ABSTRACT
The purpose of this report was to systematically review the radiation enhancement factor (REF) effects of immunotherapy on radiotherapy (RT) to the local tumor in comparison with other traditional radiation sensitizers such as cisplatin. PubMed and Medline databases were searched until February 2019. Reports with abscopal effect in the results were excluded. Graphs of the selected papers were digitized using Plot Digitizer (Sourceforge.net) in order to calculate the tumor growth delay (TGD) caused by immunotherapy. To enable comparison between different studies,the TGD were used to define the REF between RT versus the RT/immunotherapy combination. Thirty-two preclinical papers, and nine clinical series were selected. Different mouse models were exposed to RT doses ranging from 1 to 10 fractions of 1.8 to 20 Gray (Gy) per fraction. Endpoints were heterogeneous, ranging from regression to complete local response. No randomized clinical studies were identified. The median preclinical REF effect of different immunotherapy was varying from 1.7 to 9.1. There was no relationship observed either with subclasses of immunotherapy orRT doses. In the clinical studies, RT doses ranged from 1 to 37 fractions of 1.8 to 24 Gy per fraction. Most clinical trials used ipilimumab and interleukin-2. Local control rate in the clinical series ranged from 66% to 100%. A strong REF of immunotherapy (1.7 to 9.1) was observed, this being higher than traditionally sensitizers such as cisplatin (1.1). This result implies that for the same RT dose, a higher local control was achieved with a combination of immunotherapy and RT in preclinical settings. This study therefore supports the use of combined RT and immunotherapy to improve local tumor control in clinical settings without exacerbation of toxicities.
Introduction
Radiotherapy (RT) is one of the three anticancer treatments, besides surgery and systemic therapies like chemotherapy, hormonal therapy, or immunotherapy. Several randomized trials and meta-analyses have shown that the addition of either cisplatin or 5-fluorouracil-based chemotherapy to RT significantly improves local control and survival over RT alone in several cancer subtypes such as esophagus, head and neck, lung, rectum, anal, cervix, and bladder cancer.Citation1–7 Although RT primarily damages the DNA of local cancer cells, it also changes the tumor microenvironment by generating local inflammatory reactions and enhancing tumor cell recognition by the host’s immune system. These local processes can even be enhanced when triggering the immune system by immunotherapy.Citation8,Citation9 RT-induced cancer cell damage exposes tumor-specific antigens to the immune system through a process called immunogenic cell death (ICD).Citation10 This process leads to improved priming and activation of cytotoxic T cells.Citation11 Furthermore, RT leads to the release of T-cell-attracting chemokines and the upregulation of surface receptors that makes tumor cells more vulnerable to T-cell-mediated cell killing. Such a combination may lead to increased effectiveness of local RT. Additionally, the RT + immunotherapy combination may even lead to an improved systemic effect, also known as the ‘abscopal’ effect (ab scopus: on a distant site) where the immune system starts to combat tumor deposits outside the radiation field more efficiently.Citation12 However, the abscopal effect is not within the scope of this review. The primary aim of this article is to systematically review the literature on the local effect of immunotherapy on RT in preclinical and clinical data. To this end, an estimation of the radiation enhancement factor (REF) for (the different forms of) immunotherapy was derived from the literature.
Materials and methods
A systematic review of the relevant literature search in the PubMed/Medline database was performed in February 2019 by BV. Search terms included ‘radiotherapy’ AND ‘immunotherapy’ AND ‘local effect(s)’. Furthermore, an additional search was performed using the terms ‘radiotherapy’ AND ‘immunotherapy’ AND ‘local’ NOT ‘review’ NOT ‘abscopal’ NOT ‘metastatic’. Results were limited to manuscripts in the English language. Preclinical and clinical data were included. A manual review of filtered records was conducted for relevance by screening on their titles and abstracts alone. Articles were excluded if solely describing the (systematic) abscopal effect, or if other concurrent cytotoxic treatments (chemotherapy, hyperthermia) were also administered. Clinical case reports on single patients were excluded. Finally, the selected clinical and preclinical papers from prior knowledge of the authors were also screened for additional papers that met the selection criteria.
To assess the quality differences of the preclinical studies, we divided these into three levels of response according to their assumed clinical relevance and reliability of the study endpoints (). Level 1 represented the highest level of response with a complete remission of the local tumor over a long follow-up period of at least 6 months to exclude regrowth.Citation13 The 6 months threshold was chosen because in several experiments this level is taken as a cutoff, f.e. in a clinical trial, results would be reported as a percentage of complete responses. This level is denoted as cure and was scored as a percentage of test animals with a complete remission after a long time. Level 2 response represented a complete remission over a shorter follow-up period of less than 6 months. This level is defined as complete disappearance of the tumor after treatment, followed by regrowth within 6 months. Level 3 response represented growth delay as the reported endpoint, without achieving cure.
