ABSTRACT
Tumor infiltrating lymphocytes (TIL), which represent host adaptive response to the tumor, were first identified at scanning magnification to select areas with the highest counts on hematoxylin and eosin slides, quantitated per high-power field (HPF), and analyzed for association with recurrence-free survival (RFS) in 848 patients. Highest TIL in a single HPF was analyzed as a continuous and categorical variable, and optimal cutoff analysis was performed to predict RFS. Highest TIL count in a single HPF ranged from 0 to 45, and the optimal cutoff for TIL high vs TIL low was determined to be ≥ 3 vs < 3 with a concordance probability estimate of 0.74. In the entire cohort, 5-year RFS was 90.2% (95% CI = 83.7–94.2) in TIL high compared to 78.9% (95% CI = 74.1–82.9) in TIL low (log rank P < .0001). TIL remained significant in the mismatch repair-proficient (pMMR) cohort where 5-year RFS was 94.6% (95% CI = 88.3–97.5) in TIL high compared to 77.9% (95% CI = 69.2–84.4) in TIL low (P = .008). On multivariable analysis, TIL and AJCC Stage were independently associated with RFS in the pMMR cohort. Qualitatively in the pMMR cohort, RFS in Stage II TIL high patients was similar to that in Stage I patients and RFS in Stage III TIL high was similar to that in Stage II TIL low patients. Assessment of TIL in a single HPF using standard H&E slides provides important prognostic information independent of MMR status and AJCC stage.
Introduction
Within the tumor microenvironment, there is a complex interplay between neoplasia, stroma, vascular and lymphatic endothelium, and tumor-infiltrating lymphocytes (TIL), including T and B lymphocytes, natural killer cells, macrophages, and dendritic cells. Especially at the tumor/host invasive front, TIL are an important component toward unlocking the potential of immunotherapy including activating the patient’s preexisting intratumoral immunity.Citation1–3
In colon cancer, TIL have long been known to be the hallmark of the microsatellite instability high (MSI) subgroup and hypothesized to represent an adaptive response to neoantigens resulting from mismatch repair enzyme deficiency (dMMR) and accumulation of frameshift mutations.Citation4–8 MSI colon cancers are more commonly right sided and associated with earlier stage and improved survival.Citation9–13
Prior to routine evaluation for MMR proteins, TIL evaluation was required by the College of American Pathologists (CAP), as the presence of high TIL was an indication for MSI testing.Citation14 Although TIL have been studied in colon cancer for over two decadesCitation2,Citation3,Citation15,Citation16 and understanding the biology of lymphocyte subsets within the stromal and intraepithelial tumor compartments is an active area of research,Citation3,Citation17–19 TIL measurement has not been standardized for routine pathologic specimens. CAP provides limited guidance on where TIL should be measured in a heterogeneous, often necrotic tumor and what constitutes a robust immune response.Citation14 Once MMR evaluation became standard, TIL measurement was removed from the CAP guidelines;Citation20 however, recent investigations have renewed interest in TIL, as they have been shown to be prognostic independent of MMR status.Citation21,Citation22
Development of TIL measurement protocols has been hindered by tumor heterogeneity, including areas of necrosis, and a lack of a universally accepted cutoff to define a robust TIL response.Citation2,Citation3,Citation15–17,Citation23 The goal of this investigation was to quantitate TIL in the tumor invasive frontCitation21 using hematoxylin-eosin-stained slides; determine whether the highest TIL count in a single high-power field (HPF) or sum TIL count in five HPFs is more closely associated with recurrence-free survival (RFS); define the TIL cutoff for designating tumors as high or low TIL in relation to RFS; and examine the association between TIL and RFS in dMMR and MMR-proficient (pMMR) colon cancer.
Materials and methods
With approval from the institutional review board, prospectively maintained institutional databases were queried for patients who underwent curative surgery for nonmetastatic invasive adenocarcinoma of the colon at Memorial Sloan Kettering Cancer Center between February 2007 and December 2014. Patients who received neoadjuvant therapy or underwent noncurative resection were excluded. Data on demographics, histopathology, and short- and long-term oncological outcomes were collected and analyzed.
