ABSTRACT
Background: The gut microbiota has a key role in the regulation of the immune system. Disruption of the gut microbiota’s composition by antibiotics might significantly affect the efficacy of immune checkpoint inhibitors. In a study of patients treated with ipilimumab, we sought to assess the relationship between overall survival and in-hospital antibiotic administration.
Methods: Patients having been treated with ipilimumab between January 2012 and November 2014 were selected from the French National Hospital Discharge Summary Database. Exposure to antibiotics was defined as the presence of a hospital stay with a documented systemic bacterial infection in the 2 months before or the month after initiation of the patient’s first ever course of ipilimumab. The primary outcome was overall survival.
Results: We studied 43,124 hospital stays involving 1585 patients from 97 centers. All patients had received ipilimumab monotherapy for advanced melanoma. Overall, 117 of the 1585 patients (7.4%) were documented as having received systemic antibiotic therapy in hospital during the defined exposure period. The median overall survival time was shorter in patients with infection (6.3 months, vs. 15.4 months in patients without an infection; hazard ratio (HR) = 1.88, 95% confidence interval [1.46; 2.43], p = 10−6). In a multivariate analysis adjusted for covariates, infection was still significantly associated with overall survival (HR = 1.68, [1.30; 2.18], p = 10−5).
Conclusions: In patients treated with ipilimumab for advanced melanoma, infection, and antibiotic administration in hospital at around the time of the patient’s first ever course of ipilimumab appears to be associated with significantly lower clinical benefit.
Introduction
Since their clinical introduction in 2010, immune checkpoint inhibitors (ICIs) have revolutionized cancer management. This drug class has become the fifth pillar of cancer treatment, along with surgery, radiotherapy, chemotherapy, and targeted therapy.Citation1 In France, the first marketing authorization for an ICI was granted in July 2011, for the monoclonal antibody ipilimumab as a second-line treatment for advanced (unresectable or metastatic) melanoma.Citation2 The drug was initially prescribed under a temporary authorization for use, and reimbursement under the national social security system was obtained in April 2012. In October 2013, ipilimumab’s marketing authorization was extended to first-line treatment. The marketing authorization and reimbursement covered up to four courses of ipilimumab monotherapy, i.e. a total of 3 months of treatment. Up until 2014, melanoma was the only approved indication for ICIs in France.Citation2 Ipilimumab binds to CTLA4, which has a major role in regulating T cell function.Citation3 The antibody lifts constraints on preexisting anticancer T cell responses and might also trigger a new immune response.Citation4
Antibiotics are widely used in oncology. Treatment with antibiotics alters the gut microbiota qualitatively and quantitatively.Citation5 The exact nature of these changes depends on many factors, including the class of antibiotic,Citation5,Citation6 the duration of administration,Citation6 and the dose administered.Citation7 Furthermore, the gut microbiota have a key role in regulating the immune balance.Citation8–10 It has therefore been hypothesized that by disturbing the gut microbiota, antibiotics may be responsible (at least in part) for the lack of an effective response to ICIs observed in some patients.Citation11–13
This hypothesis was first explored in several preclinical studies.Citation11–13 From 2017 onwards, several retrospective and prospective clinical studies then evaluated the association between antibiotics and the efficacy of immunotherapy.Citation14–30 All of these studies showed significantly worse progression-free and/or overall survival in the group of patients exposed to antibiotics. The relative decrease in median overall survival ranged from 3.8 to 16.7 months.Citation16 The only study that did not evidence lower efficacy had a small study population (n = 74 patients).Citation23 Nevertheless, the retrospective nature of these studies and their small sample sizes limit the robustness of the conclusions; additional data on this issue are required.
Routine clinical practice generates huge amounts of data.Citation31 This is notably the case for the French National Hospital Discharge Summary Database (PMSI), whose data can be reused for medical research.Citation32–34 Some drugs of interest have a specific reimbursement process that requires exhaustive reporting in the PMSI database; this is the case for certain monoclonal antibodies in the PMSI database,Citation35 and especially ipilimumab.
The primary objective of the present study was to determine whether the overall survival of patients treated with ipilimumab is influenced by antibiotics. To this end, we performed a data reuse analysis of the PMSI database and assessed the association between the overall survival of patients treated with ipilimumab for advanced melanoma on one hand and the presence of hospital stays with an infection and in-hospital antibiotic administration on the other.
Material & methods
Study design and data source
We carried out a population-based, retrospective cohort study using data extracted from the PMSI database, which contains standardized discharge reports from all patients discharged from for-profit or nonprofit hospitals in France. Each discharge report provides administrative and demographic data, and information on diagnoses, diagnostic procedures, therapeutic procedures, and some specific administered drugs. Diagnoses are encoded as primary or secondary diagnoses using the French version of the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10).Citation36 Therapeutic and diagnostic procedures are recorded according to the French “common classification of medical procedures” terminology (CCAM).Citation37 Administered drugs (when recorded) are encoded with the French common dispensing unit (CDU) terminology.Citation38 Discharge reports are mandatory and serve as the basis for the funding of both for-profit and nonprofit hospitals. This database also includes a unique, anonymous patient identifier that enables one to link together all a given patient’s inpatient stays – even when the patient has been admitted to several different healthcare facilities.