Table 1. The three levels of response according to their assumed clinical relevance and reliability of the study endpoints (Table 1)
To obtain a quantitative number of the local RT sensitizing effect of immunotherapy for the Level 3 studies, all graphs in the selected papers were digitized using Plot Digitizer (v2.6.8, Oct 2015, downloaded from https://sourceforge.net). Tumor growth delayed (TGD) was obtained for every specific immunotherapy agent and was calculated as:
TGD = [Ttv x 4] – [Tcv x4]
where Ttv x 4 and Tcv x 4 is the time to reach four fold tumor volume increase compared to treatment start, based on an exponential growth fit in treated tumors (tv) and in untreated control tumors (cv), respectively.
When Ttv x 4 was not reached due to stable disease, i.e. tumor was not growing or tumor was cured (progression-free): the volume of the last day of follow-up was used.
These calculated TGD were used to obtain the radiation enhancement factor (REF) by this formula:
REF = TGDRT + IO/TGDRT
When no graphics of tumor volume were available for calculating REF, the specific ratios are used: when survival curves were available, the REF was calculated as:
REF = Median SurvivalRT + IO/Median SurvivalRT
Again, if the median survival was not reached, the last day of follow-up was used.
When percentages of responses were available, the REF was calculated as:
REF = % DFSRT + IO/% DFSRT
where DFS is the disease-free survival.
Beside the three levels of responses in preclinical studies, the clinical results are reported as a percentage of partial responses.
All forms of immunotherapy were divided into different subclasses according to their working mechanism: immune checkpoint inhibitors: anti-PD-(L)1; anti-CTLA4; cytokines: r-IL2; vaccines/dendritic cells; CPG/Toll-like receptor; and others.
A non-parametric Kruskal–Wallis test is performed with a Dunn’s multiple comparisons test to obtain a significant differentiation of the subclasses of immunotherapy and in comparison of immunotherapy with cisplatin. A p-value <0.05 was considered statistically significant.
Results
We identified 1172 PubMed/Medline references (). Thirty-seven preclinical papers were retrieved that directly reported local effects, which are summarized in and . All experiments were performed in mice except one report described experiments performed in rats, Citation17 All selected studies used RT in combination with immunotherapy to sensitize the local radiotherapy effect. Some reports also described the systemic effect of RT.
Table 2. Overview of level 2 preclinical studies according to the search criteria
Table 3. Overview of level 3 preclinical studies according to the search criteria
Figure 1. Flowchart of studies, which were identified by the literature search, screened excluded or included from analysis
Seven different immune-competent mouse strains had been used: the C57BL/6 and Balb/c were most frequently presented. These mice had been mostly used because the tumor models were syngeneic with these genetic strains (See ). Only one report used nude mice to investigate the role of T cells in the association of RT and immunotherapy. Radiation doses varied from conventional schedules of 1.8 to 2 Gy per fraction to extreme hypofractionation, ranging from 1 to 10 fractions of 1.8 to 20 Gray (Gy) per fraction (). Responses varied from local regression to complete cure. Data were available from many different immunotherapy classes in regards to their working mechanisms, see .
Results from level 1 studies
No studies reported on level 1 outcome with a follow-up of longer than 6 months. Several studies observed a long follow-up, however, none longer than 180 days have been described.
Results from level 2 studies
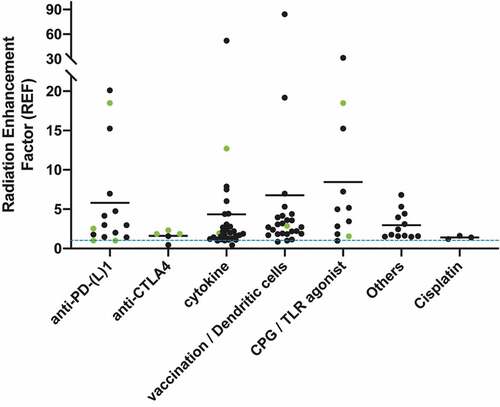
provides an overview of the 11 studies reporting Level 2 response. The preclinical reports describing complete responses in 100% of cases were using Staphylococcal enterotoxins (SEC2)-activated T lymphocytes, IL-2, CpG (intratumoral), anti-PD-1, and adenoviral vector + IL-12. The calculated REF’s are represented in . Thirty-four graphics are analyzed with median REF of 9.1, 1.7, 2.8, 7.3, and 3.1 for anti-PD-(L)1; cytokines: r-IL2; vaccines/dendritic cells; CPG/Toll-like receptor; and other immunotherapies, respectively. REF varied between 0.4 and 52.1.
