ABSTRACT
Podoplanin (PDPN) has been proved to have significant immunoregulatory effects in several types of malignancies and is considered to be a novel immune checkpoint molecule. However, the clinical significance of PDPN and its potential influence on immune contexture in gastric cancer remain obscure. Here, we aimed to investigate the clinical outcomes and immunoregulatory role of tumor-infiltrating PDPN+ cells (tPDPNs) in gastric cancer. A total of 454 tumor tissue microarray specimens and 68 fresh tumor tissues of gastric cancer patients from Zhongshan Hospital, and transcriptional data of 293 gastric cancer patients from The Cancer Genome Atlas were included. We demonstrated that tPDPNs high subgroup experienced worse overall survival and disease-free survival, and indicated inferior therapeutic responsiveness to fluorouracil-based adjuvant chemotherapy (ACT) in gastric cancer. The abundance of tPDPNs was correlated with an immunoevasive contexture characterized by pro-tumor macrophage and dysfunctional CD8+ T cell infiltration. Moreover, dysfunctional CD8+ T cells in tPDPNs high subgroup exhibited decreased interferon-γ, granzyme B and perforin-1 expression yet elevated programmed cell death-1 (PD-1) and T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) expression. Stratification of gastric cancer patients into different risk groups based on tPDPNs and CD8+ T cells showed distinct prognosis, responsiveness to ACT and molecular characteristics. This study revealed that the abundance of tPDPNs could identify an immunoevasive contexture and might be applied as an independent predictor for poor prognosis and suboptimal ACT responsiveness. Thus, we recommended tPDPNs as a promising therapeutic target in gastric cancer.
Introduction
Gastric cancer is the fifth most common malignancy and the third leading cause of cancer-related death worldwide.Citation1 Clinical guidelines have established radical gastrectomy as the most feasible curative treatment for gastric cancer.Citation2 For advanced gastric cancer patients, fluorouracil-based adjuvant chemotherapy (ACT) is recommended as the first-line postoperative treatment.Citation3 Nevertheless, chemotherapeutic resistance limits the clinical application of fluorouracil,Citation4,Citation5 and many patients still experience relapse and death after ACT treatment. In addition, targeting human epidermal growth factor receptor-2 (HER-2) and vascular endothelial growth factor receptor-2 (VEGFR-2) in gastric cancer have been proven unsatisfactory. The only two targeting agents trastuzumab and ramucirumab have a modest impact on survival.Citation6–8 Therefore, further stratification of gastric cancer patients for survival benefits and therapeutic responsiveness remains a critical challenge.
Researches on immunotherapy have stimulated interest in characterizing the host immune responses and the underlying mechanisms of tumor immune evasion.Citation9,Citation10 Re-activation of T-cell-mediated anti-tumor immunity in the tumor microenvironment (TME), such as targeting immune checkpoints (ICKs), has emerged as a novel treatment paradigm in several types of malignancies.Citation11,Citation12 Notably, pembrolizumab and nivolumab have been applied for the treatment of advanced or metastatic gastric cancer based on clinical trials such as KEYNOTE-059 and ATTRACTION-2.Citation13,Citation14 However, only approximately 10%-20% of gastric cancer patients benefit from ICK inhibitors that block programmed cell death-1/programmed cell death-ligand 1 (PD-1/PD-L1) signaling. This may, partly, be attributed to the heterogeneous TME, T-cell-inflamed gene-expression profile and different molecular subtypes.Citation15,Citation16 Consequently, in order to further improve response rates and expand therapeutic effects, novel predictive biomarkers and potential immunoevasive mechanisms should be identified in gastric cancer.
Podoplanin (PDPN) is a cell-surface mucin-type transmembrane glycoprotein with various functions.Citation17 Physiologically, PDPN interacts with C-type lectin receptor type-2 (CLEC-2) to promote the separation of blood vessels and lymphatic vessels during embryonic development.Citation18 PDPN may be a novel potential therapeutic target due to its involvement in inflammation and cancer.Citation19 Besides being highly expressed by lymphatic endothelial cells, PDPN is also widely found in tumor cells, fibroblasts, and several immune cells.Citation20 Although the implications of PDPN+ tumor cells or fibroblasts in tumor progression and invasion have been well documented,Citation21,Citation22 the role of tumor-infiltrating PDPN+ cells (tPDPNs) in gastric cancer is unclear. Consequently, we aim to investigate the clinical outcomes of tPDPNs and its association with the immune contexture in gastric cancer.
In the current study, we demonstrated that tPDPNs could indicate poor prognosis and inferior responsiveness to ACT in gastric cancer patients. Further analysis showed that the abundance of tPDPNs was positively associated with an immunoevasive contexture. Moreover, we also comprehensively analyzed the clinical significance of tPDPNs and CD8+ T cells for patient stratification. Our results shed light on targeting tPDPNs as a promising therapeutic strategy in gastric cancer.