This study had been conducted in compliance with the French Regulation (MR004), and was approved by the French data protection agency (Commission nationale de l’informatique et des libertés (Paris, France), approval number: DE-2017-277). All data were anonymized. According to French Regulation, obtaining written consent does not apply to research work using such a database.
Study population
We searched the PMSI database for hospital discharges of adult (over-18) patients who being admitted for a first course of ipilimumab (hereafter denoted as “C1”) between January 1st, 2012, and November 30th, 2014 (CDU codes: 9374067 and 9374050). It should be noted that at this time, ipilimumab was only authorized as a monotherapy for malignant melanoma. The selected patients were included at C1 and followed up until December 2014. We also extracted data on all hospital stays by the selected patients between January 1st, 2008, and C1.
Study variables
The following items of information were extracted for each patient: age, sex, ICD-10 diagnoses, CCAM procedures, CDU drug codes, and inpatient stays (admission and discharge dates). Using feature extraction, the patients were classified according to their ICD-10 diagnosis.Citation34
The proxy for systemic in-hospital exposure to antibiotics was defined as any hospital admission with a diagnosis of a systemic bacterial infection (see the list of codes in the supplementary material: Appendix 1) beginning in the 2 months before or in the month following the study inclusion date (i.e. C1). We defined two study groups: the “infection” group comprised all patients with at least one hospital admission with a systemic bacterial infection, and the “no infection” group comprised all other selected patients.
The study outcome was death from any cause. The primary endpoint was overall survival, defined as the time interval between C1 and the date of death from any cause. For non-deceased patients, data were censored at the end of the exhaustive follow-up period (December 2014).
The study consisted of three parts. Firstly, our main analysis took account of the period of possible antibiotic exposure most commonly used in the literatureCitation16 (i.e. the 2 months before C1 and in the month following C1). Secondly, we performed a sensitivity analysis that considered only exposure during the 2 months before C1. Lastly, we performed a negative control analysis by considering antibiotic exposure far from C1 (i.e. between 12 and 2 months before C1).
Statistical analysis
Continuous variables were described as the mean (standard deviation (SD)) or the median [interquartile range (IQR)], as appropriate. Categorical variables were described as the frequency (percentage). To compare the characteristics of the “infection” vs. “no infection” groups, we used the chi-squared test or Fisher’s exact tests for categorical variables and Welch’s two-sample T-test for quantitative variables.
The Kaplan-Meier method was used to estimate survival rates overall and in subgroups. The hazard ratio (HR) for death associated with infection was estimated using Cox models – first in a bivariate analysis, and then in a multivariate analysis adjusted for possible confounders. The following covariates (all available in the PMSI) were considered: age (18–39 vs. 40–65 vs. >65 years), sex, severe malnutrition at C1, brain metastases at or before C1, previous chemotherapy, and the cumulative length of hospital stays before C1 (<50 days vs. ≥50 days, taking into account all possible stays in the period 2008–2014). The initial multivariate model included all covariates associated with a p-value <0.20 in bivariate analyses. The final multivariate model included only covariates associated with p-value <0.05.
All estimates are quoted with their 95% confidence intervals [95%CI]. All tests were two-sided, and the threshold for statistical significance was set to p < .05.
There were no missing data. All statistical analyses were performed using R software.Citation39
Results
Patient population and characteristics
We identified 1585 adult patients having received ipilimumab monotherapy for advanced melanoma between January 1st, 2012, and November 30th, 2014, and extracted a total of 43,124 hospital stays in 97 different French hospitals from 2008 to 2014. C1 occurred in 2012 for 121 patients (7.6%), in 2013 for 469 patients (29.6%), and in 2014 for 995 patients (62.8%).
Overall, 117 (7.4%) patients were considered to have received antibiotics in hospital during the main exposure period (i.e. the 2 months before C1 and the month following C1). It should be noted that a given patient could have several infections during the period of exposure. The following infection sites were recorded: the skin (n = 35, 21.6%), the respiratory tract (n = 34, 20.4%), the digestive system (n = 23, 14.4%), the urinary tract (n = 18, 11.1%), bones/joints (n = 12, 7.4%), catheters (n = 10, 6.2%), the cardiac valves (n = 6, 3.7%), the upper respiratory tract (n = 4, 2.5%), the brain or the meninges (n = 2, 1.2%), or an unknown site (n = 18, 11.1%). Inpatient stays with infection accounted for 42% of all stays during the defined exposure period.