Figure 2. The radiotherapy sensitizing effect of Immunotherapy is compared between different studies: therefore tumor growth delay(TGD) was calculated and used to define the Radiation Enhancement factor (REF) between radiotherapy combined with immunotherapy and radiotherapy alone. The X axis displays the various classes of immunotherapies used in the studies: (1) anti-PD-(l)-1, (2) CTLA4, (3) cytokines: r-IL-2, (4) Vaccination / Dendritic cells, (5) CPG / Toll-like receptor, and (6) others. The Y-axis represents the value of the REF, from 0 to 90. The blue-dashed line is a REF value of 1, meaning RT + immunotherapy has the same effect as RT solely. Every dot represents a single calculated REF of one preclinical study. Several dots are calculated per study. A horizontal line represents the mean REF per immunotherapy. A green dot represents the calculated REF’s based on survival curves or on response rates: these are based on the volume of the last day of follow-up because the tumor was progression free. Therefore these dots are a minimal representation of the REF because in reality it concerns a higher REF
Results from level 3 studies
shows an overview of the 21 studies reporting level 3 response. REF varied between 0.4 and 84.3. These calculated REF’s are represented in . Sixty-five graphics are analyzed with median REF of 2.5, 1.9, 1.9, 2.7, 2.3, and 1.8 for anti-PD-(L)1; anti-CTLA4; cytokines: r-IL2; vaccines/dendritic cells; CPG/Toll-like receptor; and other immunotherapies, respectively.
All forms of immunotherapy were divided into different classes to obtain more differentiation of the subclasses. However, neither a relationship was observed between the type of immunotherapy, nor in the dose, nor the timing of RT. A significant difference was observed of the immunotherapy subclasses of vaccines/dendritic cells, and others versus cisplatin; p = .0484 and 0.0324, respectively.
Results from clinical studies
No randomized clinical studies were identified. provides an overview of the clinical studies. Eight series of patients have been reported, from which melanoma and renal cell carcinoma were the most frequent tumor histology types. RT doses were widely dispersed, ranging from 1 to 37 fractions of 1.8 to 24 Gy per fraction. The two most commonly used immunotherapy agents were ipilimumab and IL-2, administered in 3 and 2 clinical reports, respectively. In four trials the immunotherapy has been prescribed during RT, whereas in two trials it was prescribed before, during, and after RT. In two other trials, the immunotherapy started several days after commencing RT. Local tumor control rates varied from 66% to 100%.
Table 4. Overview of clinical studies according to the search criteria
Discussion
Radiation sensitizers such as chemotherapy, monoclonal antibodies, and targeted agents, increase the local tumor effects of RT, without the need for higher RT doses and these have been clinically used in different cancer subtypes. These sensitizers increase the local and systemic control approximately with 10% to 20% .Citation54–57 However, with these regimens, radiation toxicity (such as oral mucositis) has been exacerbated. Many other common side effects such as myelosuppression, nausea, and vomiting have been observed.Citation11,Citation54 In this review, immunotherapy was critically analyzed as a sensitizer for RT: different multiply sensitizing factors ranging from 0.4 to 84 have been derived from the reviewed literature for different subtypes of immunotherapy. This increase is enormous compared to the 0.1 increase found for the classical radiosensitizing drug cisplatin. When comparing the combination of RT-immunotherapy with RT in preclinical studies, mostly short-term responses were observed. The complete responses in all cases for more than 6 months were not documented in any preclinical setting. However, the mean life span of a mouse is 1.5 years, which means that this cutoff time will be barely observable in the pre-clinical setting. Most reports showed tumor growth delay, which meant that the optimal combination of specified immunotherapy is not yet known. Moreover, a wide range of different immunotherapy agents with different working mechanisms have been described. However, in the preclinical setting, the experimental set-up was generally not intended to quantify complete responses over a long time period: the sensitization effect therefore still needs to be demonstrated. Therefore, conscious decisions have been made to choose low RT doses in combination with different immunotherapy.
Local radiosensitization in patients
The local immunological potential of certain tumors also comes to the forefront in different case reports that described the combination of RT + immunotherapy treatment: a number of manuscripts have already confirmed the presence of abscopal effects of RT + immunotherapy.Citation12 summarizes the local effect in patients and circumscribe the preclinical analyses, findings, and conclusions: immunotherapy is an extremely good local radiosensitizer in comparison with cisplatin or 5-Fluororacil. Ipilimumab is the most clinically cited immunotherapy in malignant melanoma. Ipilimumab causes CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) blockade leading to a decreased exhausted phenotype on CD8 T cells and decreased regulatory T-cell (Treg) activity.Citation58 This synergizes well with RT since Tregs lead to a suppressed immune response and tend to be more radio-resistant than other T cells .Citation59 These Treg inhibitions increase the CD8/Treg-ratio resulting in modest peripheral expansion of TCR (T-cell receptor)-clonotypes in the tumor. RT has the effect of diversifying the TCR repertoire of tumor-infiltrating lymphocytes and further shapes the repertoire of expanded clones, resulting in better local outcomes. Several reports of combinations of multiple immunotherapy have been are published reporting better overall survival than solely using immunotherapy: 5 years overall survival was 52% in the nivolumab-plus-ipilimumab group, in comparison with 44% in the nivolumab group, and 26% in the ipilimumab group.Citation60
Timing and dose of radiotherapy
RT induces inflammation and necrosis, attracting in-field dendritic cells (DC) and other types of Antigen Presenting Cells (APC) into the tumor micro-environment.Citation61 Immune cells appear to be highly radiosensitive: in the body, naïve lymphocytes are one of the most radiosensitive among all cells: doses of 0.5 Gy have proven to be already cytotoxic.Citation62 DC and APC may survive higher RT doses, however, more rapid function loss has been observed.Citation63 Therefore, the choice of fractionation schedule and (consequently) the time point between different fractionations could impact on the availability of local immune effectors. The results are mainly dependent on the type of immunotherapy and the RT dose. In some reports, fractionation has been useful, while in others a single high-dose of RT appears to be best. High-dose RT seems to be good at producing immunogenic modulation of tumors resulting in intense CD8+ T-cell tumor infiltration, and a loss of myeloid-derived suppressor cells (MDSC).Citation64 Due to the shorter period of treatment, this may avoid continued eradication of responding lymphocytes.Citation65 Furthermore, high-dose RT results in more vascular and stromal damage and increased apoptosis of tumor cells, thus creating a tumor microenvironment with increased levels of tumor-associated antigens.Citation66 When combining immunotherapy with RT, concurrent administration reveals a better superior sensitizing effect.
