ABSTRACT
Metastatic clear cell renal cell carcinoma (mccRCC) benefits from several treatment options in the first-line setting with VEGFR inhibitors and/or immunotherapy including anti-PD-L1 or anti-PD1 agents. Identification of predictive biomarkers is highly needed to optimize patient care. Circulating markers could reflect the biology of metastatic disease. Therefore, we evaluated soluble forms of PD-L1 (sPD-L1) and PD-1 (sPD-1) in mccRCC patients. The levels of sPD-L1 and sPD-1 were evaluated from plasma samples of mccRCC patients before they received a first-line treatment (T0) by the VEGFR inhibitor sunitinib (50 patients) or by the anti-VEGF bevacizumab (37 patients). The levels of sPD-L1 and sPD-1 were correlated to clinical parameters and progression-free survival (PFS). High levels of sPD-1 or sPDL1 were not correlated to PFS under bevacizumab while they were independent prognostic factors of PFS in the sunitinib group. Patients with high T0 plasmatic levels of sPD-L1 had a shorter PFS (11.3 vs 22.5 months, p = .011) in the sunitinib group. Equivalent shorter PFS was found with high levels of sPD-1 (8.6 vs 14.1 months, p = .009). mccRCC patients with high plasmatic levels of sPD-L1 or sPD-1 are poor responders to sunitinib. sPD-L1 or sPD-1 could be a valuable tool to guide the optimal treatment strategy including VEGFR inhibitor.
Introduction
Clear cell renal cell carcinoma (ccRCC) accounts for approximately 75% of renal cancer. Non-metastatic ccRCC is mainly treated by surgery.Citation1 Until recently, agents targeting the vascular endothelial growth factor (VEGF) or VEGF-receptors (VEGFR) pathway were the mainstay of mccRCC treatment; among them, interferon-alpha combined with bevacizumab (anti-VEGF) or sunitinib (VEGFR inhibitor) were the first agents that improved the survival of mccRCC patients. Sunitinib, which inhibits angiogenesis and induces tumor cell apoptosis, has been the most widely prescribed agent worldwide.Citation2 Recently, several other treatment options demonstrated a better efficacy as compared to sunitinib. Indeed, immunotherapy has revolutionized the first-line treatment of mccRCC, with immune-oncology (IO) drug combinations (anti-PD-1 nivolumab and anti-CTLA-4 ipilimumab) in IMDC (International Metastatic RCC database Consortium) intermediate and poor-risk patientsCitation3 or combination of VEGFR inhibitors and IO (axitinib associated to the anti-PD-1 pembrolizumab or anti-PD-L1 avelumab) in all IMDC risk groups.Citation4,Citation5 However, considering these multiple new options, the identification of the optimal treatment for a specific patient is still an unanswered question. More specifically, no available biomarker predicts the efficacy of the nivolumab-ipilimumab or the IO-VEGFR inhibitor combination. A specific marker predicting the efficacy of VEGFR inhibitors could help the administration of the optimal therapy.