The mean number of courses of ipilimumab was lower in patients with an infection than in patients without an infection (2.7 vs. 3.6, p = 10−5).
Baseline patient characteristics are described in .
Table 1. Patient population and baseline characteristics
The main analysis
According to the reverse Kaplan-Meier method, the median follow-up time was 8.7 months. 592 deaths were observed. The median [IQR] overall survival rate was 71% [69;73] at 6 months and 55% [52;58] at 12 months. As illustrated by the Kaplan-Meier curves (), overall survival from C1 was significantly lower in patients with an infection than in patients without an infection (HR = 1.88 [1.46; 2.43], p = 10−6), leading to median overall survival times of 6.3 and 15.4 months, respectively.
Figure 1. Overall survival according to the Kaplan-Meier method, main analysis (p-value from the Cox bivariate model)
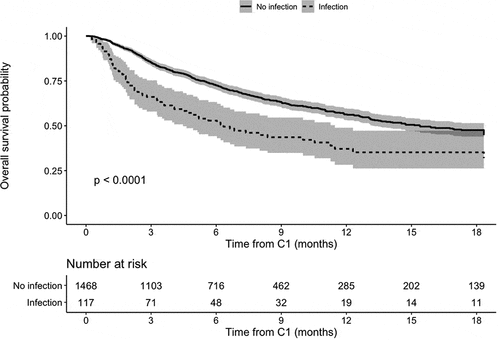
The estimated effect size associated with infection was stable and statistically significant in a multivariate analysis, with an HR of 1.68 ([1.30; 2.18], p = 10−5) after adjustment for severe malnutrition and brain metastases ().
Table 2. The Cox model
The sensitivity analysis
In the sensitivity analysis (considering the 2 months before C1 as the exposure period), 71 of the 1585 (4.5%) patients were considered to have received antibiotics in hospital.
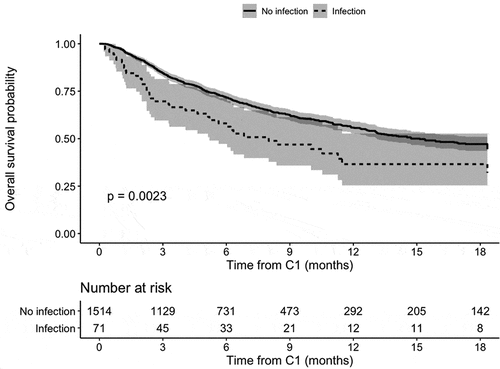
The HR (1.65, [1.19; 2.28], p = .0023) was very similar to that obtained in the main analysis. The median overall survival time was 8 months in patients with an infection and 15.1 months in patients without an infection. The Kaplan-Meier estimates are given in Appendix 2.
The negative control analysis
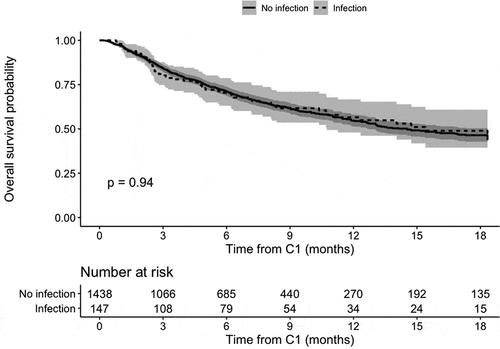
When considering an exposure period between 12 and 2 months before C1, 147 of the 1585 (9.3%) patients had received antibiotics in hospital. The median overall survival was similar in the two groups: 15.4 months in patients with an infection and 14.5 months in patients without an infection (HR = 0.99, [0.75; 1.30], p = .94). The Kaplan-Meier estimates are given in Appendix 3.
Discussion
In the present study of patients treated with ipilimumab for advanced melanoma, we examined the association between the presence of hospital stays with bacterial infection and in-hospital antibiotic administration on one hand and overall survival on the other. Our main finding is that antibiotic exposure in the 2 months before C1 and in the month following C1 was significantly associated with a decrease in overall survival (unadjusted HR = 1.88; adjusted-HR = 1.68). The median survival time was 6.3 months in patients with an infection and 15.4 months in patients without an infection. The same association was found when the exposure period was restricted to the 2 months before C1. We did not find any association when considering antibiotic exposure far from ICI treatment (i.e. between 12 and 2 months before C1).
By reducing the exposure period to the 2 months prior to C1, the sensitivity analysis reduced several sources of bias in antibiotic exposure: (i) administration related to a deterioration in general condition (e.g. the absence of early effectiveness of immunotherapy, unrelated to the infection and the administration of antibiotics), and (ii) administration for the treatment of adverse events associated with ipilimumab (e.g. colitis).