Histopathologic analysis
All available hematoxylin-eosin stained slides that included full-thickness sections of the tumor encompassing the deepest portion of the invasive front (mean, five tumor slides per patient; range, 1–13 slides per patient) were reviewed. Pathologists determined T and N classifications of the American Joint Committee on Cancer (AJCC) as well as high risk of recurrence features including venous invasion, lymphovascular invasion, or perineural invasion (VELPI). TIL in the advancing margin were measured as previously described,Citation21 which has an acceptable interobserver agreement.Citation10,Citation24,Citation25 In brief, TIL were first identified at scanning magnification to select areas with the highest counts. Using a BX51 microscope (Olympus, Tokyo, Japan) with a standard 22-mm diameter eyepiece under an objective lens with a magnification of ×40 (specimen area, 0.238 mm2), TIL were counted in five consecutive HPFs parallel to the invasive margin (Supplemental Figure 1). Two metrics of TIL density were assessed: the sum TIL in five consecutive HPFs and the highest TIL count in a single HPF within the advancing margin. Only intratumoral and stromal lymphocytes within the boundary of the tumor cell nests or glands were counted, without discriminating CD antigens by immunohistochemistry. Peritumoral lymphocytes that were outside the boundaries were not regarded as TIL, and apoptotic bodies were excluded.
Immunohistochemistry
Immunohistochemical screening was performed to assess MMR protein (MLH1, MSH2, MSH6, and PMS2) expression as previously described.Citation26 pMMR was defined as positive staining for all four antibodies.
Staging and follow-up
Patients were clinically staged with colonoscopy; CT of the chest, abdomen, and pelvis; and measurement of serum markers. Surgical staging was performed per AJCC,Citation27 and adjuvant therapy and postsurveillance follow-up performed in accordance with national guidelines.Citation28,Citation29 Recurrence was defined by imaging or biopsy.
Statistical analysis
Categorical and ordinal variables were summarized as frequencies and percentages, and quantitative variables were summarized as medians with minimum and maximum. Comparisons of TIL low vs high were made using the chi-square test and the two-sample t-test.
Kaplan-Meier analysis was used to estimate RFS, calculated from the date of surgery to the date of recurrence, death, or last follow-up. Differences in RFS were analyzed using the log-rank test. Cox regression was used to estimate hazard ratios with 95% confidence intervals and Wald p value for continuous variables and multivariable models. Kaplan-Meier analysis was also used to estimate overall survival (OS), calculated from the date of surgery to the date of death or date of the last follow-up. Total lymphocytes were investigated as a continuous variable, in quintiles to evaluate the linearity relationship with RFS and as a binary variable defined at an optimal cutpoint. To identify an optimal cutpoint for sum TIL in five consecutive HPFs in the advancing margin and for the highest TIL count in a single HPF without inflating the Type I error, we used an approach that controls the false discovery rate.Citation30 The concordance probability estimate (CPE), which computes the concordance of a variable and a censored outcome, was calculated to measure the goodness of fit.Citation31
In the absence of an external validation dataset, we conducted a bootstrap validation to ensure our findings at the optimal cutoff could be internally validated. The HR for TIL and RFS at the optimal TIL cutoff was calculated for each of 500 bootstrapped samples and plotted in a histogram.
Statistical significance was set at P < .05, and two-sided 95% confidence intervals are reported. All analyses were conducted in SAS software version 9.4 and RVersion 3.6.0.