PD-L1 is a cell surface ligand of the PD-1 receptor expressed by T lymphocytes. PD-1 is expressed on immune cells, such as activated T lymphocytes whereas PD-L1 is expressed by B cells, dendritic cells, macrophages, but also by tumor and endothelial cells. The stimulation of PD-1 by PD-L1 and PD-1 activates a mechanism of immune tolerance exerted by tumor cells which leads to the inhibition of cytotoxic T cells.Citation6 PD-L1 expression by tumor cells is associated with worse prognosis for patients with many types of tumors including ccRCC.Citation7,Citation8 Although PD-L1 staining appears to correlate with a better outcome from immune-based combinations like nivolumab-ipilimumab,Citation3 it is a poor discriminating factor for clinical decisions. Furthermore, the evaluation of PD-L1 expression by immunohistochemistry (IHC) has not been yet considered as a gold-standard. The detection of PD-L1 expression by IHC presents several flaws related to the tumor preparation, the use of antibodies with different affinities, the specificity and ability to bind to different PD-L1 epitopes, the choice of the cell population expressing it (tumor versus stromal cells) and the criteria/cutoff used in the interpretation of the results.Citation9 Finally, PD-L1 expression is usually assessed on the primary tumor and less often on metastases, although PD-1 and PD-L1 are differentially expressed in the primary tumor and the metastases.Citation10
The expression of soluble forms of PD-1 (sPD-1) and PD-L1 (sPD-L1) can be detected in the peripheral blood (serum or plasma). The exact origin of sPD-1 and sPD-L1 remains unclear. As soluble forms of other membrane proteins, they result from the cleavage of their extracellular domains or from alternative splicing of the pre-mRNA coding for the membrane form. sPD-1 and sPD-L1 were initially described in autoimmune disorders in which they are produced by immune cells upon stimulation by the proinflammatory cytokines IFN-α, IFN-γ, or TNF-α.Citation11
sPD-1 derived mainly from an alternative splicing of the pre-mRNA encoding the PD-1 Deltaex3 variant.Citation12 sPD-L1 is mainly released through proteolytic cleavage by matrix metalloproteinases of membrane-associated PD-L1 present on tumor cells, on immature dendritic cells, on mesenchymal stromal cells, on myeloid-derived suppressive cells and on T cells.Citation13,Citation14
A positive correlation between sPD-1 and sPD-L1 levels was described in hepatocellular carcinoma, oral, lung and triple-negative breast cancers (TNBC) indicating that sPD-1 and sPD-L1 play an important role in the occurrence and the development of malignant tumors.Citation14,Citation15
Increased sPD-L1 levels were correlated with poor clinical outcomes in head and neck squamous cell carcinoma,Citation16 large B-cell lymphoma,Citation17 melanoma,Citation18 pancreaticCitation19,Citation20 and ovarian cancers.Citation21
However, the prognostic role of sPD-1 seems to depend on the type of cancers, leading either to good or poor clinical outcomes. For example, high plasma levels of sPD-1 have been positively associated with inflammation and poor clinical outcomes in pancreatic cancer patients,Citation20 in TNBCCitation15 and in non-small cell lung cancer,Citation12 while it was a favorable prognostic factor in hepatocellular carcinoma.Citation22 Moreover, increased sPD-1 was correlated with prolonged survival in patients with advanced EGFR-mutated non-small cell lung cancer treated with erlotinib.Citation23
Limited results are available for ccRCC patients since sPD-L1 and sPD-1 have been studied only in a pre-operative non-metastatic setting. In these patients, sPD-L1 and sPD-1 are correlated with a poor prognosis,Citation24 tumor size and tumor grade.Citation25,Citation26 The impact of sPD-L1 and sPD-1 on the response to treatment and survival in mccRCC patients is still unknown. The aim of this study was to determine the prognostic/predictive value of sPD-L1 and sPD-1 in mccRCC patients treated with the antiangiogenic agents sunitinib or bevacizumab.
Methods
Patients
The population of the study included mccRCC patients from the prospective SUVEGIL (17 patients) and TORAVA (45 patients) trials and from a retrospective cohort (33 patients) from Pavia (Italy) and Rennes (France). See supplementary Methods.
These studies were approved by the ethic committee at each participating center (Center Antoine Lacassagne (Nice, France) for patients included in SUVEGIL trial (NCT00943839), Center Léon Bérard (Lyon, France) for patients included in TORAVA trial (NCT00619268), Rennes (France) and Pavia (Italia) hospitals for other patients) and run in agreement with the International Conference on Harmonization of Good Clinical Practice Guideline. The study was performed in accordance with the Declaration of Helsinki.
Efficacy and safety
Blood samples were collected at baseline, before the beginning of the treatment (T0) and after a 4-week period (1 cycle of sunitinib or 2 cycles of bevacizumab administration, T1) for biochemical analyses.