The purpose of the negative control analysis was to determine whether an infection long before C1 had any impact on survival. The lack of an association in the negative control analysis reinforced the hypothesis whereby recent or concomitant administration of antibiotics could disrupt the effectiveness of ICI by altering the gut microbiota.
Our present results are in agreement with most studies of the association between infections and ipilimumab. These were also retrospective studies. Elkrief et al. found shorter survival times in their group of melanoma patients with an infection during the month before the first course of ipilimumab (n = 10/74, 13.5%): the median progression-free survival was significantly shorter (2.4 months, vs 7.3 months in patients without an infection, HR = 0.28, [0.10; 0.76], p = .01), as was the median overall survival time (10.7 vs. 18.3 months, respectively; HR = 0.52, [0.21; 1.32], p = .17).Citation17 In Tinsley et al.’s study, which included a majority of melanomas (61.5%), the administration of antibiotics during the period 2 weeks before or 6 weeks after ipilimumab initiation was associated with a significantly shorter median progression-free survival time (3.1 months, vs. 6.3 months patients without antibiotics; HR = 1.56, p = .003) and median overall survival time (10.4 vs. 21.7 months, respectively; HR = 1.70, p = .002).Citation28 Most recently, Mohiuddin et al. analyzed 568 patients with stage III or IV melanoma. In a multivariate analysis of patients with stage IV melanoma, overall survival was significantly worse in the antibiotic-exposed group than in the non-exposed group (HR = 1.81, [1.27; 2.57]).Citation40
Our study had several strengths. Firstly, to the best of our knowledge, it constituted the largest yet study (in terms of the sample size) to have addressed this issue. Secondly, the study population was highly representative, with exhaustive data collection. For example, our data for 2014 covered more than 99% of patients newly treated with ipilimumab, as identified by French National Cancer Institute (Institut National du Cancer).Citation2 Thirdly, the present study is the first (to the best of our knowledge) to have used a national database to explore this issue. Lastly, this was a real-life cohort study.
However, the study also had some limitations. Firstly, we did not have access to primary data on the antibiotic administration as per or to data on outpatients. This may have led us to consider some “exposed” patients as “non-exposed” patients, i.e. classification bias. Secondly, we did not have access to detailed data on the cancer subtype (e.g. BRAF mutation status) or antibiotic exposure (e.g. drug class, combination, or not, route, duration, and dose). Consequently, our multivariate model contained a small number of covariates and therefore only partially captured the indications for antibiotics. This, even though the clinical context in which antibiotics are administered must be kept in mind: advanced disease is more likely to be complicated by infections (i.e. an indication bias).Citation24 Lastly, it should be noted that the data in this work concerned exclusively ipilimumab, and that ipilimumab is no longer the standard monotherapy for advanced melanoma. Indeed, the data available for this study only extended to December 31, 2014. This is due to the delay between the collection of the data in the PMSI database and the availability of these data for their analysis. However, this work will constitute the background and the proof-of-concept for the exploitation of the most recent PMSI data. These data will include other types of ICIs (particularly anti-PD1) and other cancer sites (including lung, kidney, and bladder).
As in previously published studies, the effect of antibiotics per se cannot be disentangled from that of the infection. Resolving this issue is both methodologically and ethically challenging.
Our study’s prime conclusion is that the administration of antibiotics close to (and especially just before) the first course of ipilimumab may explain (at least in part) the non-response observed in some cancer patients. The question is then whether these patients should receive another class of therapeutic agent. Our second conclusion is that it is important to prescribe antibiotics sparingly and to limit their use to strictly necessary situations. However, for some patients, antibiotic use appears to be unavoidable. In these cases, ongoing clinical studies are testing ways of reverting antibiotic-induced dysbiosis: fecal microbiota transplantation (NCT03353402, NCT03341143, NCT04116775, NCT04163289), microbial ecosystem therapeutics (NCT03686202) and probiotics (NCT03637803, NCT03829111), for example.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Authors’ contributions
PYC, NB, NP, and EC conceived and designed the study. PYC and EC analyzed the data. PYC, NB, MCLD, NP, and EC interpreted the results. PYC, NB, and EC drafted the manuscript. All authors critically reviewed or revised the manuscript. All the authors read and approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Abbreviations
Availability of data and material
Requests for access to the data can be submitted to the corresponding author.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Michot JM, Lazarovici J, Tieu A, Champiat S, Voisin AL, Ebbo M, Godeau B, Michel M, Ribrag V, Lambotte O. Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur J Cancer. 2019;122:72–8. doi:10.1016/j.ejca.2019.07.014.
- Denis H, Davoine C, Bermudez E, Grosjean G, Schwager M, Ifrah N, Dahan M, Negellen S. Les immunothérapies spécifiques dans le traitement des cancers. Bull Cancer. 2019;106(1):37–47. doi:10.1016/j.bulcan.2018.12.007.
- Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19(1):565–594. doi:10.1146/annurev.immunol.19.1.565.