Results
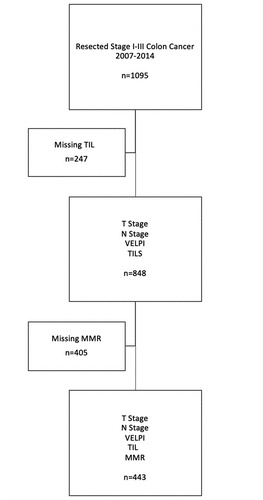
A total of 1095 patients met the inclusion criteria [541 (49%) were men; mean age, 64.5 ± 13.7 years]. TIL count in the advancing margin was available for 848 patients and MMR status was available in 443 patients (). Median follow-up was 2.8 years in the 744 patients who did not have a recurrence. Median follow-up using the inverse Kaplan-Meier method was 3.0 years (95% confidence interval, 2.7–3.1 years).Citation32
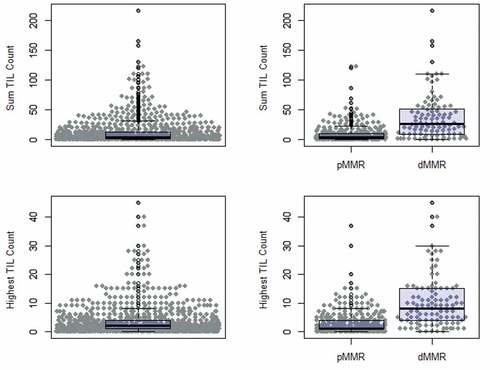
Quantitative TIL counts are shown in . The sum TIL count in five HPFs ranged from 0 to 217 with a mean of 12.0 (SD 21.5) and a median of 4. The greatest TIL count in a single HPF ranged from 0 to 45 with a mean of 3.8 (SD 5.6) and a median of 2. In the 443 patients with known MMR status, dMMR tumors had a higher mean TIL count than pMMR tumors for both measures. Using the sum TIL count in five HPFs, the mean count for pMMR was 8.7 (range 0–123) and for dMMR was 37.2 (range 0–217) (P < .001). Using the greatest TIL count in a single HPF metric, the mean count for pMMR was 2.9 (range 0–37) and for dMMR was 10.3 (range 0–45) (P < .001).
Figure 2. Quantitative TIL count including sum in five HPFs and highest TIL count in a single HPF in all patients, patients with pMMR, and patients with dMMR
Sum TIL count in five HPFs and the greatest TIL count in a single HPF were strongly correlated (Supplemental Figure 2). Univariate analysis of RFS was performed on the sum TIL count in five HPFs and separately on the greatest TIL count in a single HPF. The hazard ratio for the highest TIL count in a single HPF indicated a slightly stronger effect (0.92; 95% confidence interval [CI] = 0.87–0.98, P = .0086) than the hazard ratio for the sum TIL count in 5 HPFs (0.98; CI = 0.964–0.997, P = .0201). The CPE was also numerically higher (0.60 versus 0.58) and the RFS Kaplan Meier curves separate more cleanly for the highest TIL count than for the sum TIL count in 5 HPFs (Supplemental Table 1 and Supplemental Figure 3). Thus, we decided to further analyze the highest TIL count in a single HPF rather than sum TIL count in five HPFs.
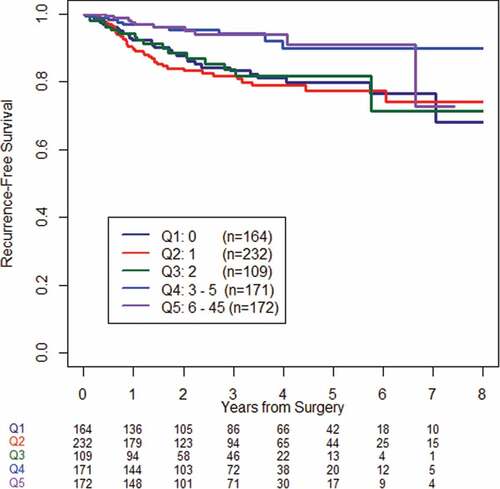
Using the highest TIL count in a single HPF, we then investigated the linearity of the relationship between TIL and RFS by dividing the patient population into quintiles based on the highest TIL count. Patients with three or more TIL in any HPF had significantly longer RFS on average than the other groups (P = .0008) (). An optimal binary cutpoint was identified at a highest TIL count of 3, where the study cohort was classified as TIL high (≥3 lymphocytes in any HPF) or TIL low (<3 lymphocytes in any HPF). The CPE for RFS at this binary cutpoint was 0.74, indicating a good fit. The bootstrapped hazard ratio distribution indicated good internal validity (bootstrapped HR 0.36, IQR 0.12). Repeat analysis of the pMMR cohort yielded similar results (data not shown).