Limitations
This review has shown that different forms of immunotherapy have large potential to improve local tumor control within the radiation field. For the first time, systematic review has been performed to compare the effectiveness of different forms of immune treatment, and doing so in a quantitative way, using Radiation Enhancement Factors. An original approach was introduced enabling comparison of the results from different studies. This was done by extracting and digitizing the growth data of tumors from different experimental setups, determining the tumor growth delay for radiotherapy as well as for the combined immune treatment. These data could then be used to determine the radiation enhancement factor as the ratio of the growth delay for combined treatment to that for radiation-only treatment. Since this methodology can be used to compare the potential of any kind or class of radiosensitizers, the methodology can be applied to address many alternative questions in this field. And, as growth delay experiments are the most widely used preclinical in vivo experiments assessing the efficacy of a radiosensitizer, our approach can move the field forward significantly in other areas, based on already available data.
However, this review has also some limitations.
Firstly, most reports were preclinical, including only small numbers of cases. The modeling of animals has biological and physical limitations, so this should be considered when interpreting preclinical RT trials. Murine tumor and normal tissue radiation response has been shown to vary from humans in regards to cellular and molecular pathways.Citation67 Secondly, as no randomized phase III trials were available, no good control groups have been reported to compare the combination therapy in the clinical reports. Thirdly, with the search strategy employed, abscopal reports were specifically excluded. Hence, it is possible that certain reports with a focus on abscopal effects but also reporting on local control have not been included in this review. Moreover, the search and screening method could be optimized.
Further, the evaluation of clinical local responses has not been consistent in every report: the disease progression is often reported without mentioning specific details of the local control. However, local control evaluation after extreme high-RT dose in combination with immunotherapy is obsolete: the tumor has already been destroyed by the RT itself. Response criteria are sometimes according to the traditional Response Evaluation Criteria In Solid Tumors (RECIST) criteria.Citation68 However, the evaluation criteria of the response of immunotherapy can differ from those with traditional therapies: a progression of known lesions or even the appearance of new lesions, before stabilization of the disease or even regression can be observed.Citation69 Therefore, consensus-based criteria for response to immunotherapy (iRECIST) have been developed recently for use in trials testing immunotherapy.Citation70 Moreover, a possible time delay could exist between the systemic treatment and the evaluation of the response to RT, and the presence or absence of control, in order to distinguish this effect of systemic treatment or RT.
Next, the levels of responses that we used to stratify the quality differences among the several preclinical studies consisted of only three levels. However, level 1 response was more a theoretical level, since no mice-related work had follow-ups of greater than 6 months which were as per our definition the highest demand for clinical work, which is described as a knowledge gap. Additionally, the review is based on a relatively small amount of papers with a broad amount of variables: seven different immune-competent mouse strains with a disease heterogeneity (cancer type and subtype) using radiation doses varying from conventional schedules to extreme hypo-fractionation, with the application of different immunotherapies at various time points during, before and after the RT. Response of immune-radiotherapy combinations further depends on total dose, and probably also other parameters like the treated tumor volume and the patients’ condition or in preclinical studies the specified immunocompetence of the animal used. This study did take such parameters into account while comparing the different results over the described experiments.
Finally, the number of clinical studies is limited and varies in methodologies. This can definitely be extended toward parameters like total dose, dose fractionation, and timing as discussed.
Perspectives
More clinical and mechanistic knowledge is needed about the precise immune reaction created by RT. This additional information will give us supplementary knowledge to individualize the best sensitizing effect of immunotherapy on RT. This can ultimately lead to decreasing RT doses, with consequently decreasing toxicity levels, while preserving excellent local control, thus leading the way forward toward new organ preservation strategies. However, immunotherapy can also lead to increased toxicities like dermatologic (rashes), colitis (diarrhea), hepatotoxicity, pneumonitis, and endocrinopathies (such as thyroid, hypophysitis). More research is therefore needed to examine these combination treatment strategies.
Conclusion
We concluded that different forms of immunotherapy can act as a local sensitizer for RT with good local control rates. Local effects were observed in a variety of tumor types, with different RT doses and fractionation schedules. Further research is needed to confirm the optimal RT-immunotherapy combination.
Role of funding source
This work was possible of a grant of Varian.