Stage score
RCC was classified according to the tumor, node and metastasis (TNM) system developed by the American Joint Committee on Cancer (AJCC). RCC was classified from stage I to stage IV according to the TNM. Stage is a prognostic score. Stage I: T1 N0 M0; stage II: T2 N0 M0; stage III: T3 or N1 M0 and stage IV: T4 or M1.
Biochemical analyses
Blood samples were centrifuged (10 000 g for 10 min) and the plasmas were collected and conserved at −80°C. Plasmatic levels of sPD-1 and sPD-L1 were determined by ELISAs produced by DYNABIO S.A. (Parc de Luminy, Marseille France) according to their specifications. See supplementary Methods.
Statistical analysis
Progression-free survival (PFS) was defined as the time between blood sample collection and progression, or death from any cause, censoring those alive and progression free at last follow-up. Overall survival (OS) was defined as the time from blood sample collection to the date of death from any cause, censoring those alive at last follow-up. The sPD-L1 and sPD-1 cutoff point (0.1 ng ml−1 and 1.67 ng ml−1 respectively) for PFS was determined using spline curve analysis. T-test was applied to compare continuous variables, and chi-square test or Fisher’s exact test (when the application condition of χ2-test was not fulfilled) was used for categorical variables. Kaplan–Meier method was used to produce survival curves and analyses of censored data were performed using the log-rank test. To guarantee the independence of sPD-L1 and sPD-1 as a predictive factor from validated predictive factors, a multivariate analysis was performed using Cox regression adjusted on stage score. Adjusted hazard ratio (HR) and 95% confidence interval (95% CI) were calculated. All analyses were performed using R software, version 3.2.2 (Vienna, Austria, https://www.r-project.org/).
Results
Patients characteristics
Fifty (50) patients with mccRCC were included and treated in the first line by sunitinib, and 37 patients were treated by bevacizumab in combination with either IFN-α or temsirolimus. The population characteristics and pathological parameters are summarized in . In the sunitinib group, median PFS and OS were of 13.9 months and 36 months, respectively. In the bevacizumab group, median PFS and OS were of 10.9 months and 24.4 months, respectively ().
Table 1. Characteristics of the patients included in the study
sPD-L1 and sPD-1 levels in the plasma of metastatic ccRCC patients
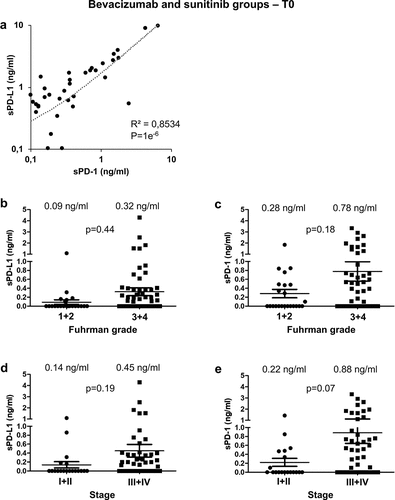
The mean pre-treatment (T0) levels of plasmatic sPD-L1 and sPD1 were 0.33 ng/ml and 0.65 ng/ml, respectively (Supplementary Figure S1a). The levels of sPD-1 correlated to those of sPD-L1 (R2 = 0.8534, p = 1e−6, ). sPD-L1 and sPD-1 levels were increased in high Fuhrman grade (3 + 4) and high stage (III+IV) tumors (but did not reach statistical significance; -e). After a 4-week period (T1) with sunitinib or bevacizumab, plasmatic levels of sPD-L1 or sPD-1 remained stable (Supplementary Figure S1b-e).