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi:10.1038/nature10673.
- Iizumi T, Battaglia T, Ruiz V, Perez Perez GI. Gut microbiome and antibiotics. Arch Med Res. 2017;48(8):727–734. doi:10.1016/j.arcmed.2017.11.004.
- Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. 2019;79(6):471–489. doi:10.1016/j.jinf.2019.10.008.
- Nord CE, Gajjar DA, Grasela DM. Ecological impact of the des-F(6)-quinolone, BMS-284756, on the normal intestinal microflora. Clin Microbio Infect. 2002;8(4):229–239. doi:10.1046/j.1469-0691.2002.00414.x.
- Kelly D, Mulder IE. Microbiome and immunological interactions. Nutr Rev. 2012;70(Suppl 1):S18–30. doi:10.1111/j.1753-4887.2012.00498.x.
- Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011;32(6):256–264. doi:10.1016/j.it.2011.04.003.
- Diehl GE, Longman RS, Zhang J-X, Breart B, Galan C, Cuesta A, Schwab SR, Littman DR. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX 3 CR1 hi cells. Nature. 2013;494(7435):116–120. doi:10.1038/nature11809.
- Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CPM, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi:10.1126/science.aad1329.
- Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Man Lei Y, Jabri B, Alegre M-L, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–1089. doi:10.1126/science.aac4255.
- Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi:10.1126/science.aan3706.
- Ahmed J, Kumar A, Parikh K, Anwar A, Knoll BM, Puccio C, Chun H, Fanucchi M, Lim SH. Use of broad-spectrum antibiotics impacts outcome in patients treated with immune checkpoint inhibitors. Oncoimmunology. 2018;7(11):e1507670. doi:10.1080/2162402X.2018.1507670.
- Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, Long N, Plodkowski AJ, Arbour KC, Chaft JE, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018;29(6):1437–1444. doi:10.1093/annonc/mdy103.
- Elkrief A, Derosa L, Kroemer G, Zitvogel L, Routy B. The negative impact of antibiotics on outcomes in cancer patients treated with immunotherapy: a new independent prognostic factor? Ann Oncol. 2019;30(10):1572–1579. doi:10.1093/annonc/mdz206.
- Elkrief A, El Raichani L, Richard C, Messaoudene M, Belkaid W, Malo J, Belanger K, Miller W, Jamal R, Letarte N, et al. Antibiotics are associated with decreased progression-free survival of advanced melanoma patients treated with immune checkpoint inhibitors. Oncoimmunology. 2019;8(4):e1568812. doi:10.1080/2162402X.2019.1568812.
- Farmakiotis D. The human microbiome and checkpoint inhibition: potential benefits from antibiotic stewardship. Clin Infect Dis. 2019. doi:10.1093/cid/ciz1003.
- Galli G, Triulzi T, Proto C, Signorelli D, Imbimbo M, Poggi M, Fucà G, Ganzinelli M, Vitali M, Palmieri D, et al. Association between antibiotic-immunotherapy exposure ratio and outcome in metastatic non small cell lung cancer. Lung Cancer. 2019;132:72–78. doi:10.1016/j.lungcan.2019.04.008.
- Guo J-C, Lin -C-C, Lin C-Y, Hsieh M-S, Kuo H-Y, Lien M-Y, Shao -Y-Y, Huang T-C, Hsu C-H. Neutrophil-to-lymphocyte ratio and use of antibiotics associated with prognosis in esophageal squamous cell carcinoma patients receiving immune checkpoint inhibitors. Anticancer Res. 2019;39(10):5675–5682. doi:10.21873/anticanres.13765.
- Hakozaki T, Okuma Y, Omori M, Hosomi Y. Impact of prior antibiotic use on the efficacy of nivolumab for non-small cell lung cancer. Oncol Lett. 2019;17:2946–2952. doi:10.3892/ol.2019.9899.
- Huemer F, Rinnerthaler G, Westphal T, Hackl H, Hutarew G, Gampenrieder SP, Weiss L, Greil R. Impact of antibiotic treatment on immune-checkpoint blockade efficacy in advanced non-squamous non-small cell lung cancer. Oncotarget. 2018;9(23):16512–16520. doi:10.18632/oncotarget.24751.
- Kaderbhai C, Richard C, Fumet JD, Aarnink A, Foucher P, Coudert B, Favier L, Lagrange A, Limagne E, Boidot R, Ghiringhelli Fet al. Antibiotic use does not appear to influence response to nivolumab. Anticancer Res. 2017;37:3195–3200. doi:10.21873/anticanres.11680.
- Milano G. Efficacy of immunotherapy, gut microbiota and impact of antibiotic use: are there confounding factors? Cancer Chemother Pharmacol. 2019;84(1):223–224. doi:10.1007/s00280-019-03833-2.