Patients and methods
Study design and patients
This study enrolled three patient cohorts and the study design was shown in Supplementary Figure S1. Cohort 1 recruited 496 patients who underwent gastrectomy from Zhongshan Hospital, Fudan University (Shanghai, China) between August 2007 and December 2008. All tumor and matched peritumor specimens were formalin-fixed, paraffin-embedded (FFPE) and constructed into tissue microarray (TMA). Clinicopathological staging was assessed according to the 7th edition of American Joint Committee on Cancer Staging Manual. A total of 42 patients were excluded due to clinical information missing, metastatic diseases, or dot loss. The remaining 454 patients were then randomly divided into two independent data sets (Discovery set, n = 200; Validation set, n = 254). After gastrectomy, patients with stage II or III were principally given routine fluorouracil-based ACT. None of the patients had received radiotherapy. All follow-up data were collected until April 2014. The overall survival (OS) was defined as the time from the date of surgery to the date of death or last follow-up. The disease-free survival (DFS) was defined as the time from the date of surgery to the date of disease recurrence or last follow-up. Cohort 2 was generated from The Cancer Genome Atlas (TCGA). RNA sequencing and clinical data of 443 gastric cancer patients were downloaded by R package TCGAbiolinks,Citation23 whereas 293 gastric cancer patients with available data were included. Cohort 3 enrolled additional 60 patients who underwent gastrectomy from Zhongshan Hospital, Fudan University between August 2018 and November 2018. Fresh tumor specimens were obtained at the surgery for flow cytometry (FCM) analysis, and corresponding FFPE tissues were constructed as an independent TMA for tPDPNs evaluation. Moreover, we analyzed cell types of CD45+PDPN+ cells by FCM in an additional eight tumor and corresponding peritumor specimens of gastric cancer collected from September, 2020 to October, 2020. Informed consent was acquired from all patients, and this study was approved by the hospital Clinical Research Ethics Committee.
Immunohistochemistry and RNA-in situ hybridization
Protocol details of TMA construction and immunohistochemistry (IHC) staining have been described elsewhere.Citation24 Briefly, the slides were deparaffinized and rehydrated, endogenous peroxidase blocked, heated with an autoclave in appropriate buffer solution for antigen retrieval, and incubated with normal goat serum to eliminate nonspecific reactions. Subsequently, the slides were incubated with primary antibodies, followed by horseradish peroxidase (HRP)-labeled secondary antibody incubation and detection using diaminobenzidine (DAB) reagent. Negative controls were applied equally, but with the primary antibody omitted. Ultimately, IHC single staining slides were counterstained with hematoxylin, dehydrated, and applied coverslip and neutral resins. For IHC double staining, after DAB visualization, the slides were incubated with the second primary antibodies before applying to alkaline phosphatase (AP)-labeled secondary antibody and Vector Blue reagent. All antibodies involved in this study were shown in Supplementary Table S1. To evaluate the presence of Epstein-Barr virus (EBV) infection, we detected EBV-encoded small RNA (EBER) by in situ hybridization (EBER-ISH) with the Digoxigenin EBER probe (Talent Biomedical, China) as manufacturer’s instructions. Strong staining within almost all tumor cell nuclei was considered to be positive.
Definition of cutoff values
All slides were independently examined by two pathologists who were blind to the clinical information. The mean number of stained cells and the intensity of cellular staining were evaluated under ×200 magnification in three randomized high power fields (HPF; Leica DM6000 B, Leica Microsystems, Wetzlar, Germany). The cutoff value for classifying tPDPNs high and low subgroups was the median value. For PDPN mRNA level, the cutoff value was determined by the minimum P-value method using X-Tile software (Version 3.6.1, Yale University). In addition, survminer package (version 0.4.8; https://CRAN.R-project.org/package=survminer) in R software version 3.6.1 was also applied to identify cutoff values of tPDPNs or PDPN mRNA, which were consistent with cutoff values mentioned above.
Flow cytometry
Fresh tumor specimens were obtained at the surgery. Single-cell suspension was isolated by collagenase IV, and then incubated with red blood cell (RBC) lysis buffer (BD Biosciences) followed by FcR-blocking reagent (Biolegend). Cells were stained with the indicated surface markers for 30 min at 4°C in dark. If necessary, cells were pre-treated with Fixation/Permeabilization Solution Kit (BD Biosciences) and then performed intracellular protein staining. Stained cells were washed and re-suspended in cell staining buffer. FCM was performed using BD FACSCelesta and analyzed by FlowJo v10.0 (Treestar). Dead cells were excluded based on the scatter profile. Antibodies involved were listed in Supplementary Table S2.