describes the association of TIL to clinicopathologic features. Compared to TIL low, TIL high tumors were associated with older age patients, right-sided lesions, earlier AJCC stage, and dMMR. TIL high tumors were less likely to demonstrate VELPI. Of the 443 patients that underwent MMR analysis by immunohistochemistry, 340 demonstrated pMMR, with 217 (64%) TIL low and 123 (36%) TIL high. Compared with pMMR TIL low tumors, pMMR TIL high tumors were associated with right-sided location, lower AJCC stage, and lack of VELPI.
Table 1. Clinicopathologic features of study cohort of 848 patients and 340 pMMR patients
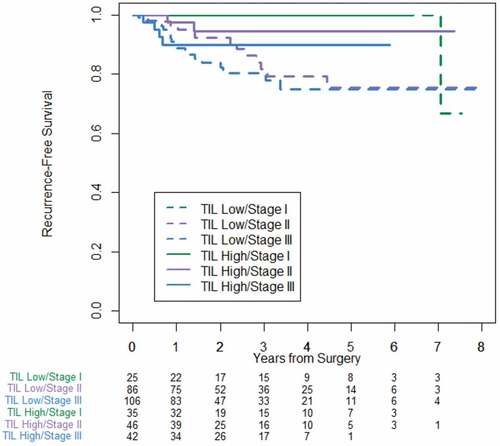
In the cohort of 848 patients with TIL measurement (), TIL high tumors were associated with improved RFS compared with TIL low tumors. Five-year RFS was 90.2% (95% CI = 83.7–94.2) in TIL high compared to 78.9% (CI = 74.1–82.9) in TIL low (P < .0001), which corresponds to a hazard ratio of 0.359 (CI = 0.22–0.585). demonstrates stage-specific RFS stratified by TIL high versus TIL low. TIL high stage II patients approached RFS of stage I patients. In a similar trend, RFS in TIL high stage III patients approached that in patients with TIL low stage II disease.
Figure 4. Stage-specific recurrence-free survival stratified by TIL high and TIL low for entire cohort of 848 patients
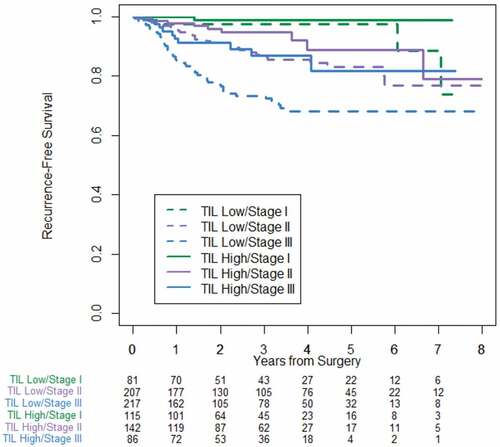
In the cohort of 340 pMMR tumors, 5-year RFS was 94.6% (CI = 88.3–97.5) in TIL high compared to 77.9% (CI = 69.2–84.4) in TIL low (P = .008). Stage-specific survival based on TIL showed similar findings to the entire cohort and is shown in .