Author Contributions
BV and EvL did the systematic review and selection of all the publications. LD did the analysis using Plot Digitizer to obtain a tumor growth delayed for every specific Immunotherapy. BV, EvL, DDR wrote the first draft of the manuscript. All authors edited and contributed to the development of the final manuscript.
Disclosure statement
All authors declare to have no conflict of interest.
References
- Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson, Jr JA, Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation therapy oncology group. JAMA. 1999;281(17):1623. doi:10.1001/jama.281.17.1623.
- Curran WJ Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, Movsas B, Wasserman T, Rosenthal SA, Gore E, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103(19):1452. doi:10.1093/jnci/djr325.
- Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. EORTC radiotherapy group trial 22921. chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114. doi:10.1056/NEJMoa060829.
- Pignon JP, le Maître A, Maillard E, Bourhis J. MACH-NC collaborative group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4. doi:10.1016/j.radonc.2009.04.014.
- Bartelink H, Roelofsen F, Eschwege F, Rougier P, Bosset JF, Gonzalez DG, Peiffert D, van Glabbeke M, Pierart M. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European organization for research and treatment of cancer radiotherapy and gastrointestinal cooperative groups. J Clin Oncol. 1997;15:2040.
- Dueñas-González A, Zarbá JJ, Patel F, Alcedo JC, Beslija S, Casanova L, Pattaranutaporn P, Hameed S, Blair JM, Barraclough H, et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol. 2011;29(13):1678. doi:10.1200/JCO.2009.25.9663.
- Mak RH, Hunt D, Shipley WU, Efstathiou JA, Tester WJ, Hagan MP, Kaufman DS, Heney NM, Zietman A. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of radiation therapy oncology group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol. 2014;32(34):3801. doi:10.1200/JCO.2014.57.5548.
- Van Limbergen EJ, De Ruysscher DK, Olivo Pimentel V, Marcus D, Berbee M, Hoeben A, Rekers N, Theys J, Yaromina A, Dubois LJ, et al. Combining radiotherapy with Immunotherapy: the past, the present and the future. Br J Radiol. 2017 Aug;90(1076):20170157. doi:10.1259/bjr.20170157.
- Demaria S, Coleman CN, Formenti SC. Radiotherapy: changing the game in immunotherapy. Trends Cancer. 2016;2(6):286–11. doi:10.1016/j.trecan.2016.05.002.
- Demaria S, Golden EB. Formenti SC role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1(9):1325–1332. doi:10.1001/jamaoncol.2015.2756.
- De Ruysscher D, Niedermann G, Burnet NG, Siva S, Lee AWM, Hegi-Johnson F. Radiotherapy toxicity. Nat Rev Dis Primers. 2019;5:14.
- Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41:503–510. doi:10.1016/j.ctrv.2015.03.011.
- Baumann M, Petersen C, Schulz P, Baisch H. Impact of overall treatment time on local control of slow growing human GL squamous cell carcinoma in nude mice treated by fractionated irradiation. Radiother Oncol. 1999 Jan;50(1):107–111. doi:10.1016/S0167-8140(98)00112-1.
- Plautz GE, Inoue M, Shu S. Defining the synergistic effects of irradiation and T-cell immunotherapy for murine intracranial tumors. Cell Immunol. 1996 Aug 1;171(2):277–284. doi:10.1006/cimm.1996.0204.
- Everse LA, Renes IB, Jürgenliemk-Schulz IM, Rutgers DH, Bernsen MR, Dullens HF, Den Otter W, Battermann JJ. Local low-dose interleukin-2 induces systemic immunity when combined with radiotherapy of cancer. A pre-clinical study. Int J Cancer. 1997 Sep 17;72(6):1003–1007. doi:10.1002/(SICI)1097-0215(19970917)72:6<1003::AID-IJC14>3.0.CO;2-5.
- Jürgenliemk-Schulz IM, Renes IB, Rutgers DH, Everse LA, Bernsen MR, Den Otter W, Battermann JJ. Anti-tumor effects of local irradiation in combination with peritumoral administration of low doses of recombinant interleukin-2 (rIL-2). Radiat Oncol Investig. 1997;5(2):54–61. doi:10.1002/(SICI)1520-6823(1997)5:2<54::AID-ROI3>3.0.CO;2-I.
- Meng Y, Carpentier AF, Chen L, Boisserie G, Simon JM, Mazeron JJ, Delattre JY. Successful combination of local CpG-ODN and radiotherapy in malignant glioma. Int J Cancer. 2005 Oct 10;116(6):992–997. doi:10.1002/ijc.21131.
- Mason KA, Neal R, Hunter N, Ariga H, Ang K, Milas L. CpG oligodeoxynucleotides are potent enhancers of radio- and chemoresponses of murine tumors. Radiother Oncol. 2006 Aug;80(2):192–198. doi:10.1016/j.radonc.2006.07.024.
- Zegers CM, Rekers NH, Quaden DH, Lieuwes NG, Yaromina A, Germeraad WT, Wieten L, Biessen EA, Boon L, Neri D, et al. Radiotherapy combined with the immunocytokine L19-IL2 provides long-lasting antitumor effects. Clin Cancer Res. 2015 Mar 1;21(5):1151–1160. doi:10.1158/1078-0432.CCR-14-2676.