Statistical analysis
Categorical variables were analyzed with Chi-squared test, and continuous variables were assessed by t test. One-way ANOVA followed by Tukey’s multiple comparisons test was performed using GraphPad Prism v8.3.0 (GraphPad Software, La Jolla, California, USA). Boxplots display a statistical summary of median, interquartile range (IQR) and possibly extreme values. Each box represents the median and IQR of the data. The end of the whiskers represents the maximum or minimum values, except for outliers. The outliers were defined as the 25th percentile minus 1.5IQR and the 75th percentile plus 1.5IQR. The individual points indicated the extreme values greater than the outliers. Scatter plots were presented as mean ± SD. Correlations were analyzed by Spearman correlation. Survival curves to compare OS and DFS were estimated by the Kaplan–Meier method and detected by log-rank test, using MedCalc 15.6.1 (MedCalc Software bvba, Ostend, Belgium). Cox regression models were performed to assess covariate effects on prognosis and interactions between covariates. IBM SPSS Statistics v20.0 (SPSS Inc., Chicago, IL) was applied for statistical analyses, and two-sided P < .05 was considered statistically significant.
Results
Association between tPDPNs and clinicopathological factors in gastric cancer patients
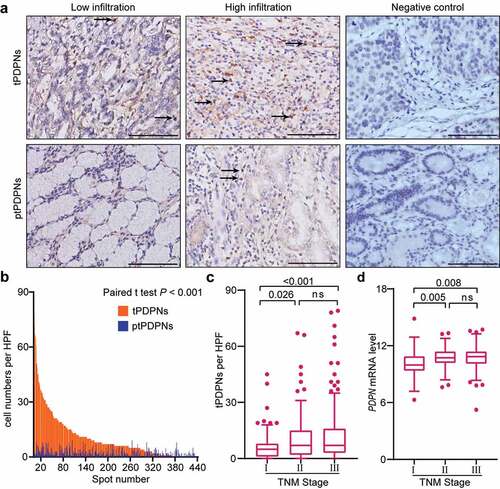
We performed IHC analysis for PDPN+ cells in gastric tissues (). The density of tPDPNs was significantly higher than that of peritumor-infiltrating PDPN+ cells (ptPDPNs) (P < .001, ). In addition, we investigated cell types of CD45+PDPN+ cells using FCM analysis. The results demonstrated that CD4+ T cells were the main component of CD45+PDPN+ cells in the tumor and peritumor tissues of gastric cancer (Supplementary Figure S2A-B). The clinicopathological characteristics of patients with high or low tPDPNs in both Discovery set and Validation set were summarized in . Higher density of tPDPNs was positively associated with T classification and tumor-node-metastasis (TNM) stage. Notably, TNM stage II or III tumors showed apparently more tPDPNs than stage I tumors ( and ). Moreover, hypoxia positive tumors contained higher tPDPNs than hypoxia negative tumors (Supplementary Figure S3A-B). However, tPDPNs showed no obvious association with age, gender, localization, tumor size, Lauren classification, grade, or adjuvant chemotherapy (). Furthermore, we found PDPN mRNA level was upregulated in advanced gastric cancer using TCGA database analysis ( and Supplementary Table S3). Together, these results indicate that the accumulation of tPDPNs in gastric cancer might be correlated with tumor progression.
Table 1. Associations between tPDPNs and clinicopathological characteristics in gastric cancer patients
Figure 1. Accumulated tPDPNs in gastric cancer are correlated with tumor progression. (a) Representative immunohistochemistry (IHC) images of tumor-infiltrating PDPN+ cells (tPDPNs) and peritumor-infiltrating PDPN+ cells (ptPDPNs) in gastric tissues. Arrow heads show tPDPNs and ptPDPNs. Scale bars, 100 μm. (b) IHC evaluation of tPDPNs versus ptPDPNs. Paired t test. (c) Distribution of tPDPNs across tumor-node-metastasis (TNM) stage. One-way ANOVA followed by Tukey’s multiple comparisons. (d) Distribution of PDPN mRNA level across TNM stage. One-way ANOVA followed by Tukey’s multiple comparisons. ns refers to not significant
Prevalence and prognostic significance of tPDPNs in gastric cancer
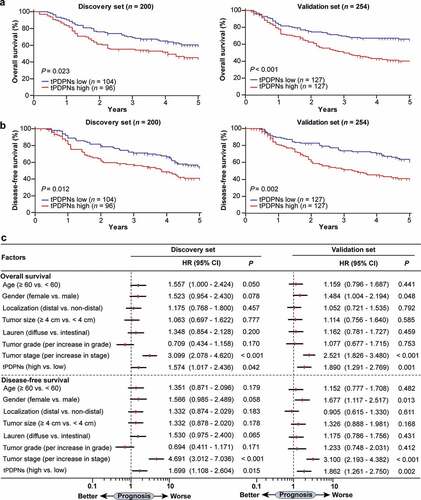
Then, we explored the prognostic significance of tPDPNs in gastric cancer. We found that in both Discovery set and Validation set, tPDPNs high subgroup experienced significantly poorer OS (P = .023 and P < .001) and DFS (P = .012 and P = .002) than tPDPNs low subgroup (). According to the multivariate analysis, tPDPNs were identified as an independent risk factor, with regard to OS (Hazard Ratio (HR): 1.574, 95% Confidence Interval (CI): 1.017–2.436, P = .042 and HR:1.890, 95% CI: 1.291–2.769, P = .001) and DFS (HR: 1.699, 95% CI: 1.108–2.604, P = .015 and HR:1.862, 95% CI: 1.261–2.750, P = .002) in both Discovery set and Validation set (). We also performed survival analysis based on PDPN mRNA level. Patients with PDPN mRNA high expression had obviously poorer OS than those with low expression (P = .006), whereas no significant difference was observed for DFS (P = .133) between PDPN mRNA high and low expression subgroups (Supplementary Figure S4A-B). Consequently, these data suggest that tPDPNs could be a promising independent prognosticator for gastric cancer patients.