Figure 5. Stage-specific recurrence-free survival stratified by TIL low and TIL high for the cohort of 340 pMMR patients
Factors associated with lower RFS are outlined in . In the entire cohort of 848 patients, greater RFS was univariately associated with TIL high, lower age, T1/T2 (vs T3/4), N0 (vs N1/2), earlier AJCC stage, and absence of VELPI. In the pMMR cohort, TIL high remained associated with greater RFS along with T1/T2 (vs T3/4), N0 (vs N1/2), AJCC stage, and absence of high-risk VELPI pathologic features. In a multivariable Cox regression analysis that included TILs, stage and VELPI, we confirmed that TIL high vs low (HR 0.407; 95% CI 0.170, 0.976; P = .04) was independently associated with RFS while Stage (P = .14) and VELPI (P = .34) were not.
Table 2. Univariate analysis of clinicopathologic factors associated with recurrence-free survival
There were a total 39 deaths at last follow-up, and 16 patients died without a recurrence. There was no significant difference in overall survival between TIL high and TIL low (Supplemental Figure 4).
Discussion
To fully realize the benefits of immunotherapy in colon cancer, defining what constitutes a robust lymphocytic response to differentiate “cold” tumors from “hot” tumors is critical. Using standard pathology processing to quantitate TIL, we found a large range of lymphocytes in the invasive front in a series of 848 patients with nonmetastatic colon cancer. We demonstrated that visually scanning hematoxylin and eosin routine pathology slides for areas of increased lymphocyte density and measuring the highest TIL count in a single HPF was more predictive of RFS than summing the total TIL count in five HPFs. Next, by analyzing TIL count in quintiles, we established that three or more TIL in a single HPF was an appropriate cutoff for high versus low TIL, as it was associated with improved RFS. Analysis of the pMMR subgroup provided similar results.
Our finding that immune density is modestly more prognostic than total immune cell count is consistent with prior studies including Immunoscore® (HalioDx, Richmond, VA), which employs immunohistochemistry, advanced proprietary image analysis, and sophisticated algorithms to calculate CD3+ and CD8+ T cell density in the tumor core and advancing margin.Citation12,Citation23,Citation33,Citation34 An international consortium recently validated Immunoscore on a large international colon cancer cohort.Citation2 A high Immunoscore had a lower risk of recurrence and improved 5-year disease-free survival and overall survival, after controlling for known prognostic factors. Nevertheless, the Immunoscore has not entered the routine pathology laboratory workflow, likely due to the requirement of additional imaging equipment, time, and effort.Citation15,Citation17,Citation35
TIL were more abundant in dMMR compared to pMMR tumors, consistent with the literature correlating MSI tumors with neoantigen load and immune response.Citation5 Interestingly, there was a wide range of TIL in both dMMR and pMMR tumors. Thirty-six percent of pMMR tumors were TIL high, in range with other studies,Citation2,Citation22 and 18% of dMMR tumors were TIL low, attesting to the complexity of the immune tumor microenvironment, referred by Binnewies and colleagues as Tumor Immunity in the MicroEnvironment (TIME).Citation36,Citation37
Our finding that dMMR tumors, which strongly correlate with MSI status,Citation38 have overall favorable outcome, is consistent with the literature.Citation9 Before routine MMR analysis, TIL association with MSICitation39 was the rationale for the required TIL measurement in colon cancer by CAP. Patients with TIL were advised to have further MSI testing. However, CAP acknowledged that absolute cutoff values were not clearly established and advised reporting TIL as none, mild to moderate (0–2 per HPF), and marked (3 or more per HPF).Citation14 Once MMR status became a CAP requirement, TIL measurement was removed from their standard synoptic reporting.Citation20 Our study adds to the growing literature that routine TIL measurement provides important prognostic information, in addition to MMR status. Specifically, TIL high pMMR stage III patients had RFS similar to stage II, and TIL high pMMR stage II had similar RFS to stage I. It should be added to the growing list of prognostic factors following colectomy for colon cancer.
More important than relaying prognosis, TIL are currently being investigated as a predictive biomarker for adjuvant chemotherapyCitation37 (NCT03422601 on clinicaltrials.gov). More salient would be the ability of TIL to inform patient selection or the efficacy of immunotherapy. Although post hoc analysis of a small study was able to show that TIL were associated with beneficial effects of adjuvant immunotherapy,Citation40 TIL have not been able to predict response to PD-1/CTLA-4 in stage IV colorectal cancer.