- Ouaknine Krief J, Helly de Tauriers P, Dumenil C, Neveux N, Dumoulin J, Giraud V, Labrune S, Tisserand J, Julie C, Emile J-F, et al. Role of antibiotic use, plasma citrulline and blood microbiome in advanced non-small cell lung cancer patients treated with nivolumab. J Immunother Cancer. 2019;7:176. doi:10.1186/s40425-019-0658-1.
- Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019;5(12):1774. doi:10.1001/jamaoncol.2019.2785.
- Sen S, Carmagnani Pestana R, Hess K, Viola GM, Subbiah V. Impact of antibiotic use on survival in patients with advanced cancers treated on immune checkpoint inhibitor phase I clinical trials. Ann Oncol. 2018;29(12):2396–2398. doi:10.1093/annonc/mdy453.
- Tinsley N, Zhou C, Tan G, Rack S, Lorigan P, Blackhall F, Krebs M, Carter L, Thistlethwaite F, Graham D, et al. Cumulative antibiotic use significantly decreases efficacy of checkpoint inhibitors in patients with advanced cancer. Oncologist. 2019. doi:10.1634/theoncologist.2019-0160.
- Yan C, Tu -X-X, Wu W, Tong Z, Liu -L-L, Zheng Y, Jiang W-Q, Zhao P, Fang W-J, Zhang H-Y, et al. Antibiotics and immunotherapy in gastrointestinal tumors: friend or foe? World J Clin Cases. 2019;7:1253–1261. doi:10.12998/wjcc.v7.i11.1253.
- Zhao S, Gao G, Li W, Li X, Zhao C, Jiang T, Jia Y, He Y, Li A, Su C, et al. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer. 2019;130:10–17. doi:10.1016/j.lungcan.2019.01.017.
- Baro E, Degoul S, Beuscart R, Chazard E. Toward a literature-driven definition of big data in healthcare. Biomed Res Int. 2015;2015:639021. doi:10.1155/2015/639021.
- Meystre SM, Lovis C, Bürkle T, Tognola G, Budrionis A, Lehmann CU. Clinical data reuse or secondary use: current status and potential future progress. Yearb Med Inform. 2017:26. doi:10.15265/IY-2017-007.
- Safran C. Reuse of clinical data. Yearb Med Inform. 2014;9:52–54. doi:10.15265/IY-2014-0013.
- Chazard E, Ficheur G, Caron A, Lamer A, Labreuche J, Cuggia M, Genin M, Bouzille G, Duhamel A, et al. Secondary use of healthcare structured data: the challenge of domain-knowledge based extraction of features. Stud Health Technol Inform. 2018;255:15–19.
- Thillard E-M, Gautier S, Babykina E, Carton L, Amad A, Bouzillé G, Beuscart J-B, Ficheur G, Chazard E. Psychiatric adverse events associated with infliximab: a cohort study from the french nationwide discharge abstract database. Front Pharmacol. 2020;11:513. doi:10.3389/fphar.2020.00513.
- CIM-10. 2008 [accessed 2018 Mar 27]. http://apps.who.int/classifications/icd10/browse/2008/fr
- CPAM. CCAM Principes généraux. 2011 [accessed 2018 Mar 27]. https://www.ameli.fr/fileadmin/user_upload/documents/CCAM-Principes_generaux_mars2011.pdf
- Assurance Maladie. Guide pratique pour la facturation des médicaments rétrocédés par les établissements de santé; 2009. [accessed 2020 Jun 17]. https://www.ameli.fr/sites/default/files/Documents/5229/document/facturation-medicaments-retrocedes_assurance-maladie.pdf
- R Development Core Team. R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2011.
- Mohiuddin JJ, Chu B, Facciabene A, Poirier K, Wang X, Doucette A, Zheng C, Xu W, Anstadt EJ, Amaravadi RK, et al. Association of antibiotic exposure with survival and toxicity in patients with melanoma receiving immunotherapy. J Natl Cancer Inst. n.d. doi:10.1093/jnci/djaa057.
Appendix 1.