Figure 2. tPDPNs predict poor prognosis in gastric cancer. (a-b) Kaplan-Meier curves for overall survival (OS) and disease-free survival (DFS) in gastric cancer patients according to tPDPNs status in Discovery set and Validation set. The OS (a) and DFS (b) were compared between tPDPNs low and high subgroups. Log-rank test was performed for Kaplan-Meier curves. (c) Multivariate analysis of OS and DFS were conducted on the basis of clinicopathological characteristics in Discovery set and Validation set. HR, hazard ratio; CI, confidence interval
Relationship between tPDPNs and responsiveness to ACT
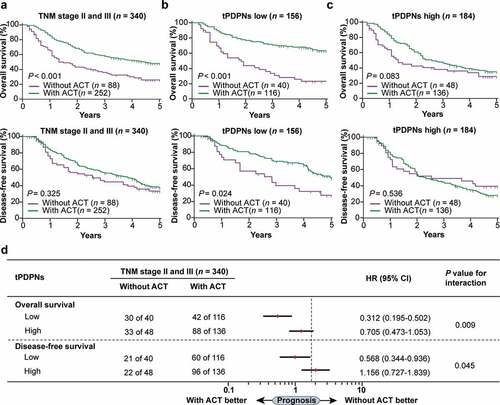
We then investigated the relationship between different tPDPNs subgroups and the responsiveness to fluorouracil-based ACT in the pooled cohort of stage II and III gastric cancer patients. The definition of tPDPNs low and high subgroups was based on the median value of the whole cohort, which has been used in . As presented in , stage II and III gastric cancer patients could benefit from ACT with regard to OS (P < .001) but not DFS (P = .325). Notably, both OS and DFS outcomes were improved after ACT application in tPDPNs low subgroup (P < .001 and P = .024; ). Contrarily, in tPDPNs high subgroup, receiving ACT just showed a trend toward OS improvement, while could not predict DFS benefits (P = .083 and P = .536; ). Furthermore, subgroup interaction analysis showed that tPDPNs high subgroup had significantly inferior chemotherapeutic responsiveness to fluorouracil, with regard to either OS or DFS (P = .009 and P = .045 for interaction; ). Collectively, these results suggest that tPDPNs enrichment could potentially impede responsiveness to fluorouracil-based ACT.
Figure 3. tPDPNs are associated with inferior therapeutic responsiveness to fluorouracil. (a) The overall survival (OS) curves and disease-free survival (DFS) curves in stage II and III gastric cancer patients according to adjuvant chemotherapy (ACT) application. (b) The OS curves and DFS curves in tPDPNs low subgroup according to ACT application. (c) The OS curves and DFS curves in tPDPNs high subgroup according to ACT application. Log-rank test was performed for Kaplan-Meier curves. (d) A test for an interaction between tPDPNs and responsiveness to ACT. HR, hazard ratio; CI, confidence interval
Association between tPDPNs and immunoevasive contexture in gastric cancer
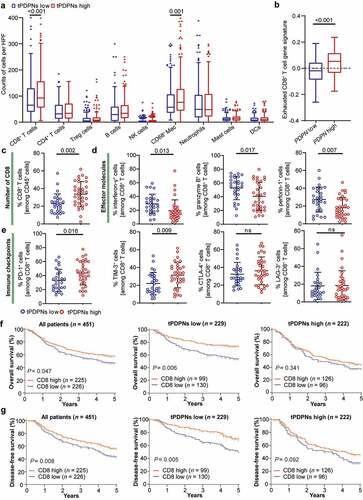
Considering tumor immune contexture could affect the prognostic information and chemotherapeutic responsiveness,Citation25 we further explored the relationship between tPDPNs and immune contexture. Through IHC analysis of nine types of immune cells in gastric cancer (Supplementary Figure S5A), we found that CD8+ T cells and CD68+ macrophages were highly enriched in tPDPNs high subgroup, compared with tPDPNs low subgroup (). Additionally, tPDPNs high subgroup contained higher numbers of CD163+ cells, DC-SIGN+ macrophages, and higher PD-L1 IHC score (Supplementary Figure S6A-B), which were identified as macrophage-related pro-tumor markers.Citation26–28 Since tPDPNs indicated elevated CD8+ T cell infiltration, which was often used as a predictor for better prognosis,Citation29 we investigated if tPDPNs could affect CD8+ T cell functional status. TCGA database analysis showed that the score of exhausted CD8+ T cell gene signature in PDPN mRNA high subgroup was higher than that in PDPN mRNA low subgroup ( and Supplementary Table S4). Consistently, FCM analysis also validated increased CD8+ T cells in tPDPNs high subgroup (). These CD8+ T cells exhibited an exhausted phenotype with decreased levels of interferon-γ, granzyme B, and perforin-1 (), yet elevated expression of PD-1 and T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) (). Moreover, PD-1+ cells and TIM-3+ cells were significantly associated with tPDPNs by IHC analysis ( and Supplementary Figure S5B). Consequently, these findings imply that tPDPNs shape an immunoevasive contexture characterized by pro-tumor macrophages and dysfunctional CD8+ T cells in gastric cancer.