Figure 1. Analysis of sPD-L1 and sPD-1 in the plasma of metastatic ccRCC patients and prognostic relevance of clinical and biological marker. (a) The plasmatic levels of sPD-L1 and sPD-1 were determined by ELISA in the plasma of mccRCC patients just before the start of the systemic treatment (T0). The correlation coefficient between the two values was calculated. (b to e) The plasmatic levels of sPD-L1 (b, d) and sPD-1 (c, e) were correlated to Fuhrman grade (1 + 2 vs 3 + 4; b, c) and to tumor stage (I+ II vs III+IV; d, e). The mean value is indicated
PFS in bevacizumab and sunitinib groups and correlation to sPD-L1 and sPD-1 plasmatic levels
Plasmatic levels of sPD-L1 and sPD-1 were correlated with PFS and OS. In the bevacizumab group, PFS was not impacted by the levels of sPD-L1 (7.7 vs 11.4 months, and Supplementary Table S1) or by the levels of sPD-1 (11.9 vs 11.5 months, and Supplementary Table S2). Equivalent results were obtained for OS (Supplementary Figure S2a, b).
Figure 2. Relationship between plasmatic levels of sPD-L1 or sPD-1 and PFS of mccRCC patients treated with bevacizumab or sunitinib and prognostic relevance of clinical and biological markers. (a to d) Kaplan–Meier analysis of PFS of patients with mccRCC treated with bevacizumab (a, b) or sunitinib (c, d). PFS was calculated from patient subgroups with plasmatic level for sPD-L1 at the diagnosis (T0) that were less than or greater than a cutoff value of 0.1 ng ml−1 (a, c) or for patient subgroups with plasmatic level for sPD-1 at the diagnosis that were less than or greater than a cutoff value of 1.67 ng ml−1 (b, d). Statistical significance (P value and Hazard ratio) and the time of the median PFS are indicated. (e) Forest plot of Hazard Ratio (CI95%) of PFS univariate analysis. The prognostic relevance of each marker was assessed by a univariate survival analysis using the Cox regression model
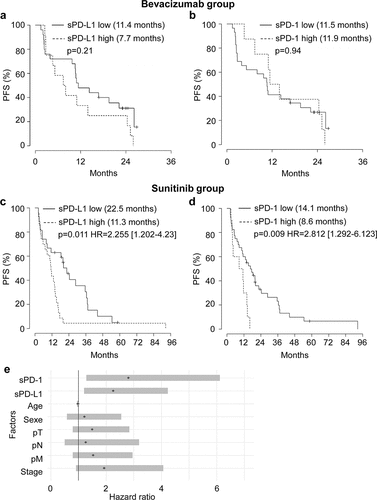
In the sunitinib group, patients with sPD-L1 plasma levels superior to 0.1 ng ml−1 (range 0.1–6.46 ng ml−1) had a shorter PFS (11.3 months) as compared to patients with plasma levels below 0.1 ng ml−1 (range 0–0.1 ng ml−1, 22.5 months, P = .011; HR 2.255 (CI 95% 1.202–4.23), ). Patients with sPD-1 plasma levels superior to 1.67 ng ml−1 (range 1.67–9.67 ng ml−1) had a shorter PFS (8.6 months) as compared to patients with plasma levels below 1.67 ng ml−1 (range 0–1.67 ng ml−1, 14.1 months, P = .009; HR 2.812 (CI 95% 1.292–6.123), ). However, sPD-L1 and sPD-1 levels did not influence OS (Supplementary Figure S2c, d) in the sunitinib group.
Correlations between sPD-1/sPD-L1 and other clinicopathological characteristics in the sunitinib group
We applied a univariate Cox regression model, including clinical characteristics (age, gender, pT, pN, pM, stage or Fuhrman grade). Stage score, sPD-L1 (˂0.1 ng ml−1) and sPD-1 (˂1.67 ng ml−1) were predictive factors of PFS ().
As shown in and , the levels of sPD-L1 or sPD-1 and clinical characteristics of patients in the sunitinib group were not correlated. Only high levels of sPD-1 were correlated to a high tumor size (pT 3/4, p = .008).