- van den Heuvel MM, Verheij M, Boshuizen R, Belderbos J, Dingemans AM, De Ruysscher D, Laurent J, Tighe R, Haanen J, Quaratino S. NHS-IL2 combined with radiotherapy: preclinical rationale and phase Ib trial results in metastatic non-small cell lung cancer following first-line chemotherapy. J Transl Med. 2015 Jan 27;13:32. doi:10.1186/s12967-015-0397-0.
- Schölch S, Rauber C, Tietz A, Rahbari NN, Bork U, Schmidt T, Kahlert C, Haberkorn U, Tomai MA, Lipson KE, et al. Radiotherapy combined with TLR7/8 activation induces strong immune responses against gastrointestinal tumors. Oncotarget. 2015 Mar 10;6(7):4663–4676. doi:10.18632/oncotarget.3081.
- Connolly KA, Belt BA, Figueroa NM, Murthy A, Patel A, Kim M, Lord EM, Linehan DC, Gerber SA. Increasing the efficacy of radiotherapy by modulating the CCR2/CCR5 chemokine axes. Oncotarget. 2016 Dec 27;7(52):86522–86535. doi:10.18632/oncotarget.13287.
- Wu CJ, Tsai YT, Lee IJ, Wu PY, Lu LS, Tsao WS, Huang YJ, Chang CC, Ka SM, Tao MH. Combination of radiation and interleukin 12 eradicates large orthotopic hepatocellular carcinoma through immunomodulation of tumor microenvironment. Oncoimmunology. 2018 Jul 23;7(9):e1477459. doi:10.1080/2162402X.2018.1477459.
- Zhuang Y, Li S, Wang H, Pi J, Xing Y, Li G. PD-1 blockade enhances radio-immunotherapy efficacy in murine tumor models. J Cancer Res Clin Oncol. 2018 Oct;144(10):1909–1920. doi:10.1007/s00432-018-2723-4.
- Buchegger F, Rojas A, Delaloye AB, Vogel CA, Mirimanoff RO, Coucke P, Sun LQ, Raimondi S, Denekamp J, Pèlgrin A, et al. Combined radioimmunotherapy and radiotherapy of human colon carcinoma grafted in nude mice. Cancer Res. 1995 Jan 1;55(1):83–89.
- Chiang CS, Hong JH, Wu YC, McBride WH, Dougherty GJ Combining radiation therapy with interleukin-3 gene immunotherapy. Cancer Gene Ther. 2000 Aug;7(8):1172–1178. doi:10.1038/sj.cgt.7700217.
- Lohr F, Hu K, Haroon Z, Samulski TV, Huang Q, Beaty J, Dewhirst MW, Li CY. Combination treatment of murine tumors by adenovirus-mediated local B7/IL12 immunotherapy and radiotherapy. Mol Ther. 2000 Sep;2(3):195–203. doi:10.1006/mthe.2000.0114.
- Teitz-Tennenbaum S, Li Q, Rynkiewicz S, Ito F, Davis MA, McGinn CJ, Chang AE. Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Res. 2003 Dec 1;63(23):8466–8475.
- Huang J, Wang Y, Guo J, Lu H, Lin X, Ma L, Teitz-Tennenbaum S, Chang AE, Li Q Radiation-induced apoptosis along with local and systemic cytokine elaboration is associated with DC plus radiotherapy-mediated renal cell tumor regression. Clin Immunol. 2007 Jun;123(3):298–310. doi:10.1016/j.clim.2007.02.005
- Meng Y, Efimova EV, Hamzeh KW, Darga TE, Mauceri HJ, Fu YX, Kron SJ, Weichselbaum RR. Radiation-inducible immunotherapy for cancer: senescent tumor cells as a cancer vaccine. Mol Ther. 2012 May;20(5):1046–1055. doi:10.1038/mt.2012.19.
- Wang YS, Liu SJ, Huang SC, Chang CC, Huang YC, Fong WL, Chi MS, Chi KH. Recombinant heat shock protein 70 in combination with radiotherapy as a source of tumor antigens to improve dendritic cell immunotherapy. Front Oncol. 2012 Oct 29;2:149. doi:10.3389/fonc.2012.00149.
- Wei S, Egenti MU, Teitz-Tennenbaum S, Zou W, Chang AE. Effects of tumor irradiation on host T-regulatory cells and systemic immunity in the context of adoptive T-cell therapy in mice. J Immunother. 2013 Feb;36(2):124–132. doi:10.1097/CJI.0b013e31828298e6.
- Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014 Oct 1;74(19):5458–5468. doi:10.1158/0008-5472.CAN-14-1258.
- Lim JY, Brockstedt DG, Lord EM, Gerber SA. Radiation therapy combined with Listeria monocytogenes-based cancer vaccine synergize to enhance tumor control in the B16 melanoma model. Oncoimmunology. 2014 Jun 3;3:e29028. doi:10.4161/onci.29028.