Table 2. tPDPNs are associated with PD-1+ cells and TIM-3+ cells
Figure 4. tPDPNs are associated with an exhausted CD8+ T-cell phenotype in gastric cancer. (a) Immunohistochemistry analysis of the immune contexture in tPDPNs low and high subgroups. Treg, regulatory T; NK, natural killer; Mac, macrophages; Neu, neutrophils; DCs, dendritic cells. (b) Relationship between PDPN mRNA level and exhausted CD8+ T cell gene signature in TCGA database. (c-e) Flow cytometry to detect the number of CD8+ T cells in CD45+ cells (c), the expression of effector molecules (interferon-γ, granzyme B and perforin-1) in CD8+ T cells (d) and immune checkpoints (PD-1, TIM-3, CTLA-4 and LAG-3) in CD8+ T cells (e) between tPDPNs low and high subgroups. Unpaired t test. ns refers to not significant. PD-1, programmed death-1; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; CTLA-4, cytotoxic T-lymphocyte-associated protein-4; LAG-3, lymphocyte-activation gene-3. (f-g) The overall survival (OS) curves (f) and disease-free survival (DFS) curves (g) in all gastric cancer patients, tPDPNs low subgroup and tPDPNs high subgroup according to CD8+ T cell status. Log-rank test was performed for Kaplan-Meier curves
tPDPN-associated dysfunctional CD8+ T cells indicate poor prognosis
As CD8+ T cells were the core of adaptive immune resistance associated with tPDPNs, we assessed the potential impact of tPDPN-related CD8+ T cell exhaustion on survival outcomes in gastric cancer patients. Kaplan-Meier analysis indicated that patients with high CD8+ T cell infiltration experienced better OS and DFS in the whole patient cohort (). Interestingly, CD8+ T cells could only predict better prognosis in tPDPNs low subgroup, rather than tPDPNs high subgroup (). These data suggest that the abundance of tPDPNs might induce the immune tolerance of CD8+ T cells and yield poor prognosis in gastric cancer.
Stratification of gastric cancer patients based on tPDPNs and CD8+ T cells predicted prognosis and chemotherapeutic responsiveness
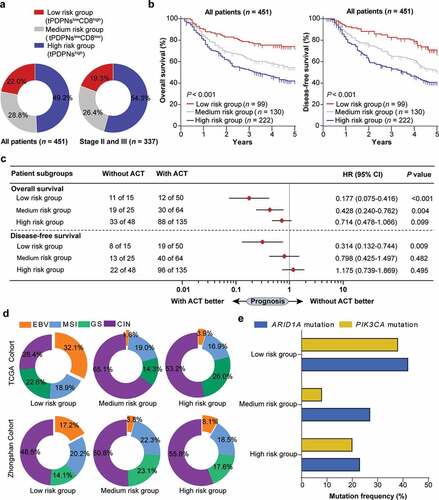
Considering CD8+ T cells could not predict better prognosis in tPDPNs high subgroup, we then classified gastric cancer patients into three distinct risk groups: low-risk group (tPDPNslowCD8hi), medium-risk group (tPDPNslowCD8low), and high-risk group (tPDPNshigh). The comprehensive proportion of the three risk groups were shown in . We demonstrated that there was a significant difference in OS and DFS among the three risk groups (P < .001 and P < .001, ). We also investigated whether the three risk groups had different chemotherapeutic responsiveness in stage II and III gastric cancer patients. Cox regression analysis indicated that high-risk group had inferior responsiveness to fluorouracil-based ACT (). Moreover, we explored the relationship between the three risk groups and molecular subtypes in gastric cancer. Interestingly, using data of molecular subtypes defined by TCGA published in Nature in 2014,Citation30 we found that EBV-positive gastric cancer was especially distributed in low-risk group (). Remarkably, this finding was confirmed in Zhongshan cohort by the approximated TCGA molecular subtyping algorithm based on EBER-ISH and IHC ( and Supplementary Figure S7). We further focused on differences in genetic mutations related to EBV-positive gastric cancer in three risk groups. As expected, ARID1A and PIK3CA gene mutations were most frequent in low-risk group (). Cumulatively, these findings suggest that a novel classification approach based on tPDPNs and CD8+ T cells could better stratify gastric cancer patients with distinct prognosis and chemotherapeutic responsiveness.