Table 2. Clinical parameters, univariate and multivariate analyses of mccRCC patients treated by sunitinib and stratified by sPD-L1
Table 3. Clinical parameters, univariate and multivariate analysis of mccRCC patients treated by sunitinib and stratified by sPD-1
Levels of sPD-L1 or sPD-1 and stage score were then analyzed in a multivariate Cox regression model on PFS. sPD-L1 expression was identified as an independent prognostic factor of PFS (P = .0053, HR 2.677 (CI 95% 1.338–5.356), ). sPD-1 expression was also identified as an independent prognostic factor of PFS (P = .008, HR 3.149 (CI 95% 1.353–7.328), ).
Discussion
Relevant prognostic markers or predictive biomarkers of treatment efficacy in mccRCC are currently lacking. sPD-1 and s-PD-L1, easily detectable in the plasma of patients, appear as a valuable tool to implement in clinical practices. Analysis of plasma levels of sPD-1 and s-PD-L1 makes it possible to assess the overall biology of the metastases (and not only partially as in the case of a biopsy). Such analysis also helps to evaluate the immune context present within these metastases and in the blood. In many cancers, the amount of sPD-L1 is not correlated with the expression of PD-L1 detected by IHC. It can be explained by the abundance of PD-L1 expressing cells in the tumor microenvironment. High PD-L1 expression by tumor cells does not predict a shorter survival of mccRCC patients receiving an antiangiogenic treatment, but its expression by stromal cells (IHC staining on primary tumor) represents a robust prognostic marker of survival.Citation27 The evaluation of sPD-L1 at the diagnosis of a metastatic disease is, therefore, more relevant as compared to the evaluation of PD-L1 by IHC especially in the primary tumor that has been surgically removed several years before the metastatic relapse.Citation13
Our results showed that sPD-L1 and sPD-1 were independent prognostic factors of PFS in mccRCC patients treated by sunitinib. Inconsistent results obtained with patients treated by bevacizumab strongly suggest that sPD-L1 and sPD-1 are predictive markers of VEGFR inhibitor efficacy. These results, if validated in independent cohorts, could be of high value to guide treatment strategy in mccRCC, even in the current context of combined VEGFR- and immune checkpoint-inhibitors.
sPD-L1 and sPD-1 have a predictive value only for sunitinib but not for all anti-angiogenic drugs including the anti-VEGF antibody, bevacizumab. This result probably reflects the pleiotropic effects of sunitinib which targets several tyrosine kinase receptors (VEGFR but also PDGFR, CSF1R and c-KIT) and which induces the death of RCC cells, unlike bevacizumab.
While high levels of sPD-L1 and sPD-1 are correlated to shorter PFS in the sunitinib group, they are not correlated to OS. These results can be explained by heterogeneous second- and third-line treatments including IO (nivolumab, anti-PD-1), everolimus (mTOR inhibitor) and other inhibitors of tyrosine kinase receptors (VEGFRs, PDGFR, c-Kit (axitinib), VEGFR2, c-MET, AXL, RET, FLT3 (cabozantinib)).
As described in several cancers, our results showed that high levels of sPD-L1 were correlated to a rapid metastatic progression linked to the exhaustion of T cell lymphocytes via the PD-1/PD-L1 signaling.
