Anticancer immunotherapy has revolutionized the clinical routine of antineoplastic regimen offering novel treatment options for patients with advanced and therapy-resistant disease. In particular immune checkpoint blockade (ICB) with monoclonal antibodies targeting the PD-1/PD-L1 interaction to restore T cell function, has shown promising effects that last beyond treatment discontinuation. Nevertheless, ICB is often overshadowed by severe side effects and only efficient in a minority of patients, resulting in an objective response rate of only 20–40% even under optimal conditions.Citation1
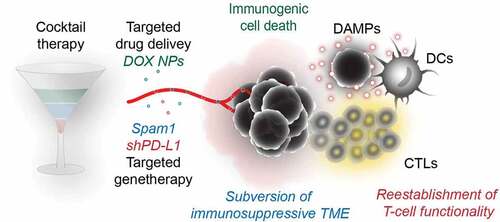
Figure 1. The cocktail therapy consists of a nanoparticle-mediated targeted delivery to the tumor of doxorubicin and a co-delivery of plasmids coding for a small hairpin RNA specific to PD-L1 (pshPD-L1) and hyaluronidase (pSpam1). The immunogenic chemotherapeutic doxorubicin induces immunogenic cell death (ICD) in the tumor and leads to the emission of danger associated molecular patterns (DAMPs) that facilitate the attraction and maturation dendritic cells (DCs) and the priming of T cells. shRNA-mediated PD-L1 downregulation in the tumor mimics the effect of immune checkpoint blockade, thus reestablishing T-cell functionality. Furthermore, hyaluronidase expression targets the immunosuppressive extracellular matrix (ECM) and facilitates the infiltration of peripheral T cells. The combination of immunogenic chemotherapy with gene therapy-mediated immune checkpoint blockade and ECM degradation results in efficient anticancer immunity
The induction of immunogenic cell death (ICD) in malignant cells is yet another immunotherapeutic approach that aims at increasing the adjuvanticity of cancers, thus favoring the dendritic cell (DC)-mediated presentation of tumor antigens to cytotoxic T lymphocytes. ICD leads to the release of danger associated molecular patterns (DAMPs) such as ATP and annexin A1 from stressed and dying cancer cells. ATP and annexin A1 facilitate the chemoattraction and chemotaxis of DCs. Moreover, the ICD-associated surface exposure of calreticulin and the exodus of HMGB1 from the malignant cells promote the transfer of tumor antigens into DCs and their subsequent processing, thus favoring the ignition of T cell-mediated adaptive immunity.Citation2 Recently, the inhibition of DNA-to-RNA transcription by cytotoxic drugs was described as a cardinal mechanism governing ICD.Citation3
Several preclinical studies have shown that ICD induced by chemotherapy or radiotherapy sensitizes to subsequent immunotherapy.Citation4 Thus, ICD induction with oxaliplatin and cyclophosphamide sensitized non-small cell lung cancers (NSCLC) expressing oncogenic Kras and lacking Trp53 to anti-CTLA-4 and anti-PD-1-mediated double ICB.Citation5 Along similar lines the combination of cisplatin with the ICD enhancer crizotinib sensitized NSCLC models to subsequent PD-1 blockade, leading to the cure of ~90% of orthotopic lung cancers.Citation6 These findings have been corroborated by a clinical trial showing that the pretreatment with the ICD inducer doxorubicin increased the likelihood of response to PD-1 blockade in women with triple negative breast cancer.Citation7,Citation8 It needs to be mentioned that this trial had only a limited number of patients in Phase I and that the results showed some degree of inter-arm variability.Citation9