Figure 5. Stratification based on tPDPNs and CD8+ T cells are associated with prognosis, therapeutic responsiveness to ACT and molecular classification. (a) Pie charts show the proportion of three stratified risk groups in all or stage II and III gastric cancer patients. (b) The overall survival (OS) curves and disease-free survival (DFS) curves for three stratified risk groups. Log-rank test was performed for Kaplan-Meier curves. (c) Cox regression analysis for the difference of responsiveness to adjuvant chemotherapy (ACT) in three risk groups. HR, hazard ratio; CI, confidence interval. (d) Pie charts show the proportion of molecular subtypes including EBV, MSI, GS and CIN in three stratified risk groups in TCGA cohort (up) and Zhongshan cohort (down). EBV, Epstein–Barr virus; MSI, microsatellite instability; GS, genomically stable; CIN, chromosomal instability. (e) ARID1A and PIK3CA gene mutation frequency in three stratified risk groups
Discussion
Currently, immunotherapy with ICKs has been proved to be a promising treatment option in several human cancers. However, the treatment responses are limited and sometimes transient in clinical trials, emphasizing the importance of finding additional or combined strategies to reverse tumor immune evasion. In this regard, previous studies have shown that PDPN can be used as a novel therapeutic target. As evidenced in experiments, neutralizing PDPN function based on chimeric antigen receptor T-cell immunotherapy and corresponding antibodies can inhibit the growth and progression of brain tumors and lung cancer.Citation31–33 Therefore, a better understanding of the clinical significance of PDPN in gastric cancer is crucial. In this study, we found that the abundance of tPDPNs was associated with adverse prognosis and inferior therapeutic responsiveness to fluorouracil-based ACT in gastric cancer, indicating that tPDPNs might be a biomarker with potential therapeutic value.
Numerous studies have shown that tumor immune profiles and immune contexture are closely related to the prognosis and therapeutic responsiveness of patients.Citation29,Citation34,Citation35 In this study, we observed that tPDPNs high subgroup in gastric cancer contained higher pro-tumor macrophages. Interestingly, our previous study revealed that M2 macrophages were positively correlated with PDPN+ cells in muscle-invasive bladder cancer.Citation36 These findings suggest that tPDPNs might collaborate with macrophages in orchestrating the immunoevasive TME. As the main effector cells, CD8+ T cells play an important role in anti-tumor immunity.Citation29 However, emerging studies have indicated two main aspects of CD8+ T cells promoting tumor immune evasion, including T cell exclusion or T cell dysfunction.Citation37 Our study further demonstrated that accumulated CD8+ T cells in tPDPNs high subgroup exhibited dysfunctional status with reduced effector molecules (interferon-γ, granzyme B and perforin-1) and elevated immune checkpoints (PD-1 and TIM-3) using FCM analysis. We also validated that tPDPNs were significantly correlated with total PD-1+ cells and TIM-3+ cells. Consistently, previous studies found that PDPN co-expressed with PD-1 and TIM-3 could promote T cell dysfunction or exhaustion.Citation38,Citation39 Therefore, tPDPNs-associated immunoevasive immune contexture might provide an explanation for adverse clinical outcomes in tPDPNs high subgroup.
Here, we found that CD8+ T cells could only predict good OS and DFS in tPDPNs low subgroup. Therefore, we applied a new classification approach based on tPDPNs and CD8+ T cells to better stratify gastric cancer patients into three risk groups with different prognosis and therapeutic responsiveness to fluorouracil-based ACT. Additionally, we also evaluated the differences in molecular subtypes of these three risk groups and found that low-risk group (tPDPNslowCD8high) had the most EBV-positive gastric cancer and corresponding EBV-related mutation. EBV-positive gastric cancer patients had an improved survival as described previously, which might better explain our findings.Citation40 These results may be clinically valuable, but need further confirmation by larger and independent cohorts.
In summary, our large cohort study identified tPDPNs as an independent predictor for adverse prognosis and inferior responsiveness to ACT. Moreover, tPDPNs play an important role in orchestrating an immunoevasive contexture with dysfunctional CD8+ T cells. The combination of tPDPNs and CD8+ T cells could stratify patients into subgroups with diverse clinical outcomes and molecular classification features. Thus, tPDPNs might serve as a predictive biomarker and a therapeutic target for gastric cancer.
Authors’ contributions
X. Liu, Y. Cao, K. Lv and Y. Gu for acquisition of data, analysis and interpretation of data, statistical analysis, and drafting of the manuscript; K. Jin, X. He, H. Fang, Y. Fei, M. Shi, C. Lin, H. Liu, H. Li and H. He for technical and material support; J. Xu, R. Li and H. Zhang for study concept and design, analysis and interpretation of data, drafting of the manuscript, obtained funding and study supervision. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Clinical Research Ethics Committee of Zhongshan Hospital, Fudan University. Written informed consent was obtained from each patient included and this study was performed in accordance with the Declaration of Helsinki.
Consent for publication
All authors provide their consent for publication of the manuscript.
Supplemental Material
Download ()Acknowledgments
We thank Dr. Lingli Chen (Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai, China) and Dr. Peipei Zhang (Department of Pathology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China) for their excellent pathological technology help.