Our results clearly showed that patients with high levels of sPD-1 had a shorter PFS when treated with sunitinib. This result is unexpected since sPD-1 should have served as a decoy inactive receptor trapping and limiting PD-1/PD-L1-dependent signaling and subsequent T cell exhaustion. Different unexpected roles of sPD-1 depending on the type of cancers probably explain the different prognostic value of sPD-1. Nevertheless, some hypotheses emerged from our observations:
1) Consistent with our results, a positive correlation between sPD-1 and sPD-L1 levels was described in hepatocellular carcinoma, oral, lung and TNB cancers indicating that sPD-1 and sPD-L1 play an important role in the occurrence and development of malignant tumors;Citation14,Citation15
2) High levels of sPD-1 are correlated to inflammation in cancers but also in acute respiratory distress syndromes. In ccRCC, chronic inflammation is correlated to a poor prognosis and to a limited response to sunitinib.Citation28
3) sPD-1 is essentially expressed by CD4+ and CD8 + T lymphocytes. In ccRCC, the majority of CD8 + T lymphocytes are exhausted and expressed PD-1, Tim-3 and Lag-3.Citation29,Citation30 Moreover, sPD-1 favors the proliferation of exhausted PD-1-expressing CD8 + T lymphocytes that also expressed Tim-3+ and Lag3 +. Hence, high levels of CD8 + T lymphocytes are correlated to a poor prognosis.Citation31
Our study showed that mccRCC patients displaying high sPD-L1 or sPD-1 levels had shorter PFS on sunitinib. Thus, patients with high levels of sPD-L1 or sPD-1 could theoretically be eligible for the nivolumab and ipilimumab combination while patients with low levels of sPD-L1 or sPD-1 could benefit of the VEGFR inhibitor/IO combination. This therapeutic strategy based on sPD-L1/sPD-1 detection needs to be validated by future clinical trials.
In conclusion, plasmatic levels of sPD-L1 or sPD-1 were independent prognostic markers and relevant predictive markers of PFS in mccRCC patients treated with sunitinib in the first line. The detection of these two plasmatic markers could be a valuable tool to guide the optimal treatment strategy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors’ contribution
Conceptualization: JI, MD and GP. Methodology: CM, AH and MD. Formal Analysis: BT, EC and MD. Investigation and data acquisition: CM, AH and MD. Resources: DB, DA, NRL, CP, SN and JMF. Writing: GP and MD. Writing Review and Editing: DB, JI and CP. Funding Acquisition: GP and MD.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Ethics approval
These studies were approved by the ethic committee at each participating center (Centre Antoine Lacassagne (Nice, France) for patients included in SUVEGIL trial (NCT00943839), Centre Léon Bérard (Lyon, France) for patients included in TORAVA trial (NCT00619268), Rennes (France) and Pavia (Italia) hospitals for other patients) and run in agreement with the International Conference on Harmonization of Good Clinical Practice Guideline. The study was performed in accordance with the Declaration of Helsinki.
Supplementary Materials
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, Grünwald V, Gillessen S, Horwich A. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2019;30(5):706–8. doi:10.1093/annonc/mdz056.
- Giuliano S, Cormerais Y, Dufies M, Grépin R, Colosetti P, Belaid A, Parola J, Martin A, Lacas-Gervais S, Mazure NM, et al. Resistance to sunitinib in renal clear cell carcinoma results from sequestration in lysosomes and inhibition of the autophagic flux. Autophagy. 2015;11(10):1891–1904. doi:10.1080/15548627.2015.1085742.
- Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018;378(14):1277–1290. doi:10.1056/NEJMoa1712126.
- Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019;380(12):1116–1127. doi:10.1056/NEJMoa1816714.
- Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S, Uemura M, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019;380(12):1103–1115. doi:10.1056/NEJMoa1816047.
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–274. doi:10.1038/nrc1586.
- Kahlmeyer A, Stöhr CG, Hartmann A, Goebell PJ, Wullich B, Wach S, Taubert H, Erlmeier F. Expression of PD-1 and CTLA-4 are negative prognostic markers in renal cell carcinoma. JCM. 2019;8(5):743. doi:10.3390/jcm8050743.
- Chipollini J, da Costa WH, Werneck da Cunha I, de Almeida E Paula F, Salles P GO, Azizi M, Spiess PE, Abreu D, Zequi S de C. Prognostic value of PD-L1 expression for surgically treated localized renal cell carcinoma: implications for risk stratification and adjuvant therapies. Ther Adv Urol. 2019;11:175628721988260. doi:10.1177/1756287219882600.
- Torlakovic E, Lim HJ, Adam J, Barnes P, Bigras G, Chan AWH, Cheung CC, Chung J-H, Couture C, Fiset PO, et al. “Interchangeability” of PD-L1 immunohistochemistry assays: a meta-analysis of diagnostic accuracy. Mod Pathol. 2020;33(1):4–17. doi:10.1038/s41379-019-0327-4.