- Rekers NH, Zegers CM, Yaromina A, Lieuwes NG, Biemans R, Senden-Gijsbers BL, Losen M, Van Limbergen EJ, Germeraad WT, Neri D, et al. Combination of radiotherapy with the immunocytokine L19-IL2: additive effect in NK cell dependent tumour model. Radiother Oncol. 2015 Sep;116(3):438–442. doi:10.1016/j.radonc.2015.06.019.
- Blanchard M, Shim KG, Grams MP, Rajani K, Diaz RM, Furutani KM, Thompson J, Olivier KR, Park SS, Markovic SN, et al. Definitive management of oligometastatic melanoma in a murine model using combined ablative radiation therapy and viral immunotherapy. Int J Radiat Oncol Biol Phys. 2015 Nov 1;93(3):577–587. doi:10.1016/j.ijrobp.2015.07.2274.
- Mondini M, Nizard M, Tran T, Mauge L, Loi M, Clémenson C, Dugue D, Maroun P, Louvet E, Adam J, et al. Synergy of radiotherapy and a cancer vaccine for the treatment of HPV-associated head and neck cancer. Mol Cancer Ther. 2015 Jun;14(6):1336–1345. doi:10.1158/1535-7163.MCT-14-1015.
- Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, Deweese TL, Drake CG. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res. 2015 Apr;3(4):345–355. doi:10.1158/2326-6066.CIR-14-0196.
- Monjazeb AM, Kent MS, Grossenbacher SK, Mall C, Zamora AE, Mirsoian A, Chen M, Kol A, Shiao SL, Reddy A, et al. Blocking Indolamine-2,3-dioxygenase rebound immune suppression boosts antitumor effects of radio-immunotherapy in murine models and spontaneous canine malignancies. Clin Cancer Res. 2016 Sep 1;22(17):4328–4340. doi:10.1158/1078-0432.CCR-15-3026.
- Young KH, Baird JR, Savage T, Cottam B, Friedman D, Bambina S, Messenheimer DJ, Fox B, Newell P, Bahjat KS, et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS One. 2016 Jun 9;11(6):e0157164. doi:10.1371/journal.pone.0157164.
- Zheng W, Skowron KB, Namm JP, Burnette B, Fernandez C, Arina A, Liang H, Spiotto MT, Posner MC, Fu YX. Weichselbaum RR combination of radiotherapy and vaccination overcomes checkpoint blockade resistance. Oncotarget. 2016 Jul 12;7(28):43039–43051. doi:10.18632/oncotarget.9915.
- Oweida A, Lennon S, Calame D, Korpela S, Bhatia S, Sharma J, Graham C, Binder D, Serkova N, Raben D, Karam SD, et al. Ionizing radiation sensitizes tumors to PD-L1 immune checkpoint blockade in orthotopic murine head and neck squamous cell carcinoma. Oncoimmunology. 2017 Aug 3;6(10):e1356153. doi:10.1080/2162402X.2017.1356153.
- Weiss T, Weller M, Guckenberger M, Sentman CL, Roth P. NKG2D-Based CAR T cells and radiotherapy exert synergistic efficacy in Glioblastoma. Cancer Res. 2018 Feb 15;78(4):1031–1043. doi:10.1158/0008-5472.CAN-17-1788.
- Choi CW, Jeong MH, Park YS, Son CH, Lee HR, Koh EK. Combination treatment of stereotactic body radiation therapy and immature dendritic cell vaccination for augmentation of local and systemic effects. Cancer Res Treat. 2019 Apr;51(2):464–473. doi:10.4143/crt.2018.186.
- Wang H, Lin X, Luo Y, Sun S, Tian X, Sun Y, Zhang S, Chen J, Zhang J, Liu X, et al. α-PD-L1 mAb enhances the abscopal effect of hypo-fractionated radiation by attenuating PD-L1 expression and inducing CD8+ T-cell infiltration. Immunotherapy. 2019 Feb;11(2):101–118. doi:10.2217/imt-2018-0049.
- Brinkmann OA, Bruns F, Gosheger G, Micke O, Hertle L. Treatment of bone metastases and local recurrence from renal cell carcinoma with immunochemotherapy and radiation. World J Urol. 2005 Jul;23(3):185–190. doi:10.1007/s00345-004-0479-8.
- Jacobs JJ, Hordijk GJ, Jürgenliemk-Schulz IM, Terhaard CH, Koten JW, Battermann JJ, Den Otter W. Treatment of stage III-IV nasopharyngeal carcinomas by external beam irradiation and local low doses of IL-2. Cancer Immunol Immunother. 2005 Aug;54(8):792–798. doi:10.1007/s00262-004-0641-6.
- Seung SK, Curti BD, Crittenden M, Walker E, Coffey T, Siebert JC, Miller W, Payne R, Glenn L, Bageac A, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2–tumor and immunological responses … . Sci Transl Med. 2012 Jun 6;4(137):137ra74. doi:10.1126/scitranslmed.3003649.