The analysis is subject to the limitations and bias inherent in observational retrospective studies. Quantitative TIL measurement was not available in all cases; however, RFS was similar between those with TIL quantification and those without TIL quantification (data not shown), indicating no apparent bias, and we therefore assume data are missing at random. Consistent with practice at the time, immunohistochemistry was performed selectively, and MMR expression was biased toward younger patients and those with TIL. However, there were sufficient events in the pMMR group to identify TIL as a prognostic factor. In the 103 dMMR patients with overall favorable outcome, there was insufficient power to assess the prognostic value of TIL as others have reported.Citation2,Citation22
In summary, we show that in addition to MMR analysis, TIL quantification in a single HPF using routine hematoxylin and eosin-stained slides provides important prognostic information.
Disclosure of potential conflicts of interest
Z. Stadler’s immediate family member serves as a consultant for Adverum Biotechnologies, Genentech/Roche, Novartis, Neurogene, Gyroscope Tx, Optos Plc, Regeneron, RegenexBio, and Spark Therapeutics. R. Yaeger has served as a consultant for Array BioPharma and Natera. N. H. Segal has served as a consultant for Boehringer Ingelheim, Roche/Genentech, Revitope, PsiOxus, Immunocore, PureTech Ventures, Amgen, GSK, CStone Pharmaceuticals, and Synlogic and has received research funding from Roche/Genentech, Pfizer, Merck, BMS, AstraZeneca, Incyte, and Immunocore. J. Joshua Smith has received travel support from Intuitive Surgical Inc. for fellow education and served as a clinical advisor for Guardant Health, Inc. J. Garcia-Aguilar holds equity in Intuitive Inc. and has received honoraria from Medtronic and Johnson & Johnson. The other authors had no potential conflicts of interest.
Supplemental Material
Download ()Acknowledgments
We gratefully acknowledge Arthur Gelmis for editing the manuscript.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Angelova M, Charoentong P, Hackl H, Trajanoski Z. The colorectal cancer immune paradox revisited. Oncoimmunology. 2016;5(2):e1078058. doi:10.1080/2162402X.2015.1078058.
- Pages F, Mlecnik B, Marliot F, Bindea G, Ou F-S, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391(10135):2128–8. doi:10.1016/S0140-6736(18)30789-X.
- Zhao Y, Ge X, He J, Cheng Y, Wang Z, Wang J, Sun L. The prognostic value of tumor-infiltrating lymphocytes in colorectal cancer differs by anatomical subsite: a systematic review and meta-analysis. World J Surg Oncol. 2019;17(1):85. doi:10.1186/s12957-019-1621-9.
- Eriksen AC, Sorensen FB, Lindebjerg J, Hager H, dePont Christensen R, Kjær-Frifeldt S, Hansen TF. The prognostic value of tumor-infiltrating lymphocytes in stage II colon cancer. A nationwide population-based study. Transl Oncol. 2018;11(4):979–987. doi:10.1016/j.tranon.2018.03.008.
- Giannakis M, Mu XJ, Shukla SA, Qian Z, Cohen O, Nishihara R, Bahl S, Cao Y, Amin-Mansour A, Yamauchi M, et al. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep. 2016;15(4):857–865. doi:10.1016/j.celrep.2016.03.075.
- Innocenti F, Ou F-S, Qu X, Zemla TJ, Niedzwiecki D, Tam R, Mahajan S, Goldberg RM, Bertagnolli MM, Blanke CD, et al. Mutational analysis of patients with colorectal cancer in CALGB/SWOG 80405 identifies new roles of microsatellite instability and tumor mutational burden for patient outcome. J Clin Oncol. 2019;37(14):1217–1227. doi:10.1200/JCO.18.01798.