- Zhang X, Yin X, Zhang H, Sun G, Yang Y, Chen J, Zhu X, Zhao P, Zhao J, Liu J, et al. Differential expressions of PD-1, PD-L1 and PD-L2 between primary and metastatic sites in renal cell carcinoma. BMC Cancer. 2019;19(1):360. doi:10.1186/s12885-019-5578-4.
- Zhu X, Soluble LJ. PD-1 and PD-L1: predictive and prognostic significance in cancer. Oncotarget. 2017;8(57):97671–97682. doi:10.18632/oncotarget.18311.
- Tiako Meyo M, Jouinot A, Giroux-Leprieur E, Fabre E, Wislez M, Alifano M, Leroy K, Boudou-Rouquette P, Tlemsani C, Khoudour N, et al. Predictive value of soluble PD-1, PD-L1, VEGFA, CD40 ligand and CD44 for nivolumab therapy in advanced non-small cell lung cancer: a case-control study. Cancers. 2020;12(2):473. doi:10.3390/cancers12020473.
- Aghajani MJ, Roberts TL, Yang T, McCafferty CE, Caixeiro NJ, DeSouza P, Niles N. Elevated levels of soluble PD-L1 are associated with reduced recurrence in papillary thyroid cancer. Endocrine Connections. 2019;8(7):1040–1051. doi:10.1530/EC-19-0210.
- Gu D, Ao X, Yang Y, Chen Z, Xu X. Soluble immune checkpoints in cancer: production, function and biological significance. J Immunotherapy Cancer. 2018;6(1):132. doi:10.1186/s40425-018-0449-0.
- Li Y, Cui X, Yang Y-J, Chen -Q-Q, Zhong L, Zhang T, Cai R-L, Miao J-Y, Yu S-C, Zhang F. Serum sPD-1 and sPD-L1 as biomarkers for evaluating the efficacy of neoadjuvant chemotherapy in triple-negative breast cancer patients. Clin Breast Cancer. 2019;19(5):326–332.e1. doi:10.1016/j.clbc.2019.03.008.
- Theodoraki M-N, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL. Clinical significance of PD-L1 + exosomes in plasma of head and neck cancer patients. Clin Cancer Res. 2018;24(4):896–905. doi:10.1158/1078-0432.CCR-17-2664.
- Cho I, Lee H, Yoon SE, Ryu KJ, Ko YH, Kim WS, Kim SJ. Serum levels of soluble programmed death-ligand 1 (sPD-L1) in patients with primary central nervous system diffuse large B-cell lymphoma. BMC Cancer. 2020;20(1):120. doi:10.1186/s12885-020-6612-2.
- Zhou J, Mahoney KM, Giobbie-Hurder A, Zhao F, Lee S, Liao X, Rodig S, Li J, Wu X, Butterfield LH, et al. Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol Res. 2017;5(6):480–492. doi:10.1158/2326-6066.CIR-16-0329.
- Park H, Bang J-H, Nam A-R, Eun Park J, Hua Jin M, Bang Y-J, Oh D-Y. Prognostic implications of soluble programmed death-ligand 1 and its dynamics during chemotherapy in unresectable pancreatic cancer. Sci Rep. 2019;9(1):11131. doi:10.1038/s41598-019-47330-1.
- Bian B, Fanale D, Dusetti N, Roque J, Pastor S, Chretien A-S, Incorvaia L, Russo A, Olive D, Iovanna J. Prognostic significance of circulating PD-1, PD-L1, pan-BTN3As, BTN3A1 and BTLA in patients with pancreatic adenocarcinoma. OncoImmunology. 2019;8(4):e1561120. doi:10.1080/2162402X.2018.1561120.