- Barker CA, Postow MA, Khan SA, Beal K, Parhar PK, Yamada Y, Lee NY, Wolchok JD. Concurrent radiotherapy and ipilimumab immunotherapy for patients with melanoma. Cancer Immunol Res. 2013 Aug;1(2):92–98. doi:10.1158/2326-6066.CIR-13-0082.
- Abei M, Okumura T, Fukuda K, Hashimoto T, Araki M, Ishige K, Hyodo I, Kanemoto A, Numajiri H, Mizumoto M, et al. A phase I study on combined therapy with proton-beam radiotherapy and in situ tumor vaccination for locally advanced recurrent hepatocellular carcinoma. Radiat Oncol. 2013 Oct;16(8):239. doi:10.1186/1748-717X-8-239.
- Kiess AP, Wolchok JD, Barker CA, Postow MA, Tabar V, Huse JT, Chan TA, Yamada Y, Beal K. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys. 2015 Jun 1;92(2):368–375. doi:10.1016/j.ijrobp.2015.01.004.
- Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015 Apr 16;520(7547):373–377. doi:10.1038/nature14292.
- Nardin C, Mateus C, Texier M, Lanoy E, Hibat-Allah S, Ammari S, Robert C, Dhermain F. Tolerance and outcomes of stereotactic radiosurgery combined with anti- programmed cell death-1 (pembrolizumab) for melanoma brain metastases. Melanoma Res. 2018 Apr;28(2):111–119. doi:10.1097/CMR.0000000000000413.
- Bourhis J, Sire C, Graff P, Grégoire V, Maingon P, Calais G, Gery B, Martin L, Alfonsi M, Desprez P, et al. Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99-02): an open-label phase 3 randomised trial. Lancet Oncol. 2012;13(2):145. doi:10.1016/S1470-2045(11)70346-1.
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck.N. Engl J Med. 2006 Feb 9;354(6):567–578. doi:10.1056/NEJMoa053422.
- James ND, Hussain SA, Hall E, Jenkins P, Tremlett J, Rawlings C, Crundwell M, Sizer B, Sreenivasan T, Hendron C, et al. BC2001 investigators. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012 Apr 19;366(16):1477–1488. doi:10.1056/NEJMoa1106106.
- Northover J, Glynne-Jones R, Sebag-Montefiore D, James R, Meadows H, Wan S, Jitlal M. Ledermann JChemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I). Br J Cancer. 2010 Mar 30;102(7):1123–1128. doi:10.1038/sj.bjc.6605605.
- Rudd CE. CTLA-4 co-receptor impacts on the function of Treg and CD8+ T-cell subsets. Eur J Immunol. 2009 Mar;39(3):687–690. doi:10.1002/eji.200939261.
- Kachikwu EL, Iwamoto KS, Liao YP, DeMarco JJ, Agazaryan N, Economou JS, McBride WH, Schaue D. Radiation enhances regulatory T cell representation. Int J Radiat Oncol Biol Phys. 2011;81(4):1128–1135. doi:10.1016/j.ijrobp.2010.09.034.
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019 Oct 17;381(16):1535–1546. doi:10.1056/NEJMoa1910836.
- Serre R, Barlesi F, Muracciole X, Barbolosi D. Immunologically effective dose: a practical model for immuno-radiotherapy. Oncotarget. 2018 Aug 7;9(61):31812–31819. doi:10.18632/oncotarget.25746.
- Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31:140–144. doi:10.3109/07357907.2012.762780.
- Merrick A, Errington F, Milward K, O’Donnell D, Harrington K, Bateman A, Pandha H, Vile R, Morrison E, Selby P, et al. Immunosuppressive effects of radiation on human dendritic cells: reduced IL-12 production on activation and impairment of naive T-cell priming. Br J Cancer. 2005;92:1450. doi:10.1038/sj.bjc.6602518.
- Filatenkov A, Baker J, Mueller AM, Kenkel J, Ahn GO, Dutt S, Zhang N, Kohrt H, Jensen K, Dejbakhsh-Jones S, et al. Ablative tumor radiation can change the tumorimmune cell microenvironment to induce durable complete remissions. Clin Cancer Res. 2015;21:3727–3739. doi:10.1158/1078-0432.CCR-14-2824.
- Honeychurch J, Illidge TM. The influence of radiation in the context of developing combination immunotherapies in cancer. Ther Adv Vaccines Immunother. 2017 Dec;5(6):115–122. doi:10.1177/2051013617750561.
- Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat Res. 2012;177(3):311–327. doi:10.1667/RR2773.1.
- Koontz BF, Verhaegen F, De Ruysscher D. Tumour and normal tissue radiobiology in mouse models: how close are mice to mini-humans? Br J Radiol. 2017;90:20160441. doi:10.1259/bjr.20160441.
- Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, et al. 1.1-Update and clarification: from the RECIST committee. Eur J Cancer. 2016 Jul;62:132–137. doi:10.1016/j.ejca.2016.03.081.
- Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immunearelated response criteria. Clin Cancer Res. 2009;15(23):7412. doi:10.1158/1078-0432.CCR-09-1624.
- Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et al. RECIST working group. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143. doi:10.1016/S1470-2045(17)30074-8.