In a recent issue of Science Advances, Wu and colleagues described a novel approach for the functional combination of ICD and ICB with the additional expression of extracellular matrix (ECM)-degrading hyaluronidase, together yielding elevated levels of tumor associated CD8+ T-cells and resulting anticancer immunity in several subcutaneous models of cancer.Citation10 This so-called “cocktail therapy” consists of an acid-responsive nanoparticle-delivery system facilitating the tumor-specific delivery of ICD-inducing doxorubicin and the parallel distribution of a plasmid encoding a small hairpin RNA targeting PD-L1 as an alternative to antibody-mediated ICB. Indeed, this cocktail therapy can be administered intravenously, and the nanoparticles (which either contain doxorubicin or DNA) are then preferentially taken up by tumor cells located in an acidic milieu, thus avoiding the cardiotoxicity of doxorubicin and the immune-related (systemic) adverse effects of PD-L1 blockade, but achieving a local ICD and ICB effect.Citation10 However, the immunogenic chemotherapy and PD-L1-targeting gene therapy had to be combined with the delivery of DNA encoding for hyaluronidase to be optimally efficient, pointing to an important immunosuppressive role for the ECM that inhibits tumor infiltration by peripheral T cells (). Cocktail therapy achieved tumor eradication of B16F10 melanoma in 5 out of 6 mice and allowed prolonged tumor growth control in several other models of subcutaneous cancers. Furthermore, cocktail therapy led to the generation of CD8+ effector memory T cells in the spleen, inhibited the establishment of lung metastasis and prolonged overall survival. Altogether, this study underlines the efficacy of combinations of ICD and ICB, that can be optimized by tumor-targeted delivery systems as well as by approaches targeting ECM.Citation10
In sum, the work by Wu et al. introduces a toolbox for the targeted induction of ICD, subversion of the immunosuppressive tumor microenvironment, as well as the restoration of T-cell mediated immunity. It will be interesting to witness if all (or some) of these strategies will have translational value and hence will be successfully evaluated in cancer patients.
Disclosure of potential conflicts of interest
G.K. and O.K. are cofounders of Samsara Therapeutics.
Additional information
Funding
References
- Hwang WL, Pike LRG, Royce TJ, Mahal BA, Loeffler JS. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat Rev Clin Oncol. 2018;15:477–2. doi:10.1038/s41571-018-0046-7.
- Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. doi:10.1038/nri.2016.107.
- Humeau J, Sauvat A, Cerrato G, Xie W, Loos F, Iannantuoni F, Bezu L, Lévesque S, Paillet J, Pol J, et al. Inhibition of transcription by dactinomycin reveals a new characteristic of immunogenic cell stress. EMBO Mol Med. 2020;12(5):e11622. doi:10.15252/emmm.201911622.
- Rodriguez-Ruiz ME, Vitale I, Harrington KJ, Melero I, Galluzzi L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat Immunol. 2020;21:120–134.
- Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, Yamazaki T, Poirier-Colame V, Newton A, Redouane Y, et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity. 2016;44(2):343–354. doi:10.1016/j.immuni.2015.11.024.
- Liu P, Zhao L, Pol J, Levesque S, Petrazzuolo A, Pfirschke C, Engblom C, Rickelt S, Yamazaki T, Iribarren K, et al. Crizotinib-induced immunogenic cell death in non-small cell lung cancer. Nat Commun. 2019;10(1):1486. doi:10.1038/s41467-019-09415-3.
- Voorwerk L, Slagter M, Horlings HM, Sikorska K, van de Vijver KK, de Maaker M, Nederlof I, Kluin RJC, Warren S, Ong S, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25(6):920–928. doi:10.1038/s41591-019-0432-4.
- Kepp O, Zitvogel L, Kroemer G. Clinical evidence that immunogeniccell death sensitizes to PD-1/PD-L1 blockade. Oncoimmunology. 2019;8:e1637188. doi:10.1080/2162402X.2019.1637188.
- Demaria S, Romano E, Brackstone M, Formenti SC. Immune induction strategies to enhance responses to PD-1 blockade: lessons from the TONIC trial. J Immunother Cancer. 2019;7:318. doi:10.1186/s40425-019-0783-x.
- Wu J, Chen J, Feng Y, Zhang S, Lin L, Guo Z, Sun P, Xu C, Tian H, Chen X, et al. An immune cocktail therapy to realize multiple boosting of the cancer-immunity cycle by combination of drug/gene delivery nanoparticles. Sci Adv. 2020;6(40):eabc7828. doi:10.1126/sciadv.abc7828.