Data availability
All data generated that are relevant to the results presented in this article are included in this article.
Disclosure statement
The authors declare no conflict of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–11. doi:10.3322/caac.21492.
- Songun I, Putter H, Kranenbarg EM-K, Sasako M, van de Velde CJH. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11(5):439–449. doi:10.1016/S1470-2045(10)70070-X.
- De Vita F, Orditura M, Matano E, Bianco R, Carlomagno C, Infusino S, Damiano V, Simeone E, Diadema MR, Lieto E, et al. A phase II study of biweekly oxaliplatin plus infusional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J Cancer. 2005;92(9):1644–1649. doi:10.1038/sj.bjc.6602573.
- Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. New Engl J Med. 2007;357(18):1810–1820. doi:10.1056/NEJMoa072252.
- Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387–4393. doi:10.1200/JCO.2011.36.5908.
- Bang Y-J, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. The Lancet. 2010;376(9742):687–697. doi:10.1016/S0140-6736(10)61121-X.
- Wilke H, Muro K, Van Cutsem E, Oh S-C, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim T-Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–1235. doi:10.1016/S1470-2045(14)70420-6.
- Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. The Lancet. 2014;383(9911):31–39. doi:10.1016/S0140-6736(13)61719-5.
- Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–723. doi:10.1016/j.cell.2017.01.017.
- Spranger S, Gajewski TF. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat Rev Cancer. 2018;18(3):139–147. doi:10.1038/nrc.2017.117.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi:10.1038/nrc3239.
- Callahan MK, Postow MA, Wolchok JD. Targeting T cell co-receptors for cancer therapy. Immunity. 2016;44(5):1069–1078. doi:10.1016/j.immuni.2016.04.023.
- Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges J-P, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013–e180013. doi:10.1001/jamaoncol.2018.0013.
- Kang Y-K, Boku N, Satoh T, Ryu M-H, Chao Y, Kato K, Chung HC, Chen J-S, Muro K, Kang WK, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. 2017;390(10111):2461–2471. doi:10.1016/S0140-6736(17)31827-5.
- Zeng D, Li M, Zhou R, Zhang J, Sun H, Shi M, Bin J, Liao Y, Rao J, Liao W, et al. Tumor microenvironment characterization in gastric cancer identifies prognostic and immunotherapeutically relevant gene signatures. Cancer Immunol Res. 2019;7(5):737–750. doi:10.1158/2326-6066.CIR-18-0436.
- Cristescu R, Lee J, Nebozhyn M, Kim K-M, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21(5):449–456. doi:10.1038/nm.3850.
- Ugorski M, Dziegiel P, Suchanski J. Podoplanin - a small glycoprotein with many faces. Am J Cancer Res. 2016;6:370–386.
- Uhrin P, Zaujec J, Breuss JM, Olcaydu D, Chrenek P, Stockinger H, Fuertbauer E, Moser M, Haiko P, Fässler R, et al. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood. 2010;115(19):3997–4005. doi:10.1182/blood-2009-04-216069.
- Krishnan H, Rayes J, Miyashita T, Ishii G, Retzbach EP, Sheehan SA, Takemoto A, Chang Y-W, Yoneda K, Asai J, et al. Podoplanin: an emerging cancer biomarker and therapeutic target. Cancer Science. 2018;109(5):1292–1299. doi:10.1111/cas.13580.
- Quintanilla M, Montero-Montero L, Renart J, Martin-Villar E. Podoplanin in Inflammation and Cancer. Int J Mol Sci. 2019;20(3):707. doi:10.3390/ijms20030707.
- Kawaguchi H, El-Naggar AK, Papadimitrakopoulou V, Ren H, Fan YH, Feng L, Lee JJ, Kim E, Hong WK, Lippman SM, Mao L, et al. Podoplanin: a novel marker for oral cancer risk in patients with oral premalignancy. J Clin Oncol. 2008;26(3):354–360.
- Yoshida T, Ishii G, Goto K, Neri S, Hashimoto H, Yoh K, Niho S, Umemura S, Matsumoto S, Ohmatsu H, et al. Podoplanin-positive cancer-associated fibroblasts in the tumor microenvironment induce primary resistance to EGFR-TKIs in lung adenocarcinoma with EGFR mutation. Clin Cancer Res. 2015;21(3):642–651. doi:10.1158/1078-0432.CCR-14-0846.
- Colaprico A, Silva TC, Olsen C, Garofano L, Cava C, Garolini D, Sabedot TS, Malta TM, Pagnotta SM, Castiglioni I, et al. TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016;44(8):e71. doi:10.1093/nar/gkv1507.
- Cao Y, Liu H, Li H, Lin C, Li R, Wu S, Zhang H, He H, Zhang W, Xu J, et al. Association of O 6-methylguanine-DNA methyltransferase protein expression with postoperative prognosis and adjuvant chemotherapeutic benefits among patients with stage II or III gastric cancer. JAMA Surg. 2017;152(11):e173120. doi:10.1001/jamasurg.2017.3120.