- Schwitalle Y, Kloor M, Eiermann S, Linnebacher M, Kienle P, Knaebel HP, Tariverdian M, Benner A, von Knebel Doeberitz M. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology. 2008;134(4):988–997. doi:10.1053/j.gastro.2008.01.015.
- Tougeron D, Fauquembergue E, Rouquette A, Le Pessot F, Sesboüé R, Laurent M, Berthet P, Mauillon J, Fiore F, Sabourin J-C, et al. Tumor-infiltrating lymphocytes in colorectal cancers with microsatellite instability are correlated with the number and spectrum of frameshift mutations. Mod Pathol. 2009;22(9):1186–1195. doi:10.1038/modpathol.2009.80.
- Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, French AJ, Kabat B, Foster NR, Torri V, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28(20):3219–3226. doi:10.1200/JCO.2009.27.1825.
- Shia J, Ellis NA, Paty PB, Nash GM, Qin J, Offit K, Zhang X-M, Markowitz AJ, Nafa K, Guillem JG, et al. Value of histopathology in predicting microsatellite instability in hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer. Am J Surg Pathol. 2003;27(11):1407–1417.
- Mei Z, Liu Y, Liu C, Cui A, Liang Z, Wang G, Peng H, Cui L, Li C. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. 2014;110(6):1595–1605. doi:10.1038/bjc.2014.46.
- Mlecnik B, Bindea G, Kirilovsky A, Angell HK, Obenauf AC, Tosolini M, Church SE, Maby P, Vasaturo A, Angelova M, et al. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci Transl Med. 2016;8(327):327ra26. doi:10.1126/scitranslmed.aad6352.
- Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi:10.1038/nrc3245.
- College of American Pathologists. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum, version 3.4.0.0. www.cap.org
- Ko SY, Pyo J-S. Clinicopathological significance and prognostic role of tumor-infiltrating lymphocytes in colorectal cancer. Int J Biol Markers 2019;34(2):132–138.
- Roelands J, Kuppen PJK, Vermeulen L, Maccalli C, Decock J, Wang E, Marincola F, Bedognetti D, Hendrickx W. Immunogenomic classification of colorectal cancer and therapeutic implications. Int J Mol Sci. 2017;18(10):2229. doi:10.3390/ijms18102229.
- Kong JC, Guerra GR, Pham T, Mitchell C, Lynch AC, Warrier SK, Ramsay RG, Heriot AG. Prognostic impact of tumor-infiltrating lymphocytes in primary and metastatic colorectal cancer: a systematic review and meta-analysis. Dis Colon Rectum. 2019;62(4):498–508. doi:10.1097/DCR.0000000000001332.
- Kuwahara T, Hazama S, Suzuki N, Yoshida S, Tomochika S, Nakagami Y, Matsui H, Shindo Y, Kanekiyo S, Tokumitsu Y, et al. Intratumoural-infiltrating CD4 + and FOXP3 + T cells as strong positive predictive markers for the prognosis of resectable colorectal cancer. Br J Cancer. 2019;121(8):659–665. doi:10.1038/s41416-019-0559-6.
- Shang B, Liu Y, Jiang S-J, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep. 2015;5(1):15179. doi:10.1038/srep15179.
- College of American Pathologists. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum, version 4.0.1.0. www.cap.org
- Konishi T, Shimada Y, Lee LH, Cavalcanti MS, Hsu M, Smith JJ, Nash GM, Temple LK, Guillem JG, Paty PB, et al. Poorly differentiated clusters predict colon cancer recurrence: an in-depth comparative analysis of invasive-front prognostic markers. Am J Surg Pathol. 2018;42(6):705–714. doi:10.1097/PAS.0000000000001059.
- Williams DS, Mouradov D, Jorissen RN, Newman MR, Amini E, Nickless DK, Teague JA, Fang CG, Palmieri M, Parsons MJ, et al. Lymphocytic response to tumour and deficient DNA mismatch repair identify subtypes of stage II/III colorectal cancer associated with patient outcomes. Gut. 2018. doi:10.1136/gutjnl-2017-315664.
- Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27(35):5944–5951. doi:10.1200/JCO.2008.19.6147.
- Swisher SK, Wu Y, Castaneda CA, Lyons GR, Yang F, Tapia C, Wang X, Casavilca SAA, Bassett R, Castillo M, et al. Interobserver agreement between pathologists assessing tumor-infiltrating lymphocytes (TILs) in breast cancer using methodology proposed by the international TILs working group. Ann Surg Oncol. 2016;23(7):2242–2248. doi:10.1245/s10434-016-5173-8.
- van Putten PG, van Lier MG, Hage M, Biermann K, van Rijssel RH, Westenend PJ, Morreau H, Steyerberg EW, Dinjens WNM, Kuipers EJ, et al. Limited diagnostic value of microsatellite instability associated pathology features in colorectal cancer. Fam Cancer. 2014;13(3):351–359. doi:10.1007/s10689-014-9705-8.
- Shia J, Black D, Hummer AJ, Boyd J, Soslow RA. Routinely assessed morphological features correlate with microsatellite instability status in endometrial cancer. Hum Pathol. 2008;39(1):116–125. doi:10.1016/j.humpath.2007.05.022.
- Amin MB, Gress DM, Vega M, et al. AJCC cancer staging manual, 8th ed. NewYork, NY: Springer;2016.
- American Society of Clinical Oncology. Guidelines for gastrointestinal cancer. https://www.asco.org/research-guidelines/quality-guidelines/guidelines/gastrointestinal-cancer.
- National Comprehensive Cancer Network. Guidelines for colon and rectal cancer. https://www.nccn.org/professionals/physician_gls/default.aspx#site.
- Williams BA, Mandrekar JN, Mandrekar SS. Finding optimal cutpoints for continuous covariates with binary and time-to-event outcomes. Mayo Clinic: Rochester (Minn.). www.mayo.edu/research/documents/biostat-79pdf/doc-10027230
- Gönen M, Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika. 2005;92(4):965–970. doi:10.1093/biomet/92.4.965.
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–346. doi:10.1016/0197-2456(96)00075-X.
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi:10.1126/science.1129139.
- Wirta E-V, Seppala T, Friman M, Väyrynen J, Ahtiainen M, Kautiainen H, Kuopio T, Kellokumpu I, Mecklin J-P, Böhm J, et al. Immunoscore in mismatch repair-proficient and -deficient colon cancer. J Pathol Clin Res. 2017;3(3):203–213. doi:10.1002/cjp2.71.
- Ogino S, Giannakis M. Immunoscore for (colorectal) cancer precision medicine. Lancet. 2018;391(10135):2084–2086. doi:10.1016/S0140-6736(18)30953-X.
- Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Catherine C Hedrick CC, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541–550.
- Pagès F, André T, Taieb J, Vernerey D, Henriques J, Borg C, Marliot F, Ben Jannet R, Louvet C, Mineur L, et al. Prognostic and predictive value of the Immunoscore in stage III colon cancer patients treated with oxaliplatin in the prospective IDEA France PRODIGE-GERCOR cohort study. Ann Oncol. 2020;31(7):921–929. doi:10.1016/j.annonc.2020.03.310.
- Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn. 2008;10(4):293–300. doi:10.2353/jmoldx.2008.080031.
- Smyrk TC, Watson P, Kaul K, Lynch HT. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001;91(12):2417–2422. doi:10.1002/1097-0142(20010615)91:12<2417::AID-CNCR1276>3.0.CO;2-U.
- Turksma AW, Coupe VM, Shamier MC, Lam KLH, de Weger VA, Belien JAM, van den Eertwegh AJ, Meijer GA, Meijer CJLM, Hooijberg E, et al. Extent and location of tumor-infiltrating lymphocytes in microsatellite-stable colon cancer predict outcome to adjuvant active specific immunotherapy. Clin Cancer Res. 2016;22(2):346–356. doi:10.1158/1078-0432.CCR-13-2462.