40% of observers, and a total absence of concordance (50% discordance) was evident in 45% of the cases.Citation9 By contrast, 88% perfect concordance between observers was evident for Immunoscore. Thus, Immunoscore performance surpassed greatly those from visual assessment. Furthermore, tumor-infiltrating immune cells evaluated on HE images and Immunoscore did not measure the same parameters. Indeed, 48% discordance was found between the Immunoscore and the density of tumor-infiltrating immune cells, as determined by visual assessment. Overall, the consensus Immunoscore quantification was standardized, reproducible, robust, and quantitative.
Conclusion
Immunoscore can provide new information on host-defense against the tumor, which is an essential element in the success of immunotherapy and of any cancer therapy mobilizing the immune response.Citation11–14 Indeed, the international Immunoscore consortium recently demonstrated that Immunoscore predicted survival and response to chemotherapy in 763 Stage III colon cancer (CC) patients. Citation15 The prognostic value of Immunoscore was confirmed in two independent phase 3 clinical trials (NCCTG-N0147, n = 559; Prodige-IDEA, n = 1062).Citation16,Citation17 Moreover, results from IDEA phase 3 randomized trial revealed the predictive value of Immunoscore for response to adjuvant FOLFOX chemotherapy duration.Citation16 The consensus Immunoscore assay has been developed as an in vitro diagnostic test (CE-IVD) to help guide treatment strategies, and is available in FDA CLIA-certified laboratories for routine use. Given the very high level of evidence of the clinical utility of Immunoscore and given its robustness, Immunoscore is an essential and desirable assay for patient care management.
Disclosure of potential conflicts of interest
JG and FP have patents associated with the immune prognostic biomarkers. JG is co-founder of HalioDx biotech company. Immunoscore® a registered trademark from the National Institute of Health and Medical Research (INSERM) licensed to HalioDx.
Acknowledgments
The work was supported by INSERM, AP-HP, University Paris Descartes, the Cancéropole Ile-de-France, the Cancer Research for Personalized Medicine (CARPEM), Paris Alliance of Cancer Research Institutes (PACRI), the LabEx Immunooncology, the National Cancer Institute of France (INCa; ref 2012-218), HalioDx for Immunoscore®, La Ligue Contre le Cancer, Association pour la Recherche contre le Cancer (ARC), The authors thank the patients, their caregivers, and the SITC Immunoscore consortium investigators.
References
- Bruni D, Angell HK, Galon J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. 2020. PMID: 32753728. doi:10.1038/s41568-020-0285-7.
- Galon J, Bruni D. Tumor immunology and tumor evolution: intertwined histories. Immunity. 2020;52:55–4. doi: 10.1016/j.immuni.2019.12.018.
- Ascierto PA, Capone M, Urba WJ, Bifulco CB, Botti G, Lugli A, Marincola FM, Ciliberto G, Galon J, Fox BA. The additional facet of immunoscore: immunoprofiling as a possible predictive tool for cancer treatment. J Transl Med. 2013;11:54. doi: 10.1186/1479-5876-11-54.
- Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y.
- Galon J, Fox BA, Bifulco CB, Masucci G, Rau T, Botti G, Marincola FM, Ciliberto G, Pages F, Ascierto PA, et al. Immunoscore and Immunoprofiling in cancer: an update from the melanoma and immunotherapy bridge 2015. J Transl Med. 2016;14:273. doi: 10.1186/s12967-016-1029-z.
- Kirilovsky A, Marliot F, El Sissy C, Haicheur N, Galon J, Pages F. Rational bases for the use of the Immunoscore in routine clinical settings as a prognostic and predictive biomarker in cancer patients. Int Immunol. 2016;28:373–382. doi: 10.1093/intimm/dxw021.
- Pages F, Galon J, Fridman WH. The essential role of the in situ immune reaction in human colorectal cancer. J Leukoc Biol. 2008;84:981–987.doi: 10.1189/jlb.1107773.
- Fridman WH, Dieu-Nosjean MC, Pagès F, Cremer I, Damotte D, Sautès-Fridman C, Galon J. The immune microenvironment of human tumors: general significance and clinical impact. Cancer Microenviron. 2013;6:117–122. doi: 10.1007/s12307-012-0124-9.
- Pages F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–2139.
- Marliot F, Chen X, Kirilovsky A, Sbarrato T, El Sissy C, Batista L, Van den Eynde M, Haicheur-Adjouri N, Anitei MG, Musina AM, et al. Analytical validation of the Immunoscore and its associated prognostic value in patients with colon cancer. J Immunother Cancer. 2020;8:e000272. doi: 10.1136/jitc-2019-000272.
- Aranda F, Vacchelli E, Eggermont A, Galon J, Sautes-Fridman C, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: peptide vaccines in cancer therapy. Oncoimmunology. 2013;2:e26621. doi: 10.4161/onci.26621.
- Galluzzi L, Vacchelli E, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Zucman-Rossi J, Zitvogel L, Kroemer G. Trial watch: monoclonal antibodies in cancer therapy. Oncoimmunology. 2012;1:28–37. doi: 10.4161/onci.1.1.17938.
- Vacchelli E, Eggermont A, Galon J, Sautes-Fridman C, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: monoclonal antibodies in cancer therapy. Oncoimmunology. 2013;2:e22789. doi: 10.4161/onci.22789.
- Vacchelli E, Galluzzi L, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Kroemer G. Trial watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2012;1:179–188. doi: 10.4161/onci.1.2.19026.
- Mlecnik B, Bifulco C, Bindea G, Marliot F, Lugli A, Lee JJ, Zlobec I, Rau TT, Berger MD, Nagtegaal ID, et al. Multicenter international society for immunotherapy of cancer study of the consensus immunoscore for the prediction of survival and response to chemotherapy in stage III colon cancer. J Clin Oncol. 2020;JCO1903205. doi: 10.1200/JCO.19.03205.
- Pagès F, André T, Taieb J, Vernerey D, Henriques J, Borg C, Marliot F, Ben Jannet R, Louvet C, Mineur L, et al. Prognostic and predictive value of the Immunoscore in stage III colon cancer patients treated with oxaliplatin in the prospective IDEA France PRODIGE-GERCOR cohort study. Ann Oncol PMID: 32294529. 2020;31:921–929. doi: 10.1016/j.annonc.2020.03.310.
- Sinicrope FA, Shi Q, Hermitte F, Zemla TJ, Mlecnik B, Benson AB, Gill S, Goldberg RM, Kahlenberg MS, Nair SG, et al. Contribution of immunoscore and molecular features to survival prediction in stage III colon cancer. JNCI Cancer Spectr. 2020;4:pkaa023. doi: 10.1093/jncics/pkaa023.