- Jiang Y, Xie J, Han Z, Liu W, Xi S, Huang L, Huang W, Lin T, Zhao L, Hu Y, et al. Immunomarker support vector machine classifier for prediction of gastric cancer survival and adjuvant chemotherapeutic benefit. Clin Cancer Res. 2018;24(22):5574. doi:10.1158/1078-0432.CCR-18-0848.
- Yamaguchi T, Fushida S, Yamamoto Y, Tsukada T, Kinoshita J, Oyama K, Miyashita T, Tajima H, Ninomiya I, Munesue S, et al. Tumor-associated macrophages of the M2 phenotype contribute to progression in gastric cancer with peritoneal dissemination. Gastric Cancer. 2016;19(4):1052–1065. doi:10.1007/s10120-015-0579-8.
- Liu X, Cao Y, Li R, Gu Y, Chen Y, Qi Y, Lv K, Wang J, Yu K, Lin C, Liu H, et al. Poor clinical outcomes of intratumoral dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin-positive macrophages associated with immune evasion in gastric cancer. Eur J Cancer. 2020;128:27–37. doi:10.1016/j.ejca.2020.01.002.
- Lin C, He H, Liu H, Li R, Chen Y, Qi Y, Jiang Q, Chen L, Zhang P, Zhang H, et al. Tumour-associated macrophages-derived CXCL8 determines immune evasion through autonomous PD-L1 expression in gastric cancer. Gut. 2019;68(10):1764–1773. doi:10.1136/gutjnl-2018-316324.
- Fridman WH, Zitvogel L, Sautès-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14(12):717–734. doi:10.1038/nrclinonc.2017.101.
- Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–209. doi:10.1038/nature13480.
- Shiina S, Ohno M, Ohka F, Kuramitsu S, Yamamichi A, Kato A, Motomura K, Tanahashi K, Yamamoto T, Watanabe R, et al. CAR T cells targeting podoplanin reduce orthotopic glioblastomas in mouse brains. Cancer Immunol Res. 2016;4(3):259–268. doi:10.1158/2326-6066.CIR-15-0060.
- Chandramohan V, Bao X, Kato Kaneko M, Kato Y, Keir ST, Szafranski SE, Kuan C-T, Pastan IH, Bigner DD. Recombinant anti-podoplanin (NZ-1) immunotoxin for the treatment of malignant brain tumors. Int J Cancer. 2013;132(10):2339–2348. doi:10.1002/ijc.27919.
- Kaneko MK, Yamada S, Nakamura T, Abe S, Nishioka Y, Kunita A, Fukayama M, Fujii Y, Ogasawara S, Kato Y, et al. Antitumor activity of chLpMab-2, a human-mouse chimeric cancer-specific antihuman podoplanin antibody, via antibody-dependent cellular cytotoxicity. Cancer Med. 2017;6(4):768–777. doi:10.1002/cam4.1049.
- Cao Y, He H, Li R, Liu X, Chen Y, Qi Y, Yu K, Wang J, Lin C, Liu H, et al. Latency-associated peptide identifies immunoevasive subtype gastric cancer with poor prognosis and inferior chemotherapeutic responsiveness. Ann Surg. 2020;Publish Ahead of Print. doi:10.1097/SLA.0000000000003833
- Li R, Liu H, Cao Y, Wang J, Chen Y, Qi Y, Lv K, Liu X, Yu K, Lin C, et al. Identification and validation of an immunogenic subtype of gastric cancer with abundant intratumoural CD103+CD8+ T cells conferring favourable prognosis. Br J Cancer. 2020;122(10):1525–1534. doi:10.1038/s41416-020-0813-y.
- Zhou Q, Wang Z, Zeng H, Zhang H, Liu Z, Huang Q, Wang J, Chang Y, Bai Q, Liu L, et al. Identification and validation of poor prognosis immunoevasive subtype of muscle-invasive bladder cancer with tumor-infiltrating podoplanin + cell abundance. OncoImmunology. 2020;9(1):1747333. doi:10.1080/2162402X.2020.1747333.
- Jiang P, Gu S, Pan D, Fu J, Sahu A, Hu X, Li Z, Traugh N, Bu X, Li B, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24(10):1550–1558. doi:10.1038/s41591-018-0136-1.
- Peters A, Burkett PR, Sobel RA, Buckley CD, Watson SP, Bettelli E, Kuchroo VK. Podoplanin negatively regulates CD4+ effector T cell responses. J Clin Invest. 2015;125(1):129–140. doi:10.1172/JCI74685.
- Chihara N, Madi A, Kondo T, Zhang H, Acharya N, Singer M, Nyman J, Marjanovic ND, Kowalczyk MS, Wang C, et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature. 2018;558(7710):454–459. doi:10.1038/s41586-018-0206-z.
- Camargo MC, Kim WH, Chiaravalli AM, Kim K-M, Corvalan AH, Matsuo K, Yu J, Sung JJY, Herrera-Goepfert R, Meneses-Gonzalez F, et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut. 2014;63(2):236–243. doi:10.1136/gutjnl-2013-304531.