ICD-10 codes for the diagnosis of bacterial infections considered to be very strongly associated with the administration of antibiotics
A00 A000 A001 A009 A01 A010 A011 A012 A013 A014 A02 A020 A021 A022 A028 A029 A03 A030 A031 A032 A033 A038 A039 A04 A040 A041 A042 A043 A044 A045 A046 A047 A048 A049 A05 A050 A051 A052 A053 A054 A058 A059 A06 A060 A061 A062 A063 A064 A065 A066 A067 A068 A069 A070 A071 A072 A073 A085 A15 A150 A151 A152 A153 A154 A155 A156 A157 A158 A159 A16 A160 A161 A162 A163 A164 A165 A167 A168 A169 A17 A170 A171 A178 A179 A18 A180 A181 A182 A183 A184 A185 A186 A187 A188 A19 A190 A191 A192 A198 A199 A20 A200 A201 A202 A203 A207 A208 A209 A21 A210 A211 A212 A213 A217 A218 A219 A22 A220 A221 A222 A227 A228 A229 A23 A230 A231 A232 A233 A238 A239 A24 A240 A241 A242 A243 A244 A25 A250 A251 A259 A26 A260 A267 A268 A269 A27 A270 A278 A279 A28 A280 A281 A282 A288 A289 A30 A300 A301 A302 A303 A304 A305 A308 A309 A31 A310 A311 A318 A319 A32 A320 A321 A327 A328 A329 A33 A34 A35 A36 A360 A361 A362 A363 A368 A369 A37 A370 A371 A378 A379 A38 A39 A390 A391 A392 A393 A394 A395 A398 A399 A40 A400 A401 A402 A403 A408 A409 A41 A410 A411 A412 A413 A414 A415 A418 A419 A42 A420 A421 A422 A427 A428 A429 A43 A430 A431 A438 A439 A44 A440 A441 A448 A449 A46 A48 A480 A481 A482 A483 A484 A488 A49 A490 A491 A492 A493 A498 A499 A50 A500 A501 A502 A503 A504 A505 A506 A507 A509 A51 A510 A511 A512 A513 A514 A515 A519 A52 A520 A521 A522 A523 A527 A528 A529 A53 A530 A539 A54 A540 A541 A542 A543 A544 A545 A546 A548 A549 A55 A56 A560 A561 A562 A563 A564 A568 A57 A58 A59 A590 A598 A599 A63 A630 A6300 A6308 A638 A64 A65 A66 A660 A661 A662 A663 A664 A665 A666 A667 A668 A669 A67 A670 A671 A672 A673 A679 A68 A680 A681 A689 A69 A690 A691 A692 A698 A699 A70 A71 A710 A711 A719 A74 A740 A748 A749 A75 A750 A751 A752 A753 A759 A77 A770 A771 A772 A773 A778 A779 A78 A79 A790 A791 A798 A799 B50 B500 B508 B509 B51 B510 B518 B519 B52 B520 B528 B529 B53 B530 B531 B538 B54 B55 B550 B551 B552 B559 B56 B560 B561 B569 B57 B570 B571 B572 B573 B574 B575 B58 B580 B581 B582 B583 B588 B589 B59 B60 B600 B601 B602 B608 B6080 B6088 B64 B65 B650 B651 B652 B653 B658 B659 B66 B660 B661 B662 B663 B664 B665 B668 B669 B67 B670 B671 B672 B673 B674 B675 B676 B677 B678 B95 B950 B951 B952 B953 B954 B955 B956 B957 B958 B96 B960 B961 B962 B963 B964 B965 B966 B967 B968 B9680 B9681 B9688 B98 B980 B99 B99 + 0 B99 + 1 D733 E321 G00 G000 G001 G002 G003 G008 G009 G01 G039 G04 G042 G050 G06 G060 G061 G062 G07 H600 H602 H620 H66 H660 H664 H669 H670 I320 I33 I330 I339 I38 I39 I398 I40 I400 I401 I408 I409 I410 I520 J01 J010 J011 J012 J013 J014 J018 J019 J03 J030 J038 J039 J04 J040 J041 J042 J13 J14 J15 J150 J151 J152 J153 J154 J155 J156 J157 J158 J159 J16 J160 J168 J170 J181 J189 J200 J201 J202 J340 J390 J391 J85 J850 J851 J852 J853 J86 J860 J869 K046 K047 K113 K122 K223 K251 K252 K255 K256 K261 K262 K265 K266 K271 K272 K275 K276 K281 K282 K285 K286 K35 K350 K351 K352 K353 K358 K359 K36 K37 K570 K572 K574 K578 K61 K610 K611 K612 K613 K614 K630 K65 K650 K658 K659 K670 K671 K672 K673 K750 K800 K801 K803 K804 K81 K810 K830 K831 K832 K833 L02 L020 L021 L022 L023 L024 L028 L029 L03 L030 L031 L032 L033 L038 L039 L04 L040 L041 L042 L043 L048 L049 L050 L080 L88 M00 M000 M0000 M0001 M0002 M0003 M0004 M0005 M0006 M0007 M0008 M0009 M001 M0010 M0011 M0012 M0013 