- Buderath P, Schwich E, Jensen C, Horn PA, Kimmig R, Kasimir-Bauer S, Rebmann V. Soluble programmed death receptor ligands sPD-L1 and sPD-L2 as liquid biopsy markers for prognosis and platinum response in epithelial ovarian cancer. Front. Oncol. 2019;9:1015. doi:10.3389/fonc.2019.01015.
- Chang B, Huang T, Wei H, Shen L, Zhu D, He W, Chen Q, Zhang H, Li Y, Huang R, et al. The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2019;68(3):353–363. doi:10.1007/s00262-018-2271-4.
- Sorensen SF, Demuth C, Weber B, Sorensen BS, Meldgaard P. Increase in soluble PD-1 is associated with prolonged survival in patients with advanced EGFR -mutated non-small cell lung cancer treated with erlotinib. Lung Cancer. 2016;100:77–84. doi:10.1016/j.lungcan.2016.08.001.
- Fukuda T, Kamai T, Masuda A, Nukui A, Abe H, Arai K, Yoshida K-I. Higher preoperative serum levels of PD-L1 and B7-H4 are associated with invasive and metastatic potential and predictable for poor response to VEGF-targeted therapy and unfavorable prognosis of renal cell carcinoma. Cancer Med. 2016;5(8):1810–1820. doi:10.1002/cam4.754.
- Kushlinskii NE, Gershtein ES, Morozov AA, Goryacheva IO, Filipenko ML, Alferov AA, Bezhanova SD, Bazaev VV, Kazantseva IA. Soluble ligand of the immune checkpoint receptor (sPD-L1) in blood serum of patients with renal cell carcinoma. Bull Exp Biol Med. 2019;166(3):353–357. doi:10.1007/s10517-019-04349-8.
- Wang Q, Zhang J, Tu H, Liang D, W CD, Ye Y, Wu X. Soluble immune checkpoint-related proteins as predictors of tumor recurrence, survival, and T cell phenotypes in clear cell renal cell carcinoma patients. J Immunotherapy Cancer. 2019;7(1):334. doi:10.1186/s40425-019-0810-y.
- Yao J-X, Chen X, Xi W, Zhu Y-J, Wang H, Hu X-Y, Guo J-M. Immunoscore system for predicting clinical outcome of metastatic renal cell carcinoma patients treated with tyrosine kinase inhibitors. J. Cancer. 2018;9(22):4099–4107. doi:10.7150/jca.27408.
- Beuselinck B, Job S, Becht E, Karadimou A, Verkarre V, Couchy G, Giraldo N, Rioux-Leclercq N, Molinié V, Sibony M, et al. Molecular subtypes of clear cell renal cell carcinoma are associated with sunitinib response in the metastatic setting. Clin. Cancer Res. 2015;21(6):1329–1339. doi:10.1158/1078-0432.CCR-14-1128.
- Chevrier S, Levine JH, Zanotelli VRT, Silina K, Schulz D, Bacac M, Ries CH, Ailles L, Jewett MAS, Moch H, et al. An immune atlas of clear cell renal cell carcinoma. Cell. 2017;169(4):736–749.e18. doi:10.1016/j.cell.2017.04.016.
- Ghatalia P, Gordetsky J, Kuo F, Dulaimi E, Cai KQ, Devarajan K, Bae S, Naik G, Chan TA, Uzzo R, et al. Prognostic impact of immune gene expression signature and tumor infiltrating immune cells in localized clear cell renal cell carcinoma. J Immunotherapy Cancer. 2019;7(1):139. doi:10.1186/s40425-019-0621-1.
- Mella M, Kauppila JH, Karihtala P, Lehenkari P, Jukkola-Vuorinen A, Soini Y, Auvinen P, Vaarala MH, Ronkainen H, Kauppila S, et al. Tumor infiltrating CD8 + T lymphocyte count is independent of tumor TLR9 status in treatment naïve triple negative breast cancer and renal cell carcinoma. OncoImmunology. 2015;4(6):e1002726. doi:10.1080/2162402X.2014.1002726.