M0014 M0015 M0016 M0017 M0018 M0019 M002 M0020 M0021 M0022 M0023 M0024 M0025 M0026 M0027 M0028 M0029 M008 M0080 M0081 M0082 M0083 M0084 M0085 M0086 M0087 M0088 M0089 M009 M0090 M0091 M0092 M0093 M0094 M0095 M0096 M0097 M0098 M0099 M01 M010 M0100 M0101 M0102 M0103 M0104 M0105 M0106 M0107 M0108 M0109 M011 M0110 M0111 M0112 M0113 M0114 M0115 M0116 M0117 M0118 M0119 M012 M0120 M0121 M0122 M0123 M0124 M0125 M0126 M0127 M0128 M0129 M013 M0130 M0131 M0132 M0133 M0134 M0135 M0136 M0137 M0138 M0139 M13 M462 M4620 M4621 M4622 M4623 M4624 M4625 M4626 M4627 M4628 M4629 M463 M4630 M4632 M4633 M4634 M4635 M4636 M4637 M4638 M4639 M464 M4640 M4642 M4643 M4644 M4645 M4646 M4647 M4648 M4649 M465 M4650 M4651 M4652 M4653 M4654 M4655 M4656 M4657 M4658 M4659 M492 M4920 M4921 M4922 M4923 M4924 M4925 M4926 M4927 M4928 M4929 M630 M650 M6500 M6501 M6502 M6503 M6504 M6505 M6506 M6507 M6508 M6509 M680 M710 M7100 M7101 M7102 M7103 M7104 M7105 M7106 M7107 M7108 M7109 M725 M7250 M7251 M7252 M7253 M7254 M7255 M7256 M7257 M7258 M7259 M726 M7260 M7261 M7262 M7263 M7264 M7265 M7266 M7267 M7268 M7269 M86 M860 M8600 M8601 M8602 M8603 M8604 M8605 M8606 M8607 M8608 M8609 M861 M8610 M8611 M8612 M8613 M8614 M8615 M8616 M8617 M8618 M8619 M900 M9000 M9001 M9002 M9003 M9004 M9005 M9006 M9007 M9008 M9009 N110 N111 N151 N30 N300 N309 N330 N340 N410 N412 N45 N450 N70 N700 N709 N730 N731 N732 N733 N734 N735 N740 N741 N742 N743 N744 N748 N75 N751 N760 N762 N764 N771 O23 O230 O231 O232 O233 O234 O235 O239 S01 S010 S011 S012 S013 S014 S015 S017 S018 S019 S0201 S0211 S0221 S0231 S0241 S0251 S0261 S0271 S0281 S0291 S0601 S0611 S0621 S0631 S0641 S0651 S0661 S0671 S0681 S0691 S11 S110 S111 S112 S117 S118 S119 S1201 S1211 S1221 S1271 S1281 S1291 S21 S210 S211 S212 S217 S218 S219 S2201 S2211 S2221 S2231 S2241 S2281 S2291 S2681 S2691 S2701 S2711 S2721 S2731 S2741 S2751 S2761 S2771 S2781 S2791 S31 S310 S311 S312 S313 S314 S315 S317 S318 S3201 S3211 S3221 S3231 S3241 S3251 S3271 S3281 S3611 S3621 S3631 S3641 S3651 S3661 S3671 S3681 S3691 S3701 S3711 S3721 S3731 S3741 S3751 S3761 S3771 S3781 S37810 S3791 S41 S410 S411 S417 S418 S4201 S4211 S4221 S4231 S4241 S4271 S4281 S4291 S51 S510 S517 S518 S519 S5201 S5211 S5221 S5231 S5241 S5251 S5261 S5271 S5281 S5291 S61 S610 S611 S617 S618 S619 S6201 S6211 S6221 S6231 S6241 S6251 S6261 S6271 S6281 S71 S710 S711 S717 S718 S7201 S7211 S7221 S7231 S7241 S7271 S7281 S7291 S81 S810 S817 S818 S819 S8201 S8211 S8221 S8231 S8241 S8251 S8261 S8271 S8281 S8291 S91 S910 S911 S912 S913 S917 S9201 S9211 S9221 S9231 S9241 S9251 S9271 S9291 T01 T010 T011 T012 T013 T016 T018 T019 T0201 T0211 T0221 T0231 T0241 T0251 T0261 T0271 T0281 T0291 T08 + 1 T091 T10 + 1 T111 T12 + 1 T131 T141 T1421 T36 T360 T361 T362 T363 T364 T365 T366 T368 T369 T802 T814 T826 T827 T835 T836 T845 T846 T847 T857 T874 T880 U80 U800 U801 U808 U81 U810 U818 U82 U820 U820 + 0 U821 U8210 U82100 U8218 U82180 U822 U822 + 0 U822 + 1 U828 U828 + 0 U829 U829 + 0 U83 U830 + 0 U831 U831 + 0 U832 U832 + 0 U837 U8370 U83700 U8371 U83710 U8378 U83780 U838 U838 + 0U839 U839 + 0.
Appendix 2.
Kaplan-Meier overall survival in a sensitivity analysis
Appendix 2. Kaplan-Meier overall survival in a sensitivity analysis
Appendix 3.
Kaplan-Meier overall survival in a negative control analysis
Appendix 3. Kaplan-Meier overall survival in a negative control analysis